Abstract
Quantum chemistry methods were used to calculate the energy parameters of an elementary unit and a cellulose macromolecule dimer (cellobiose), and structure simulation was performed and the energy of interaction between the fragments of native cellulose macromolecules was calculated. It was established that the trans conformation of cellobiose is more stable than the cis conformation by 6.7 kcal/mol. Differences in the calculated and real (according to literature data) IR spectra of cellulose were related to the presence of intramolecular and intermolecular hydrogen bonds in the native structure. It was shown that the interaction of individual fragments of cellulose macromolecules from eight monomer units is due to the manifestation of intramolecular hydrogen bonds. It was found that the energies of intermolecular interactions ∆Е essentially depend on the terminal groups X in the cellulose macromolecule fragments, and they are –26, 49, and ‒32 kcal/mol for X = –H, –COOH, and –COH, respectively. The structure of the interacting fragments of cellulose macromolecules can be regulated by replacing the hydrogen atoms of hydroxyl or terminal groups of the macromolecules with functional groups that do not form intramolecular hydrogen bonds and impede self-organization into fibrillar structures. It was shown that compounds with a high electron affinity or a negative energy of the lower vacant molecular orbital are the best reagents for complexation reactions with cellulose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The advantages of cellulose as a potential component of composite materials are biodegradability and valuable structural and mechanical properties. Recently, special attention has been paid to nanofibrillar cellulose [1–3] because of its increased strength, which is due to the intra- and intermolecular hydrogen bonds of cellulose macromolecules. In this case, to ensure the effective interaction of fibrillar nanocellulose macromolecules with the matrix of a dispersion medium, problems should be solved to ensure the successive replacement of the aqueous phase of the initial nanocellulose hydrogels by an oleophilic phase. This can be achieved by selecting appropriate solvents and by chemically modifying the nanocellulose in order to ensure its hydrophobization. These problems are general in nature, and they are significant in the manufacture of nanocomposite polymeric materials based on nanocellulose for use in various branches of industry (construction, oil, structural, aviation and automotive, medical, etc.). Nanocellulose can be a component of biodegradable lubricant compositions as an organic thickener. It is well known [4, 5] that biodegradable greases can be prepared based on nanocellulose and vegetable oils, which are of exceptional importance for applications at low temperatures. Experimental data on the structure and properties of cellulose and its derivatives have been published in the current scientific literature, the interpretation of which should be carried out on the basis of fundamental concepts of the structure and properties of substances with the use of modern quantum chemistry methods.
Native cellulose is a semirigid hydrophilic amorphous–crystalline polymer, which has a complex supramolecular structure with an elementary fibril as the simplest element. In turn, the fibril is an associate of macromolecules with a length of less than 1 μm and a transverse diameter of 1.5–3.5 nm. A macromolecule of cellulose is a linear chain consisting of a sequence of elementary pyranose units, in which the links of D-glucose are connected by 1,4-β-glycosidic bonds rotated 180 deg relative to each other.
Thus, considering cellulose as a semirigid hydrophilic polymer in terms of the chemistry of macromolecular compounds on the one hand and quantum chemistry apparatus on the other can be useful for characterizing possible structures formed upon the interaction of cellulose macromolecule fragments, the spectroscopic properties, and the effect of cellulose modification with appropriate reagents on these properties.
Note that Haensel et al. [8] interpreted the X-ray photoelectron spectrum (XPS) of the valence band of cellulose and lignin based on quantum chemical calculations using the density functional theory (DFT). They found that oxygen predominates in the structure of the valence band up to 10 eV, and carbon has a significant contribution in the spectrum at binding energies above 13 eV. They concluded that an analysis of the valence band according to the DFT method provides a powerful basis for a detailed interpretation of the spectral data and allows one to more deeply understand the reasons of the formation of spectra. Akman [9] used the DFT method to study the electronic structure of cellulose dimers and chitosan. The geometric structure parameters and various reactivity descriptors were determined, which are valuable for analyzing the reactivity of these compounds in various nucleophilic and electrophilic reactions. Kocheva [10] successfully used the semiempirical quantum chemical methods CNDO/2 and MINDO/3 to study the electronic structure of model compounds of cellulose and lignin.
The aim of this work was to use quantum chemistry and molecular mechanics methods for simulating the conformational structures of both the single fragments of cellulose macromolecules and the structures formed between the fragments of various cellulose macromolecules consisting of several linear chains, including a study of intra- and intermolecular interactions.
To determine the geometric parameters, the electron density distribution, and the energy characteristics of an elementary unit and a cellulose dimer (cellobiose), we used the DFT B3LYP/6-31G(d) quantum-chemical method with full optimization of geometric parameters and the calculation of the frequencies of normal vibrations. The fragment of a linear chain of cellulose macromolecule was constructed step-by-step: at the first stage, the geometric structure of the elementary cellulose unit was created, in which the broken bonds that ensure the translation of the elementary fragment in the macromolecule were compensated by hydrogen atoms or another X atom. Then, the structural fragments of a cellulose macromolecule were considered as the model structures of a linear chain consisting of 8 and 16 elementary units in order to evaluate the effect of hydrogen bonds on the conformation of single macromolecules and their interaction with each other. The interaction was simulated for two to four linear chains. The universal force field (UFF) molecular mechanics method was used for calculating the conformations; this method makes it possible to find a total energy minimum and to optimize the geometric parameters using a computer program [11].
Table 1 summarizes the results of the calculations of the geometrical parameters, electron density distributions, and energy characteristics of an elementary unit of cellulose (Fig. 1) obtained by the DFT B3LYP/6-31G(d) method. The following energies were calculated:
where \({{E}_{{{\text{elec}}}}}\) is the electron energy of the molecule, ZPE is the energy of zero oscillations, \({{E}_{{{\text{vib}}}}}\) is the molecular vibration energy at the temperature T = 298 K, \({{E}_{{{\text{rot}}}}}\) is the rotational energy, \({{E}_{{{\text{transl}}}}}\) is the translational energy, H is the enthalpy, S is the entropy, and G is the Gibbs free energy.
As can be seen in Table 1, the oxygen atoms of hydroxyl groups capable of forming hydrogen bonds have a maximum negative charge. The values of thermodynamic parameters can be used to estimate the probability of the occurrence of structure modification reactions with various functional groups. The energies of the highest occupied and lowest unoccupied molecular orbitals EHO and ELU, respectively, are also given. The value of ELU > 0 means that the presence of an electron on this orbital is energetically unprofitable and, entering it when excited, the electron will tend to leave the molecule; that is, the molecule will be an electron donor. Therefore, cellulose can react with the electrophilic centers of molecules—electron acceptors: to participate in nucleophilic substitution reactions and to form complexes with a donor–acceptor bond [13].
By calculating the frequencies of normal vibrations with the use of second derivatives, it was confirmed that the stationary points determined during geometry optimization are energy minimums, as reflected in Fig. 1 in the form of a vibrational spectrum in the middle IR region. An analysis of the calculated IR spectrum of cellulose shows that the following wavenumber regions (cm–1) correspond to the stretching vibrations of bonds: 3598 for O–H bonds, 3078 for C–H bonds, and 1030 for C–C bonds. The wave numbers in the region of 1500 cm–1 and below correspond to complex combined vibrations of the bonds of a glucopyranose ring and the deformation vibrations of the C–H, C–O, and O–H bonds. A comparative analysis of the calculated and experimental IR spectra presented in numerous publications for different types of cellulose [12–17] showed that there are significant differences in the real spectra of cellulose due to many factors, primarily, differences in the conformations of macromolecules rather than the crystallinity of the polymer structures. In this case, absorption bands in the experimental spectra can be shifted relative to those indicated in the calculated spectrum [15].
Therefore, a theoretical evaluation of the energy characteristics of cellulose macromolecules, namely, the energies of intramolecular hydrogen bonds, which affect the thermodynamic flexibility of a linear chain, and intermolecular interactions between chains due to hydrogen bonds, is important for the interpretation of experimental (including spectroscopic) data. These values were estimated based on the model structures of a linear chain consisting of n = 8 H–(C6H10O5)8–H and n = 16 H–(C6H10O5)16–H elementary units, respectively. The calculations were performed based on the UFF molecular mechanics method with parameterization using a computer program [11] with the optimization of geometric parameters. The method is applicable to the calculations of the potential energy surfaces of molecules with the inclusion of almost all of the atoms from the periodic table of elements and taking into account their valence states (the hybridization of valence shells) and atomic environments. To calculate the total energy E of a molecule, the following equations containing a set of parameters including bond stretch, deformation of bond and torsion angles, and van der Waals interactions are used:
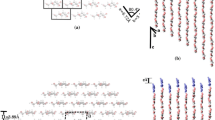
In the calculation of the difference between the energies of two states ΔЕ, errors in the calculation procedure are eliminated. Figure 2 shows the results of calculating the rotation of units in the dimer of the linear part of a cellulose (or cellobiose) macromolecule relative to the C–O bond by torsion angle. Two energy minimums were found: the first minimum E = 0.111586 au corresponds to the trans conformation, and the second E = 0.12225 au, to the cis conformation. The difference in the energy of conformations ΔE = E(cis) – E(trans) shows that the trans conformation is 6.7 kcal/mol more stable than the cis conformation.
As an example, we estimated the energy of intermolecular interactions using the model structures of linear chains consisting of n = 8 and 16 elementary units: H–(C6H10O5)8–H and H–(C6H10O5)16–H. Figures 3a and 3b show the optimized structures of the initial linear chains with n = 8 (corresponds to four dimers) and 16 (corresponds to eight dimers), respectively. The total energy with n = 8 at a minimum is E1 = 0.7540808 au. As can be seen in Fig. 3, in both cases, a twisting of the linear structure was observed due to the formation of intramolecular hydrogen bonds with the formation of more energy-stable conformations. Upon twisting, the length of a structural fragment of eight units shortened from 40 to 27 Å. Figure 4a shows that the lengths of internal hydrogen bonds H...O in the places of linear structure twisting were 1.7–2.5 Å. In this case, the structure of a six-membered ring was significantly distorted. In the linear (nontwisted) part of the molecule, the distance between the bridging oxygen atoms was 4.6–5.4 Å, and it was 2.6–3.0 Å in places where hydrogen bonds were formed. The bond lengths of bridging oxygen with carbon atoms remained almost unchanged: 1.43 Å. The above example shows that the observed conformations of the macromolecular fragments of cellulose can be described by numerous local minimums with the corresponding geometry and differ little in energy because of the large size of the modeled system of atoms and the presence of single C–O–C chemical glycosidic bonds between the adjacent units of elementary fragments and intramolecular interactions. Consequently, the experimentally measured physicochemical quantities, especially spectroscopic data, are sensitive to temperature changes.
We simulated the interaction between two and four structural fragments of a cellulose macromolecule consisting of eight elementary units (or four dimers). Within the framework of the model considered, the influence of the terminal groups X = –H, –COOH, and –COH in the structural fragments X–(С6Н10О5)8–X on the energy of intermolecular interactions ∆Е in their dimers [X–(С6Н10О5)8–Х]2. The energy of intermolecular interactions was calculated by the formula

Figure 4 shows the optimized structure of two interacting fragments of macromolecules with the empirical formulas X–(C6H10O5)8–X, where X = –H, –COOH, and –COH, and gives the energies of fragments E and the energies of intermolecular interactions ∆Е. As follows from the data in Fig. 4, the energies of intermolecular interactions ∆E essentially depend on the nature of the terminal groups, and they are –26, 49, and –32 kcal/mol at X = –H, –COOH, and –COH, respectively.
Figure 5 shows the optimized structure of four interacting model molecules with the total energy E4 = 2.834858 au. The energy of intermolecular interactions is

As follows from the above data, the energy of intermolecular interactions does not additively depend on the number of interacting fragments.
CONCLUSIONS
The model studies of the energy and geometric parameters of the structural fragments of a cellulose macromolecule based on quantum chemical calculations showed the following: differences between the calculated and experimental IR spectra of native cellulose are mainly due to differences in the conformation of its macromolecules and, as a consequence, the formation of a supramolecular structure of various shapes; to prevent the structural transition to a coil conformation, it is necessary to ensure the replacement of the hydrogen atoms of hydroxyl or terminal groups in the cellulose macromolecule by functional groups that do not form intramolecular hydrogen bonds and impede its self-organization into fibrillar structures; compounds with high electron affinity or a negative energy of the lowest unoccupied molecular orbitals are the best reagents for complexation reactions.
These conclusions were confirmed in many publications with practical recommendations oriented to the chemical modification of cellulose [18–20] using the reactions of esterification, acylation, etc.
FUNDING
This work was supported by the Ministry of Education and Science of the Russian Federation (unique project identifier RFMEFI60717X0181; agreement no. 14.607.21.0181).
REFERENCES
Bledzki, A.K. and Gassan, J., Progr. Polym. Sci., 1999, vol. 24, no. 2, p. 221.
Mohanty, A.K., Misra, M., and Hinrichsen, G., Macromolec. Mater. Eng., 2000, vol. 276, no. 1, p. 1.
Moon, R.J., Martini, A., Nairn, J., Simonsen, J., and Youngblood, J., Chem. Soc. Rev., 2011, vol. 40, no. 7, p. 3941.
Martin-Alfonso, J.E., Nunez, N., Valencia, C., Franco, J.M., and Diaz, M.J., J. Industr. Eng. Chem., 2011, no. 17, p. 818.
Sanchez, R., Franco, J.M., Delgado, M.A., Valencia, C., and Gallegos, C., Carbohydr. Polym., 2011, vol. 83, p. 151.
Nevell, T.P. and Zeronian, S.H., Cellulose Chemistry and Its Applications, New York: Wiley, 1985.
Battista, O.A., Industr. Eng. Chem., 1950, vol. 42, no. 3, p. 502.
Haensel, T., Reinmoller, M., Lorenz, P., Beenken, W.J.D., Krischok, S., and Syed Imad-Uddin, A., Cellulose, 2012, vol. 19, no. 3, p. 1005.
Akman, F., Cellulose Chem. Technol., 2017, vol. 51, nos. 3–4, p. 253.
Kocheva, L.S., Extended Abstract of Doctoral (Chem.) Dissertation, Arkhangel’sk: Arkh. State Techn. Univ., 2008.
Granovsky, A.A., GAMESS v.7.1. http://classic.chem.msu.su/gran/games/index.html
Ivanov-Omskii, V.I., Gerasyuta, S.M., and Ivanova, E.I., Izv. St.-Peterb. Lesotekhn. Akad., 2017, no. 218, p. 199.
Kačuráková, M. and Wilson, R.H., Carbohydrate Polym., 2001, vol. 44, no. 4, p. 291. https://doi.org/10.1016/S0144-8617(00)00245-9
Ali, M., et al., Polymer., 2001, vol. 42, no. 7, p. 2893.
Nugmanov, O.K., Grigor’eva, N.P., and Lebedev, N.A., Khim. Rastit. Syr’ya, 2013, no. 1, p. 40.
Langkilde, F.W. and Svantesson, A., J. Pharm. Biomed. Anal., 1995, vol. 13, nos. 4–5, p. 409.
Jiang, J.H., et al., Anal. Chem., 2002, vol. 74, no. 14, p. 3555.
Peng, B.L., Dhar, N., Liu, H.L., and Tam, K.C., Can. J. Chem. Eng., 2011, vol. 89, no. 5, p. 1191.
Eichhorn, S.J., et al., J. Mater. Sci., 2010, vol. 45, no. 1, p. 1.
Dufresne A., Curr. Opin. Colloid In. Sci., 2017, vol. 29, p. 1.
Dufresne, A., Current Opinion in Colloid & Interface Sci, 2017, vol. 29, p. 1.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Makhlyarchuk
About this article
Cite this article
Gyul’maliev, A.M., Safieva, R.Z., Vinokurov, V.A. et al. Structure Simulation and Calculation of the Energy of Interaction of the Fragments of Cellulose Macromolecules. Solid Fuel Chem. 53, 190–196 (2019). https://doi.org/10.3103/S0361521919030030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521919030030