Abstract
Gastrointestinal diseases and eating disorders are among the most common pathologies in the world. One of the most widespread and dangerous consequence of many eating disorders is an uncontrolled weight gain that often leads to obesity. This review focuses on the 15-year-long studies of obestatin, one of the potential regulators of eating behavior. This peptide contains 23 amino-acids and appears due to the processing of the preproghrelin gene responsible for the coding of another orexigenic protein ghrelin. Obestatin and ghrelin have multiple physiological functions, including appetite regulation. Obestatin was originally obtained from the gastric mucosa in rats, but subsequent studies showed that it could be expressed in various tissues and had different effects in various organs and tissues. This review emphasizes possible anorexigenic effects of this peptide and their mechanisms. Despite the 15 years of research on obestatin, its influence on different organs and the mechanism of anorexigenic effects in particular bring about a lot of discussion. This is primarily due to the ambiguity of the peptide receptors’ determination and is also related to the possible degradation of the molecule into small fragments, which, in turn, can have their own effects. The local effects of obestatin and its derivatives in peripheral tissues and the possible effect at the central level indicate the potential of these peptides for further studies. For example, these compounds can be considered as the potential therapeutic compounds for eating disorders’ treatment. The aim of this work was to describe the relevance of the problem associated with obesity treatment and to summarize the results of numerous studies on obestatin and its fragments and their effects on appetite regulation in order to explain its possible mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
GENERAL REVIEW OF THE PROBLEM OF EATING DISORDERS
Diseases of the gastrointestinal tract (GIT) and eating disorders are among the most common pathologies in the world. The annual increase in the number of patients with gastrointestinal pathologies is, on average, 1.14 per 1000 people per year. According to the forecasts of the World Health Organization (WHO), diseases of the digestive system will take one of the leading positions by the middle of the 21st century; they now already affect 15–25% of the adult working population with varying degrees of severity according to some sources and more than 50–60% according to others [1].
The main risk factors of the manifestation and development of various pathologies of the digestive system, in addition to the hereditary ones, include a large number of stressors faced in the modern society, leading to disruption of diet, decrease in nutrition quality (snacks on the go, fast foods, etc.), sedentary lifestyle, poor ecology, and consumption of low-quality food.
Not only physical but also mental health of humans largely depends on the state and functioning of the digestive tract, the disruption of the normal functioning of which underlies many health problems in modern society. For example, eating disorders have long been considered only as a medical diagnosis or genetic predisposition. However, currently this problem is investigated and treated as a mental disorder. Eating disorders are a group of behavioral disorders characterized by a pronounced concern about the weight and shape of one’s own body, which is accompanied by excessive attempts to control them. Most often, “eating disorders” are understood as diseases, such as anorexia nervosa, bulimia nervosa, psychogenic overeating, and unspecified disorders, which are diagnosed in approximately 40–50% of all cases of eating disorders. According to clinical studies in 2001–2003, the frequency of pathologies of various types of eating disorders among women and men was, respectively, as follows: 0.9 and 0.3% for anorexia nervosa, 1.5 and 0.5% for bulimia, 3.5 and 2.0% for uncontrolled overeating-type disorders, and 4.5 and 4% for eating disorders of unspecified etiology [2]. The high degree of distribution is exacerbated by the fact that, in 90% of cases, these pathologies occur even in adolescence, and young women account for up to 95% of the total number of patients [2, 3]. These pathologies are often characterized by severe mental disorders leading to catastrophic consequences for the normal functioning of the human body. For example, anorexia nervosa has one of the highest mortality rates among all mental disorders; death occurs both as a result of physiological disorders and because of suicides due to aggravated depressive states [2, 4].
The social significance for diseases of the digestive system and eating disorders is determined not only by the high degree of their spread but also by the chronic relapsing course; they often lead to long-term inability to work and sometimes disability and entail great direct and indirect economic costs.
PROBLEM OF OBESITY IN THE MODERN WORLD
One of the most common and dangerous consequences of many eating disorders (except anorexia nervosa) is uncontrolled weight gain, which often leads to obesity. WHO defines overweight and obesity as pathological or excessive accumulation of fat and fatty deposits that can be harmful to health, and it considers the spread of these diseases as a pandemic affecting millions of people. According to the UN data, approximately 15% of adults, 5.9% of all children under the age of 5 years, and approximately 7% of children and adolescents 5–19 years old suffered from obesity by 2018 (report of the UN Economic and Social Council on the Implementation of the Sustainable Development Goals until 2030). According to WHO estimates, these numbers are 650 million patients with diagnosed obesity among adults and 340 million among children [5].
A dangerous factor is that overweight and obesity are associated with a large number of various complications affecting many organs and organ systems. According to WHO, overweight and obesity determine the development of nearly 57% of all cases of type 2 diabetes mellitus, 23% cases of coronary heart disease, 17% cases of arterial hypertension, 30% cases of gallstone disease, and 14% cases of osteoarthritis as well as increased risk of developing reproductive disorders and cancer [6]. Overweight and obesity rank fifth among the most frequent risk factors for death in the world. According to the WHO data, mild and severe obesity reduce life expectancy by an average of 3–5 years and up to 15 years, respectively, since obesity increases the risk of mortality from cardiovascular diseases and oncology four times and two times, respectively [7, 8]. Approximately 2.8 million adults die each year due to overweight and obesity [9].
The cause of obesity is an imbalance between consumed and expended energy, which is based on metabolic disorders, the use of high-calorie foods, impaired behavioral responses, the influence of external factors, and genetic predisposition. In particular, the development of obesity depends by 35–80% on 32 key genes, the majority of which are expressed in the central nervous system (CNS). These key genes ensure the functioning of the positive reinforcement and appetite control systems by the feedback principle [10, 11]. According to WHO data, more than 80 environmental and lifestyle factors that contribute to the development of obesity have been identified; some of them are presented in Table 1.
Obesity is not only a global medical problem but also a huge socio-economic problem. Many patients suffer not only from diseases but also from numerous neurological disorders, such as low self-esteem, depression, emotional distress, and other psychological problems. According to McKinsey Global Institute data, an international consulting company, the world economy annually loses approximately $2 trillion, which are spent to solve problems directly or indirectly associated with obesity.
Today, obesity is a key global problem that requires an integrated comprehensive intervention. One of the ways to solve this problem may be the regulation of the appetite system (and, hence, eating behavior).
REGULATION OF EATING BEHAVIOR—A PROMISING DIRECTION IN TREATMENT OF EATING DISORDERS
Regulation of eating behavior is a complex physiological process. Regulation is realized through both peripheral (stretching of the walls of the stomach and intestines, detection of decomposition products of certain compounds in the chyme, the level of glucose in the blood, etc.) and central mechanisms. A number of endogenous signaling compounds, which can be considered in the future as the key targets of pharmacological effects, play a major role in the coordination of regulatory mechanisms.
Signal compounds—regulators of the appetite system—can be divided into two main groups: orexigenic (stimulating appetite) and anorexigenic (suppressing appetite) [14].
Appetite regulators are expressed at the level of both the gastrointestinal tract and the CNS. In the CNS, a number of structures are involved in the regulation of eating behavior, but the main processes take place in the hypothalamus, which receives information from mechanoreceptors about the degree of extension of the walls of various sections of the gastrointestinal tract, the level of metabolites in the blood and chyme (glucose, amino acids, etc.), and endocrine factors and various other signaling compounds in the blood. For example, starvation causes changes in the activity of the neurons coexpressing orexigenic regulators (including the neuropeptide Y (NPY) and the agouti-related peptide (AgRP)) in the arcuate nucleus (ARC) of the hypothalamus. The activity of the neurons coexpressing anorexigenic regulators—α-melanocyte stimulating hormone (α-MSH) and cocaine-amphetamine-regulated transcript (CART)—also changes [15].
Other hypothalamic nuclei are also involved in the regulation of food intake. It is known that the destruction of the dorsomedial (DMN) and ventromedial (VMN) nuclei, which are the “satiety center” components, leads to obesity and that the destruction of the lateral hypothalamus (LH, the “hunger center”) causes aphagia, whereas its stimulation increases food intake.
Changes in food intake also occur as a result of destruction of the suprachiasmatic (SCN) and paraventricular (PVN) nuclei and the perifornical hypothalamus (PHF). In addition, it was shown that these areas have different sensitivity to orexigenic and anorexigenic compounds [14].
Many hypothalamic peptide hormones that directly or indirectly affect eating behavior are known. The regulation of appetite by the brain directly depends on the signals coming from the periphery, especially from the gastrointestinal tract. Mechanoreceptors in the hollow organs of the gastrointestinal tract send a signal about the degree of stretching of the walls (i.e., about the fullness of the stomach and intestines). These signals are then transduced to the hypothalamic hunger and satiety centers and change their activity. In addition, many hormonal and endocrine factors involved in the regulation of appetite, including the peptide YY (PYY), the glucagon-like peptide 1 (GLP-1), and oxyntomodulin (OXM), are also synthesized in the digestive tract. These regulators cause a decrease in food motivation and a delay in gastric emptying. Cholecystokinin, guanidine, and pancreatic polypeptide (PP) are anorexigenic compounds. Amylin and insulin, which are produced by pancreatic β-cells, cause a decrease in blood glucose and suppress food motivation. Ghrelin, in turn, is an appetite stimulator [16].
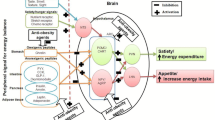
Figure 1 shows a generalized scheme of eating behavior regulation.
Generalized scheme of food motivation regulation with some peptide regulators. Designations: MCH—melanin-concentrating hormone; AgRP—agouti-related peptide; NPY—neuropeptide Y; PP—pancreatic polypeptide; GLP-1—glucagon-like peptide 1; OXM—oxyntomodulin; PYY—peptide YY; α-MSH—α-melanocyte-stimulating hormone; MC3R—melanocortin 3 receptor; MC4R—melanocortin 4 receptor; CCK—cholecystokinin; Y1R— neuropeptide Y receptor type 1; POMC—proopiomelanocortin; CART—cocaine- and amphetamine-regulated transcript; DMN—dorsomedial hypothalamic nucleus; VMN—ventromedial hypothalamic nucleus; PVN—paraventricular hypothalamic nucleus.
OBESTATIN—AN ENDOGENOUS REGULATOR OF APPETITE AND BODY WEIGHT
Structure of Obestatin and Its Distribution in the Body
The study into the mechanisms of action and the content of hormones that regulate eating behavior is one of the most promising directions in the development of new approaches to creating drugs aimed at treating obesity, both diet-induced and those associated with various pathological states that are accompanied by metabolic, hormonal, and energy disorders. In this regard, the use of hormones of eating behavior or their analogues for the regulation and correction of pathogenetic mechanisms of metabolic disorders seems quite optimistic.
Among the endogenous appetite regulators discovered in the past 15 years, obestatin is of particular interest. Obestatin (from “obedere” (to devour) and “statin” (to suppress)) consists of 23 amino acid residues and is one of the products of posttranslational processing of preprogreline, the precursor of ghrelin, the “hunger hormone.” Peripheral (systemic) and central (intraventricular) administration of the latter leads to rapid stimulation of food-procuring behavior and weight gain as well as stimulates the contraction of stomach walls and secretion of acid [17].
The amino acid sequence of obestatin in different animal species is fairly conserved. In humans and primates, the amino acid sequences of this peptide coincide completely, whereas the similarity with obestatin of rats, mice, dogs, and cats is 87%. Among the nonconserved amino acids, there are structurally or biochemically similar pairs (for example, alanine-3/valine-3, leucine-11/isoleucine-11, serine-12/threonine-12, and glutamine-18/glutamic acid-18), which additionally emphasizes the conserved nature of this peptide [18] (Table 2). The C terminus of obestatin is translationally modified with the amide group in Leu-23, which probably has functional significance. It was assumed at first that it is required for the binding of the peptide to the receptor [19]; however, it was shown later that it is important for stabilizing the general conformation of the peptide molecule [20]. Table 2 presents the data on the amino acid composition of obestatin in different organisms in comparison with the structure of human obestatin.
Obestatin was first isolated from the rat stomach, where it is synthesized in relatively large amounts [19]. It was also shown that the intracellular localization of obestatin and ghrelin is the same, which means that obestatin and ghrelin are contained in the same vesicles [21]. It was later discovered that obestatin is secreted primarily in the gastrointestinal mucosa cells. However, it was also found in the cells of the testes, skeletal muscles, lungs, adipose tissue, liver, pancreatic islets of Langerhans, and mammary gland as well as in sperm, breast milk, saliva, and blood plasma [21–28]. In the human gastrointestinal tract, obestatin-immunoreactive cells were found in the mucosa of the cardiac sphincter of the stomach to the ileum in the following distribution: most of the corresponding cells are concentrated in the stomach, fewer cells in the duodenum and jejunum, scarce cells in the ileum, and no such cells in the colon [21, 29]. These data are approximately correlated with the distribution of ghrelin-immunoreactive cells in the digestive tract [21, 30]. In the gastric mucosa, obestatin-immunoreactive cells were identified primarily in the bottom and, to a lesser extent, in the body and pyloric region of the stomach [25, 30, 31]. In addition, obestatin is expressed with different intensity not only in the gastric mucosa cells but also in the enteric nervous system ganglia. The distribution of obestatin in the Auerbach plexus cells, which controls the motor activity of the gastrointestinal tract, may be one of the factors that determine the effect of this peptide on the rate of emptying of the digestive tract [29].
Effect of Obestatin on the Functional Activity of the Gastrointestinal Tract
One of the earliest recorded effects of obestatin was the suppression of contraction of isolated jejunum muscles [19]. This effect was considered as a potential mechanism for reducing food intake and body weight. Data obtained later either confirmed this theory [32–37] or disputed it [38–41]. Such contradictions in the results obtained by different researchers may be associated with the short lifetime of obestatin in blood, its low concentration and stability, or poor purification during synthesis [42]. For example, in the case of intravenous administration of 10 μg of obestatin, its half-life is 22 min [39]. The physiological concentrations of obestatin are fairly low: the content of this peptide in blood plasma and in the stomach in rats is ≤0.2 and 0.18 fmol/mg, respectively; in humans, these values reach 6.9 and 0.17 fmol/mg, respectively [43]. According to other data, the content of obestatin in blood plasma may be 317 fmol/mL in rats [39] and 288–830 pg/mL in humans [44, 45]. The secretion of obestatin is characterized by daily fluctuations, similar to those for ghrelin and somatostatin; however, the concentrations of ghrelin and obestatin in blood plasma are not interrelated. In addition, the number of events of obestatin secretion may be less than that of ghrelin and somatostatin [39]. The secretion of obestatin does not depend on the caloric content of the diet [43, 46] nor on the feeling of hunger or satiety [46]. Obestatin inhibits the antrum motility after eating; however, this effect is not observed against the background of fasting [47]. In longitudinal smooth muscle cells of the stomach bottom, obestatin can reduce the conductivity of resting specific membrane and inhibit Ca2+ entry, thereby increasing the threshold value of action potentials [48], which ultimately leads to a decrease in the contractile activity of the stomach. There is a correlation between the effect of obestatin on the intestinal contractility (especially in the jejunum region) and the age of the animal: the older the animal, the less pronounced the effect of the peptide [47]. It is assumed that the effects of obestatin in the duodenum are realized through the vagal afferent pathways as well as through the receptors of type 1 and type 2 corticotropin releasing factor (CRF1, CRF2) in the brain, which indicates multiple possible pathways of the peptide’s action [29]. Parenteral administration of obestatin affects the intestinal mucosa regeneration [49]. There is no evidence of obestatin expression in the large intestine, and there is no data demonstrating the peptide’s effect on the motility of this digestive tract section, which may be due to the absence of specific receptors or the distribution of nerve plexuses [29]. At the same time, it was shown that obestatin may be present in saliva, although its effect on the secretory activity of salivary glands was not found [50]. In addition, no direct effect of obestatin on the secretion of gastric juice was detected. However, it was noted that obestatin at concentrations of 0.1–10 nM inhibits the somatostatin secretion in the pancreas in vitro. Somatostatin, in turn, regulates the secretion of gastrointestinal hormones (in particular, gastrin) [51]. Thus, it can be assumed that obestatin can indirectly (through the action of other hormones) affect the secretion of digestive juices.
In pancreatic islets of Langerhans, obestatin is colocalized with ghrelin [52], which contributed to developing the theory of the potential therapeutic effect of obestatin on pancreatic islet cells. For example, it was shown that obestatin increases insulin secretion by inhibiting β-cell apoptosis and reducing glucagon secretion by inhibiting α-cell proliferation in type 2 diabetes mellitus [53, 54]. It was also shown that obestatin can increase insulin secretion by increasing the number of insulin-producing cells in the pancreas [55].
Obestatin and Pathologies Associated with the Digestive Tract Organs
Analysis of plasma obestatin content under various physiological conditions showed that the levels of obestatin in obese people (in particular, those with type 2 diabetes mellitus) and in anorexia patients are significantly lower and higher, respectively, than in healthy subjects [46, 56, 57]. Obestatin levels are also reduced in patients with metabolic syndrome but increased in patients with steatohepatitis and Barde–Beadle syndrome [58, 59].
The development of gastrointestinal tract pathologies (in particular, peptic ulcer disease) is often associated with Helicobacter pylori, and the development of this infection is associated with a change in obestatin concentration [45]. This fact suggests that proper regulation of obestatin levels in peptic ulcer models can accelerate the pathology correction process [60].
In the model of necrotizing ulcerative colitis, obestatin reduced the necrotic focus area [61], and preliminary administration of obestatin significantly reduced the area of lesion of the colon mucosa caused by the application of acetic acid in the acute colitis model [62]. These data suggest that obestatin exerts antiapoptotic and anti-inflammatory effects. There is also evidence of hepatoprotective properties of obestatin. For example, in animals kept on a high-fat diet, chronic intraperitoneal administration of obestatin (80 nmol/kg) significantly decelerated the course of nonalcoholic fatty liver disease, reduced the development of hepatomegaly and hyperlipidemia, and slowed down the accumulation of lipids in the liver and the development of insulin resistance [63]. The authors attribute this effect of obestatin to a change in the activity of ghrelin and adiponectin as well as a decrease in the amount of food consumed against the background of the peptide administration.
Obestatin can serve as a marker in diagnosing pancreatic diseases. For example, during the development of pancreatitis in humans, the concentration of obestatin in blood serum increases, and the peptide content directly depends on the severity of the disease [64].
In addition, obestatin can be involved in the regeneration of the pancreas, in particular, improving the recovery of islet clusters and increasing the expression of insulin genes during differentiation of pancreatic progenitor endocrine cells [53]. It was also shown that the administration of obestatin significantly inhibits the death of islet cells in a diabetes model, increases insulin expression, and decreases glucagon release, which allows this peptide to be considered as a potential medicine for treatment of type 2 diabetes mellitus [63].
Obestatin and Appetite
A number of studies showed that obestatin administered at different doses leads to a decrease in body weight and reduces food and water consumption. It was shown that a single intraperitoneal administration of obestatin (1 μmol/kg) caused a decrease in food consumption in rodents (rats and mice); a similar result was obtained in mice after intraventricular administration of obestatin (8 nmol/kg) [19]. In addition, obestatin had a similar effect in the case of chronic administration [19, 34]. It was also shown that intraperitoneal administration of obestatin at a dose of 1 μmol/kg reduced the orexigenic effect of ghrelin [39]. There is a large amount of data confirming the anorexigenic effect of obestatin [32, 65, 66]. However, in the study performed by other authors, neither a single subcutaneous infusion of obestatin at a dose of up to 500 nmol/kg nor a daily administration of this compound for 7 days at a dose of 1000 nmol/kg had an effect on the body weight and food consumption [40]. Anorexigenic effects of obestatin were also not confirmed in a number of other studies [67, 68].
The secretion of obestatin does not depend on the calorie content of the diet nor on feelings of hunger or satiety [46]. However, there is an inverse correlation between the body mass, blood glucose, and insulin and leptin levels, on the one hand, and the blood obestatin level, on the other hand, which suggests that obestatin is involved in the regulation of nutrition [69]. On average, the plasma obestatin level in obese people is 64.19 pg/mL lower than that in people with normal weight [70]. The decrease in obestatin content is associated with obesity not only in adults but also in children. For example, children suffering from obesity have a lower content of obestatin as well as a positive correlation between the decrease in the concentration of the peptide and fat consumption [71]. There are clinical data showing that the level of obestatin increases significantly in patients with anorexia [69] as well as bulimia [57], although there are also data disproving the relationship between the development of bulimia and changes in the level of obestatin [72].
Possible Mechanisms of Anorexigenic Effects of Obestatin
The observed anorexigenic effects of obestatin imply primarily the central mechanisms of action; this, in turn, suggests that this peptide may cross the blood–brain barrier (BBB). However, there is no unequivocal evidence to support this assumption. For example, studies by Pan et al. [73] showed that radioactively labeled obestatin actively passes through the BBB capillaries into parenchymal cells at a fairly high velocity, greater than that of many known peptides. However, the authors of that study failed to determine the exact mechanism of BBB passage by obestatin (the specific transport system by which it is carried out) as well as to detect it in the CNS. Moreover, it was shown that most of the labeled obestatin decomposes into smaller peptide fragments as early as 10 min after intravenous injection, which also does not allow unequivocally postulating whether or not obestatin itself can overcome the BBB [73]. Some researchers interpret this fact as the inability of obestatin to overcome the BBB [74], whereas others, on the contrary, interpret it as the ability of obestatin to overcome the BBB at a fairly high velocity [75].
Determining the mechanisms and targets of obestatin’s directed action is also hampered by the fact that the receptors with which it interacts have not yet been determined and, accordingly, there are no adequate tools (agonists or antagonists) for further study of its function. Initially, obestatin was discovered as a ligand of the G protein-coupled receptor 39 (GPR39), because it contains a domain for its activation [76]. These data were confirmed in in vitro experiments on activation of this type of receptors by obestatin in mouse C2C12 myoblasts [77] and in vivo in sheep in which intraventricular administration of obestatin increases the GPR39 mRNA expression in the mediobasal hypothalamus [78, 89]. Kolodzeiski et al. showed that the increase in the GPR39 mRNA expression in the liver and the decrease in it in the pancreas in obesity and type 2 diabetes mellitus correlate with the corresponding level of obestatin expression [37]. These data suggest that obestatin can realize its metabolic effects in the CNS through this receptor. However, in a number of studies, the binding of obestatin to GPR39 was disproved and its binding to the glucagon-like peptide 1 receptor (GLP-1R) was shown [79, 80]. Gargantini et al. showed that obestatin competes with GLP-1R agonists in hippocampal progenitor cells [81]. A high level of GLP-1R expression was found in various tissues, including the CNS, where it can function as an appetite-regulating factor. However, studies by Uniappan et al. showed that obestatin does not bind to GLP-1R and does not replace the binding of GLP-1 to INS-1 cells (rat insulinoma cells) and HEK293 cells, which actively express GLP-1R [40]. These data, conversely, indicate the absence of convincing evidence of obestatin’s action through GLP-1R. In 2017, Pradhan et al. put forward a hypothesis according to which obestatin acts through the growth hormone receptor (GHS-R) in pancreatic β-cells, because the effect of this peptide is not observed in the areas with inactivated GHS-R [82]. The results obtained in the study by Szakas et al., in which they investigated the combined effect of the above-mentioned compounds on sleep, memory, and analgesia mechanisms contradict the initial assumption that obestatin functions primarily as a ghrelin antagonist. As a result, it was assumed that obestatin can act through the ghrelin receptor (GHRP6) [83]; however, there is no convincing evidence for this either. Another possible mechanism of action of obestatin is associated with the corticotropin releasing factor, which also exhibits anorexigenic properties and suppresses appetite [84]. That is, obestatin is, possibly, capable of activating CRF1 and CRF2 receptors [85].
The main mechanisms for food intake control include the interaction between the brain, the digestive system, and adipose tissue. The data on the central effects of obestatin are very fragmentary and contradictory, which follows from the contradictory data on the receptor mechanisms and the possibility of overcoming the BBB. For example, Brunetti et al. showed that a direct injection of 1 nmol/kg obestatin into the arcuate hypothalamic nucleus caused a significant decrease in the amount of consumed calories and in the weight gain [66]. It was also shown that intravenous injection of obestatin induces c-fos expression in PVN, and immunofluorescence staining also showed activation of PVN neurons containing CRF and urocortin-2 [85].
A number of studies showed that hypothalamic control of eating behavior can manifest itself in modulating the activity of catecholaminergic neurons (dopaminergic, noradrenergic, and serotonergic) in the brain stem. Both afferent and efferent innervation from the hypothalamic structures involved in the homeostatic regulation of energy balance and eating behavior is distributed mainly through the serotonergic (5-HT1B and 5-HT2C receptors), catecholaminergic (α1, β1, и β2-adrenergic receptors), and dopaminergic (D1 receptors) mediator systems [86, 87]. For example, the well-known anorectic hormones leptin, amylin, and PYY inhibit the release of dopamine from rat hypothalamic synaptosomes [88–90]. This suggests that it is dopamine that may play the key role (both stimulatory and inhibitory) in the CNS in the regulation of eating behavior. Brunetti’s studies showed that the administration of obestatin also inhibits the depolarization-induced release of obestatin and, in opinion of some researchers, may be one of the central mechanisms of obestatin’s action. It was also shown that obestatin blocks the ghrelin-dependent inhibition of serotonin secretion in hypothalamic cells [66].
The anorexigenic effects of obestatin may be associated not only and not so much with food intake as with a decrease in fluid intake [91] against the background of a decrease in the secretion of vasopressin, the most important regulator of the water–salt balance. According to some researchers, such effects of obestatin allow it to be considered an endogenous regulator of the level of fluid in the body [92]. A decrease in water consumption may lead to intensification of lipolysis processes (in order to maintain the water–salt balance), also leading to a decrease in food intake. Taken together, these effects may lead to a sufficiently intense weight loss. It was also shown that obestatin can be involved in the regulation of lipid metabolism by inhibiting lipolysis in adipocytes [80], and chronic administration of stabilized obestatin significantly reduces the level of triglycerides in blood plasma, which indicates a possible role of obestatin in lipid homeostasis [93].
The regulation of many functions of the body (eating behavior, in particular) is increasingly often regarded in the context of the GIT–brain system, which is a complex neurohumoral communication network supporting metabolic homeostasis [94]. Therefore, GIT is a no less important target of obestatin than the brain in the regulation of eating behavior. Zhang et al. showed that obestatin reduces the peristaltic activity of the antrum of the stomach and duodenum in adult rats, predominantly in the state of satiety, subsequently preventing the return of the initial level of peristalsis [19, 95] and, thereby, helping to reduce food intake. Slupek et al. also confirmed the suppression of duodenal and jejunum motility in adult rats caused by obestatin administration. However, at an early age (before maturity of rats), these authors observed opposite effects (improved gastrointestinal motility) [47]. It should be noted that, as is the case with the central mechanisms, the effect of obestatin on the GIT is also disputed by a number of researchers [96, 97]. The data on the effect of obestatin on insulin secretion and, accordingly, on the blood glucose level, are also contradictory, which may also be one of the mechanisms for food intake regulation. In vitro experiments showed that obestatin increases the secretion of the C peptide [98], which is a marker of insulin synthesis in clinical practice. However, in many in vivo experiments, no effect of obestatin on the level of glucose or insulin in blood of normoglycemic mice and rats was detected [79, 91], but it was shown that glucose-induced insulin secretion in rats and in isolated islets of Langerhans of mice and rats was inhibited by obestatin [99]. This finding is consistent with the clinical data on the inverse correlation between obestatin and insulin levels [100]. The vagus nerve—the main nerve of the parasympathetic nervous system that regulates the activity of the majority of organs of the digestive tract, containing approximately 80% afferent fibers and 20% efferent fibers—can be considered the key link in the GIT–brain system. It was assumed that a change in the vagus nerve sensitivity may be one of the causes of impaired appetite regulation, which increases the risk of obesity [10]. Meyer et al. showed that, in people with normal weight and obese people, atropine (an M-cholinergic receptor blocker) and eating cause a significant decrease in the level of obestatin and ghrelin in the blood; however, the ghrelin/obestatin concentration ratio was significantly reduced only in skinny individuals. In addition, the correlation between the concentrations of ghrelin and obestatin in plasma in obese people was significantly stronger than that in skinny people, which suggests that the independent regulation of food intake by these peptides during the development of obesity is disturbed. The blockade of the afferent part of the vagus nerve by capsaicin eliminates the inhibitory effect of obestatin on the motility of the duodenal region but not the antrum [85]. The involvement of the vagal afferent pathways was confirmed by the increase in the number of c-fos-positive neurons the nucleus tractus solitarius of the medulla oblongata after an intravenous injection of obestatin. Taken together, these results show that some (but not all) of the effects of obestatin can be considered as realized through the modulation of the vagus nerve activity [101].
A number of studies on the appetite-regulating peptides showed that a possible central modulator of their activity in the hypothalamus is NO: an increase in the NO level is observed against the background of the appetite-improving peptides, whereas the peptides that reduce food intake decrease its level [102]. Obestatin, in turn, can change the content of NO [103]. However, in the majority of studies on the relationship between obestatin and NO levels, their relationships at the level of potential cardioprotective properties of obestatin, though not in the context of appetite regulation, are investigated [104].
Figure 2 shows a generalizing scheme of the possible mechanisms of the anorexigenic effects of obestatin.
Possible mechanisms of anorexigenic effects of obestatin. Designations: PVN—paraventricular hypothalamic nucleus; GPR39—G protein-coupled receptor 39; GLP-1R—glucagon-like peptide-1 receptor; GHRP6—ghrelin receptor; CRF1 and CRF2—corticotropin-releasing factor receptors; ?—assumption of the localization of receptors that may be involved in the realization of effects; ↓—direction of downward changes.
OBESTATIN FRAGMENTS—POTENTIAL REGULATORS OF EATING BEHAVIOR AND BODY WEIGHT
One of the factors that determine the difficulties in studying obestatin is its relatively low stability. Possibly, different fragments into which it decomposes under the action of proteases are responsible for the realization of its different functions. The study into the effects of obestatin fragments is of particular interest. Since they are fragmentary structures of a large protein, they may have part of the spectrum of action of the whole peptide and, at the same time, their properties may differ from the properties of the whole peptide. Due to the ambiguity of data on the obestatin’s action itself, the action of its fragments remains extremely poorly studied.
The effects of the obestatin fragment are studied, as a rule, in two directions. Firstly, this is the search for the constituent part of the obestatin molecule that is directly involved in the realization of its effect on the body weight and on food and water consumption. Secondly, this is the study into the effects of modifications of the fragments that have a more stable structure and, as a result, a more prolonged effect.
In a number of early studies of obestatin, a fairly short period of its degradation in tissues was shown [25, 39, 73]. However, it was already noted in the work by Vergot et al. that the metabolic degradation of obestatin in liver and kidney homogenates and in blood plasma had different half-life periods ranging from 12.6 to 138.0 min. In addition, these studies showed that the proteolytic hydrolysis of obestatin proceeds differently in different tissues. For example, the hydrolysis of human obestatin in mouse plasma proceeds between F1–N2 with a probability of 95%; in the liver homogenate, between P4–F5 with a probability of 41%, between Y16–Q17 with a probability of 34%, and between Q17–H18 with a probability of 10%; in the kidney homogenate, between F1–N2 with a probability of 13%, between S12–G13 with a probability of 49%, and between G13–V14 with a probability of 16%. Hydrolysis of mouse obestatin in blood plasma proceeded more often between F1–N2 (84%), while that in the liver was between P4–F5 (75%) [105].
Some researchers explain the prolonged effect of the administration of the whole peptide partly by its structure, which is sufficiently proteolysis-resistant [105], and partly by the fact that the effect of the fragments formed as a result of endopeptidase hydrolysis is similar to that of the whole peptide [106]. For example, it was shown in one of the studies that the N-terminal obestatin fragment FNAP, whose stability was increased by amidation, had a more pronounced effect on the body weight and on food and water intake than the whole peptide [106].
Published data on the effects of obestatin fragments that affect eating behavior are rather discrepant. For example, in the study by Nagaraj et al. on the effects of chronic administration of obestatin fragments 1–13, 6–18, and 11–23, it was shown that fragment 1–13 had the greatest anorexigenic effect [65]. However, Subasinghage et al. suggest that fragment 11–23, whose anorexigenic effect is comparable to that of obestatin itself, has the greatest effectiveness. According to some researchers, the effect of this fragment on the “appetite system” is not directly associated with the effect on glucose and insulin homeostasis [32]; however, other researchers hold the opposite point of view [107]. According to Subasinghage, fragment 1–10 does not have a pronounced effect on body weight and food intake.
Khirazova et al. showed that a single administration of terminal fragments 1–4 and 16–23 of obestatin caused a significant decrease in the body weight of rats and a decrease in food and water consumption, whereas fragments 5–10 and 10–15, on the contrary, increased water and food consumption [106]. Possibly, the central fragments of obestatin have other properties than the whole peptide (either do not affect food intake or may enhance it), whereas the terminal fragments, similarly to the whole peptide, have an anorexigenic effect. Interestingly, an additional study into the chronic administration of the terminal obestatin fragments 1–4 and 16–23 showed that the anorexigenic effects were retained only by the N-terminal part of the peptide [106].
In the study by Motorykina et al., anorexigenic effects of the terminal obestatin fragment 1–4 were shown not only for males but also for females. The effects of the administration of this peptide were recorded in a delayed period. The authors assume that this delay in the manifestation of the effect is associated with triggering cascade reactions and with the effect of secondary intermediaries on the body [108]. This type of action is a common property of regulatory peptides and can serve for inducing not only rapid direct responses but also relatively long-term regulatory effects.
Khirazova et al. studied the effect of obestatin fragment 1–4 in a model of rats with increased body weight and showed a decrease in food and water consumption without change in the body weight. The authors explained the absence of changes in the body weight after the peptide administration by an insufficiently long monitoring time of this index and assumed that the effect may be manifested later [106]. A study into the dose dependence of the manifestation of the anorexigenic effect of the obestatin fragment 1–4 showed its effectiveness at lower doses of 100 and 300 nmol/kg [108].
The summary data on the effect of obestatin fragments on the body weight and on food and water consumption are presented in Table 3.
As mentioned above, the anorexigenic effects of obestatin may be associated with a decrease in water intake [92]. However, this was not shown in the studies by Motorykina et al.: neither obestatin nor its fragment 1–4 on the background of deprivation caused by its single and chronic administration caused changes in drinking motivation indices [108]. In this regard, it can be assumed that the anorexigenic effect of obestatin and its fragment is not directly associated with a change in drinking motivation but is realized through other mechanisms that subsequently lead to a change in fluid intake.
CONCLUSIONS
Over the 15 years since the discovery of obestatin in 2005, it was repeatedly shown that this peptide is involved in the regulation of not only eating behavior and the appetite system but also many other physiological processes in the body. At the same time, obestatin is still an “ambiguous peptide” primarily due to the discrepant data on its central effects and uncertainty of receptor mechanisms. Receptors GPR39, GLP-1R, CRF1, and CRF2 are considered by different researchers as potential receptors for obestatin and its fragments. However, there is no convincing evidence to date that the effects of obestatin or its fragments are due to the activation of a certain type of receptors. This fact allows some researchers to doubt the very question of whether obestatin can be considered a hormone. However, the proven secretion of obestatin in various types of cells, as well as its autocrine/paracrine action in different tissues, allows for obestatin to be considered a hormone even in the absence of a clearly proven receptor mechanism. Moreover, obestatin exhibits pleiotropic properties even in the eating behavior regulation: it inhibits gastrointestinal motility, regulates insulin secretion, reduces inflammation, inhibits apoptosis, and stimulates cell proliferation. This suggests that obestatin may prevent the onset and development of certain gastrointestinal diseases. Its numerous biological functions and therapeutic potential are of great interest and importance for further research, including the search for the most effective and stable obestatin fragments.
REFERENCES
Talley, N.J., Dyspepsia: Management guidelines for the millennium, Gut, 2002, vol. 50, suppl. 4, pp. iv72–iv78.
Hudson, J.I., Hiripi, E., Pope, Jr., H.G., and Kessler, R., The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication, Biol. Psychiatry, 2007, vol. 61, no. 3, pp. 348–358.
American Dietetic Association, Position of the American Dietetic Association: Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and eating disorders not otherwise specified (EDNOS), J. Am. Diet. Assoc., 2001, vol. 101, no. 7, pp. 810–819.
Millar, H.R., Wardell, F., Vyvyan, J.P., Naji, S.A., Prescott, G.J., and Eagles, J.M., Anorexia nervosa mortality in Northeast Scotland, 1965–1999, Am. J. Psychiatry, 2005, vol. 162, no. 4, pp. 753–757.
World Health Organisation Website. https://www.who.int/about. Accessed March 13, 2020.
James, W.P.T., Jackson-Leach, R., Mhurchu, C.N., Kalamara, E., Shayeghi, M., Rigby, N., Nishida, C.R.A., and Rodgers, A., in Overweight and Obesity (High Body Mass Index), World Health Organization, 2004, ch. 8.
Jungheim, E.S., Travieso, J.L., Carson, K.R., and Moley, K.H., Obesity and reproductive function, Obstet. Gynecol. Clin., 2012, vol. 39, no. 4, pp. 479–493.
Jiao, L., De Gonzalez, A.B., Hartge, P., et al., Body mass index, effect modifiers, and risk of pancreatic cancer: A pooled study of seven prospective cohorts, Cancer Causes Control, 2010, vol. 21, no. 8, pp. 1305–1314.
World Health Organisation Website. https://www.who.int/features/factfiles/obesity/ru/. Accessed March 13, 2020.
Willer, C.J., Speliotes, E.K., Loos, R.J.F., et al., Six new loci associated with body mass index highlight a neuronal influence on body weight regulation, Nat. Genet., 2009, vol. 41, no. 1, pp. 25–34.
Zheng, H., Lenard, N.R., Shin, A.C., and Berthoud, H.R., Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals, Int. J. Obes., 2009, vol. 33, suppl. 2, pp. S8–S13.
Speliotes, E.K., Willer, C.J., Berndt, S.I., et al., Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index, Nat. Genet., 2010, vol. 42, no. 11, pp. 937–948.
Gairolla, J., Kler, R., Modi, M., and Khurana, D., Leptin and adiponectin: Pathophysiological role and possible therapeutic target of inflammation in ischemic stroke, Rev. Neurosci., 2017, vol. 28, no. 3, pp. 295–306.
Smirnova, O.V., The Physiology of the Endocrine System, Cambridge: Cambride Scholars Publishing, 2019.
Bartness, T.J., Keen-Rhinehart, E., Dailey, M.J., and Teubner, B.J., Neural and hormonal control of food hoarding, Am. J. Physiol. Integr. Comp. Physiol., 2011, vol. 301, no. 3, pp. 641–655.
Pimentel, G.D., Micheletti, T.O., Pace, F., Rosa, J.C., Santos, R.V.T., and Lira, F.S., Gut-central nervous system axis is a target for nutritional therapies, Nutr. J., 2012, vol. 11, no. 1, p. 22.
Shintani, M., Ogawa, Y., Ebihara, K., Aizawa-Abe, M., Miyanaga, F., Takaya, K., Hayashi, T., Inoue, G., Hosoda, K., Kojima, M., Kangawa, K., and Nakao, K., Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway, Diabetes, 2001, vol. 50, no. 2, pp. 227–232.
Green, B.D. and Grieve, D.J., Biochemical properties and biological actions of obestatin and its relevence in type 2 diabetes, Peptides, 2018, vol. 100, pp. 249–259.
Zhang, J.V., Ren, P.G., Avsian-Kretchmer, O., Luo, C.-W., Rauch, R., Klein, C., and Hsueh, A.J.W., Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake, Science, 2005, vol. 310, no. 5750, pp. 996–999.
Scrima, M., Campiglia, P., Esposito, C., Gomez-Monterrey, I., Novellino, E., and D’Ursi, A.M., Obestatin conformational features: A strategy to unveil obestatin’s biological role?, Biochem. Biophys. Res. Commun., 2007, vol. 363, no. 3, pp. 500–505.
Grönberg, M., Tsolakis, A.V., Magnusson, L., Janson, E.T., and Saras, J., Distribution of obestatin and ghrelin in human tissues: Immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands, J. Histochem. Cytochem., 2008, vol. 56, no. 9, pp. 793–801.
Grönberg, M., Amini, R.M., Stridsberg, M., Janson, E.T., and Saras, J., Neuroendocrine markers are expressed in human mammary glands, Regul. Pept., 2010, vol. 160, nos. 1–3, pp. 68–74.
Gurriarán-Rodriguez, U., Santos-Zas, I., Al-Massadi, O., Mosteiro, C.S., Beiroa, D., Nogueiras, R., Crujeiras, A.B., Seoane, L.M., Señarís, J., García-Caballero, T., Gallego, R., Casanueva, F.F., Pazos, Y., and Camiña, J.P., The obestatin/GPR39 system is up-regulated by muscle injury and functions as an autocrine regenerative system, J. Biol. Chem., 2012, vol. 287, no. 45, pp. 38379–38389.
Moretti, E., Vindigni, C., Tripodi, S.A., Mazzi, L., Nuti, R., Figura, N., and Collodel, G., Immunolocalisation of ghrelin and obestatin in human testis, seminal vesicles, prostate and spermatozoa, Andrologia, 2014, vol. 46, no. 9, pp. 979–985.
Zhao, C.-M., Furnes, M.W., Stenstrom, B., Kulseng, B., and Chen, D., Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: An immunohistochemical and electron-microscopic study, Cell Tissue Res., 2008, vol. 331, no. 3, pp. 575–587.
Aydin, S., Ozkan, Y., Erman, F., Gurates, B., Kilic, N., Colak, R., Gundogan, T., Catak, Z., Bozkurt, M., Akin, O., Sen, Y., and Sahn, I., Presence of obestatin in breast milk: relationship among obestatin, ghrelin, and leptin in lactating women, Nutrition, 2008, vol. 24, nos. 7-8, pp. 689–693.
Cengiz, H., Dagdeviren, H., Caypinar, S.S., Kanawati, A., Yildiz, S., and Ekin, M., Plasma serotonin levels are elevated in pregnant women with hyperemesis gravidarum, Arch. Gynecol. Obstet., 2015, vol. 291, no. 6, pp. 1271–1276.
Dag, E., Aydin, S., Ozkan, Y., Erman, F., Dagli, A.F., and Gurger, M., Alteration in chromogranin A, obestatin and total ghrelin levels of saliva and serum in epilepsy cases, Peptides, 2010, vol. 31, no. 5, pp. 932–937.
Xing, Y.X., Yang, L., Kuang, H.Y., Gao, X.Y., and Liu, H.L., Function of obestatin in the digestive system, Nutrition, 2017, vol. 34, pp. 21–28.
Tsolakis, A.V., Grimelius, L., Stridsberg, M., Falkmer, S.E., Waldum, H.L., Saras, J., and Janson, E.T., Obestatin/ghrelin cells in normal mucosa and endocrine tumours of the stomach, Eur. J. Endocrinol., 2009, vol. 160, no. 6, pp. 941–949.
Dun, S.L., Brailoiu, G.C., Brailoiu, E., Yang, J., Chang, J.K., and Dun, N.J., Distribution and biological activity of obestatin in the rat, J. Endocrinol., 2006, vol. 191, no. 2, pp. 481–490.
Green, B.D., Irwin, N., and Flatt, P.R., Direct and indirect effects of obestatin peptides on food intake and the regulation of glucose homeostasis and insulin secretion in mice, Peptides, 2007, vol. 28, no. 5, pp. 981–987.
Zhang, J.V., Jahr, H., Luo, C.W., Klein, C., Van Kolen, K., Ver, DonckL., De, A., Baart, E., Li, J., Moechars, D., and Hsueh, A.J., Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39, Mol. Endocrinol., 2008, vol. 22, no. 6, pp. 1464–1475.
Brunetti, L., Leone, S., Orlando, G., Recinella, L., Ferrante, C., Chiavaroli, A., Di Nisio, C., Di Michele, P., and Vacca, M., Effects of obestatin on feeding and body weight after standard or cafeteria diet in the rat, Peptides, 2009, vol. 30, no. 7, pp. 1323–1327.
Hassouna, R., Zizzari, P., Viltart, O., Yang, S.K., Gardette, R., Videau, C., Badoer, E., Epelbaum, J., and Tolle, V., A natural variant of obestatin, Q90L, inhibits ghrelin’s action on food intake and Gh secretion and targets NPY and GHRH neurons in mice, PLoS One, 2012, vol. 7, no. 12.
Jung, J.Y., Jeong, J.B., Kim, J.W., Kim, S.H., Koh, S.-J., Kim, B.G., and Lee, K.L., Circulating ghrelin levels and obestatin/ghrelin ratio as a marker of activity in ulcerative colitis, Intest. Res., 2015, vol. 13, no. 1, pp. 68–73.
Kolodziejski, P.A., Pruszynska-Oszmalek, E., Sassek, M., Kaczmarek, P., Szczepankiewicz, D., Billert, M., Mackowiak, P., Strowski, M.Z., and Nowak, K.W., Changes in obestatin gene and GPR39 receptor expression in peripheral tissues of rat models of obesity, type 1 and type 2 diabetes, J. Diabetes, 2017, vol. 9, no. 4, pp. 353–361.
Seoane, L.M., Al-Massadi, O., Pazos, Y., Pagotto, U., and Casanueva, F.F., Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats, J. Endocrinol. Invest., 2006, vol. 29, no. 8, p. RC13–RC15.
Zizzari, P., Longchamps, R., Epelbaum, J., and Bluet-Pajot, M.T., Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents, Endocrinology, 2007, vol. 148, no. 4, pp. 1648–1653.
Unniappan, S., Speck, M., and Kieffer, T.J., Metabolic effects of chronic obestatin infusion in rats, Peptides, 2008, vol. 29, no. 8, pp. 1354–1361.
Gao, X.Y., Kuang, H.Y., Liu, X.M., and Bin, Z., Decreased gastric body mucosa obestatin expression in abdominal obesity patients with normal body mass index, Biomed. Environ. Sci., 2014, vol. 27, no. 5, pp. 385–387.
Seim, I., Walpole, C., Amorim, L., Josh, P., Herington, A., and Chopin, L., The expanding roles of the ghrelin-gene derived peptide obestatin in health and disease, Mol. Cell. Endocrinol., 2011, vol. 340, no. 1, pp. 111–117.
Mondal, M.S., Toshinai, K., Ueno, H., Koshinaka, K., and Nakazato, M., Characterization of obestatin in rat and human stomach and plasma, and its lack of acute effect on feeding behavior in rodents, J. Endocrinol., 2008, vol. 198, no. 2, pp. 339–346.
Reinehr, T., De Sousa, G., and Roth, C.L., Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight, Clin. Endocrinol. (Oxford), 2008, vol. 68, no. 2, pp. 304–310.
Ulasoglu, C., Isbilen, B., Doganay, L., Ozen, F., Kiziltas, S., and Tuncer, I., Effect of Helicobacter pylori eradication on serum ghrelin and obestatin levels, World J. Gastroenterol., 2013, vol. 19, no. 15, pp. 2388 –2394.
Huda, M.S.B., Durham, B.H., Wong, S.P., Deepak, D., Kerrigan, D., McCulloch, P., Ranganath, L., Pinkney, J., and Wilding, J.P.H., Plasma obestatin levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal, Int. J. Obes., 2008, vol. 32, no. 1, pp. 129–135.
Slupecka, M., Pierzynowski, S.G., Kuwahara, A., Kato, I., and Woliński, J., Age-dependent effect of obestatin on intestinal contractility in Wistar rats, Gen. Comp. Endocrinol., 2014, vol. 208, pp. 109–115.
Squecco, R., Garella, R., Francini, F., and Baccari, M.C., Influence of obestatin on the gastric longitudinal smooth muscle from mice: Mechanical and electrophysiological studies, Am. J. Physiol. Liver Physiol., 2013, vol. 305, no. 9, pp. G628–G637.
Slupecka-Ziemilska, M., Grzesiak, P., Jank, M., Majewska, A., Rak, A., Kowalczyk, P., Kato, I., Kuwahara, A., and Woliński, J., Small intestinal development in suckling rats after enteral obestatin administration, PLoS One, 2018, vol. 13, no. 10.
Taskin, E., Atli, B., Kiliç, M., Sari, Y., and Aydin, S., Serum, urine, and saliva levels of ghrelin and obestatin pre-and post-treatment in pediatric epilepsy, Pediatr. Neurol., 2014, vol. 51, no. 3, pp. 365–369.
Schubert, M.L., Gastric secretion, Curr. Opin. Gastroenterol., 2014, vol. 30, no. 6, pp. 578–582.
Granata, R., Volante, M., Settanni, F., Gauna, C., Ghé, C., Annunziata, M., Deidda, B., Gesmundo, I., Abribat, T., van der Lely, A.J., Muccioli, G., Ghigo, E., and Papotti, M., Unacylated ghrelin and obestatin increase islet cell mass and prevent diabetes in streptozotocin-treated newborn rats, J. Mol. Endocrinol., 2010, vol. 45, no. 1, pp. 9–17.
Baragli, A., Grande, C., Iacopo, G., Settanni, F., Marina, T., Gargantini, E., Ghigo, E., and Granata, R., Obestatin enhances in vitro generation of pancreatic islets through regulation of developmental pathways, PLoS One, 2013, vol. 8, no. 5.
Li, W., Chang, M., Qiu, M., Chen, Y., Zhang, X., Li, Q., and Cui, C., Exogenous obestatin decreases beta-cell apoptosis and alfa-cell proliferation in high fat diet and streptozotocin induced type 2 diabetic rats, Eur. J. Pharmacol., 2019, vol. 851, pp. 36–42.
El-Asfar, R.K., Kamal, M.M., EL-Razek, R.S.A., Ebtehal, E.D., and El-Mesallamy, H.O., Obestatin can potentially differentiate Wharton’s jelly mesenchymal stem cells into insulin-producing cells, Cell Tissue Res., 2018, vol. 372, no. 1, pp. 91–98.
Gao, X.Y., Kuang, H.Y., Liu, X.M., and Ma, Z.B., Decreased gastric body mucosa obestatin expression in overweight and obese patients, Peptides, 2010, vol. 31, no. 2, pp. 291–296.
Sedlackova, D., Kopeckova, J., Papezova, H., Hainer, V., Kvasnickova, H., Hill, M., and Nedvidkova, J., Comparison of a high-carbohydrate and high-protein breakfast effect on plasma ghrelin, obestatin, NPY and PYY levels in women with anorexia and bulimia nervosa, Nutr. Metab., 2012, vol. 9, no. 1, p. 52.
Büscher, A.K., Cetiner, M., Büscher, R., Wingen, A.M., Hauffa, B.P., and Hoyer, P.F., Obesity in patients with Bardet–Biedl syndrome: Influence of appetite-regulating hormones, Pediatr. Nephrol., 2012, vol. 27, no. 11, pp. 2065–2071.
Gutierrez-Grobe, Y., Villalobos-Blasquez, I., Sánchez-Lara, K., Villa, A.R., Ponciano-Rodriguez, G., Ramos, M.H., Chavez-Tapia, N.C., Uribe, M., and Méndez-Sánchez, N., High ghrelin and obestatin levels and low risk of developing fatty liver, Ann. Hepatol., 2010, vol. 9, no. 1, pp. 52–57.
Dembiński, A., Warzecha, Z., Ceranowicz, P., Cieszkowski, J., Dembiński, M., Ptak-Belowska, A., Kuwahara, A., and Kato, I., Administration of obestatin accelerates the healing of chronic gastric ulcers in rats, Med. Sci. Monit., 2011, vol. 17, no. 8, pp. BR196–BR200.
Korkut, S., Özdemir, A., Yay, A.H., Yalçn, B., Ceylan, M., Korkmaz, L., Yazici, C., Güntürk, I., and Kurtoğlu, S., Obestatin reduces intestinal damage in experimental necrotizing enterocolitis in newborn rats, Am. J. Perinatol., 2019, vol. 36, no. 11, pp. 1179–1187.
Matuszyk, A., Ceranowicz, P., Warzecha, Z., Cieszkowski, J., Gałazka, K., Bonior, J., Jaworek, J., Konturek, P.C., Gil, K., and Dembinski, A., Pretreatment with obestatin inhibits the development of acetic acid-induced colitis in rats, Arch. Med. Sci., 2018, vol. 14, no. 4, pp. 920–929.
Khaleel, E.F. and Abdel-Aleem, G.A., Obestatin protects and reverses nonalcoholic fatty liver disease and its associated insulin resistance in rats via inhibition of food intake, enhancing hepatic adiponectin signaling, and blocking ghrelin acylation, Arch. Physiol. Biochem., 2019, vol. 125, no. 1, pp. 64–78.
Kanat, B.H., Ayten, R., Aydn, S., Girgin, M., Çetinkaya, Z., Ilhan, Y.S., Yur, M., and Çatak, Z., Significance of appetite hormone ghrelin and obestatin levels in the assessment of the severity of acute pancreatitis, Turk. J. Gastroenterol., 2014, vol. 25, no. 3, pp. 309–313.
Nagaraj, S., Peddha, M.S., and Manjappara, U.V., Fragments of obestatin as modulators of feed intake, circulating lipids, and stored fat, Biochem. Biophys. Res. Commun., 2008, vol. 366, no. 3, pp. 731–737.
Brunetti, L., Michelotto, B., Orlando, G., and Vacca, M., Obestatin inhibits dopamine release in rat hypothalamus, Eur. J. Pharmacol., 2010, vol. 641, nos. 2–3, pp. 142–147.
Nogueiras, R., Pfluger, P., Tovar, S., et al., Effects of obestatin on energy balance and growth hormone secretion in rodents, Endocrinology, 2007, vol. 148, no. 1, pp. 21–26.
Gourcerol, G. and Tache, Y., Obestatin: A ghrelin-associated peptide that does not hold its promise to suppress food intake and motility, Neurogastroenterol. Motil., 2007, vol. 19, no. 3, pp. 161–165.
Shen, C., Yu, T., Tang, Z.H., and Wu, K.M., Changes in ghrelin and obestatin levels before and after a meal in children with simple obesity and anorexia, Horm. Res. Paediatr., 2013, vol. 79, no. 6, pp. 341–346.
Zhang, N., Yuan, C., Li, Z., Li, J., Li, X., Li, C., Li, R., and Wang, S.R., Meta-analysis of the relationship between obestatin and ghrelin levels and the ghrelin/obestatin ratio with respect to obesity, Am. J. Med. Sci., 2011, vol. 341, no. 1, pp. 48–55.
Aly, G.S., Hassan, N.E., Anwar, G.M., Ahmed, H.H., El-Masry, S.A., El-Banna, R.A., Ahmed, N.H., Kamal, A.N., and Tarkan, R.S., Ghrelin, obestatin and the ghrelin/obestatin ratio as potential mediators for food intake among obese children: A case control study, J. Pediatr. Endocrinol. Metab., 2020, vol. 33, no. 2, pp. 199–204.
Monteleone, P., Serritella, C., Martiadis, V., Scognamiglio, P., and Maj, M., Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa, J. Clin. Endocrinol. Metab., 2008, vol. 93, no. 11, pp. 4418–4421.
Pan, W., Tu, H., and Kastin, A.J., Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin, and adiponectin, Peptides, 2006, vol. 27, no. 4, pp. 911–916.
Sobrino Crespo, C., Perianes Cachero, A., Puebla Jiménez, L., Barrios, V., and Arilla Ferreiro, E., Peptides and food intake, Front. Endocrinol., 2014, vol. 5, p. 58.
Szlis, M. and Wojcik-Gladysz, A., Neuromodulatory action of obestatin on the secretory activity of the hypothalamic-pituitary axis, Zesz. Nauk. Uniw. Szczecin.Acta Biol., 2014, vol. 21, pp. 125–134.
Alén, B.O., Nieto, L., Gurriarán-Rodriguez, U., Mosteiro, C.S., Álvarez-Pérez, J.C., Otero-Alén, M., Camiña, J.P., Gallego, R., Garcia-Caballero, T., and Martin-Pastor, M., The NMR structure of human obestatin in membrane-like environments: Insights into the structure-bioactivity relationship of obestatin, PLoS One, 2012, vol. 7, no. 10.
Gurriarán-Rodriguez, U., Santos-Zas, I., González-Sánchez, J., et al., Action of obestatin in skeletal muscle repair: Stem cell expansion, muscle growth, and microenvironment remodeling, Mol. Ther., 2015, vol. 23, no. 6, pp. 1003–1021.
Szlis, M., Polkowska, J., Skrzeczyńska, E., Przybył, B.J., and Wojcik-Gladysz, A., Does obestatin modulate the hypothalamic appetite-regulating network in peripubertal sheep?, J. Anim. Physiol. Anim. Nutr., 2018, vol. 102, no. 3, pp. 690–700.
Granata, R., Settanni, F., Gallo, D., Trovato, L., Biancone, L., Cantaluppi, V., Nano, R., Annunziata, M., Campiglia, P., Arnoletti, E., Ghè, C., Volante, M., Papotti, M., Muccioli, G., and Ghigo, E., Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function, Diabetes, 2008, vol. 57, no. 4, pp. 967–979.
Granata, R., Gallo, D., Luque, R.M., et al., Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation, FASEB J., 2012, vol. 26, no. 8, pp. 3393–3411.
Gargantini, E., Lazzari, L., Settanni, F., Taliano, M., Trovato, L., Gesmundo, I., Ghigo, E., and Granata, R., Obestatin promotes proliferation and survival of adult hippocampal progenitors and reduces amyloid-β-induced toxicity, Mol. Cell. Endocrinol., 2016, vol. 422, pp. 18–30.
Pradhan, G., Wu, C.S., Lee, J.H., Kanikarla, P., Guo, S., Yechoor, V.K., Samson, S.L., and Sun, Y., Obestatin stimulates glucose-induced insulin secretion through ghrelin receptor GHS-R, Sci. Rep., 2017, vol. 7, no. 1, p. 979.
Szakács, J., Csabafi, K., Lipták, N., and Szabó, G., The effect of obestatin on anxiety-like behaviour in mice, Behav. Brain Res., 2015, vol. 293, pp. 41–45.
Fekete, E.M., Zhao, Y., Szucs, A., Sabino, V., Cottone, P., Rivier, J., Vale, W.W., Koob, G.F., and Zorrilla, E.P., Systemic urocortin 2, but not urocortin 1 or stressin1-a, suppresses feeding via crf2 receptors without malaise and stress, Br. J. Pharmacol., 2011, vol. 164, no. 8, pp. 1959–1975.
Ataka, K., Inui, A., Asakawa, A., Kato, I., and Fujimiya, M., Obestatin inhibits motor activity in the antrum and duodenum in the fed state of conscious rats, Am. J. Physiol. Liver Physiol., 2008, vol. 294, no. 5, pp. G1210–G1218.
Wellman, P.J., Modulation of eating by central catecholamine systems, Curr. Drug Targets, 2005, vol. 6, no. 2, pp. 191–199.
Tecott, L.H., Serotonin and the orchestration of energy balance, Cell Metab., 2007, vol. 6, no. 5, pp. 352–361.
Brunetti, L., Michelotto, B., Orlando, G., and Vacca, M., Leptin inhibits norepinephrine and dopamine release from rat hypothalamic neuronal endings, Eur. J. Pharmacol., 1999, vol. 372, no. 3, pp. 237–240.
Brunetti, L., Recinella, L., Orlando, G., Michelotto, B., Di Nisio, C., and Vacca, M., Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus, Eur. J. Pharmacol., 2002, vol. 454, nos. 2–3, pp. 189–192.
Brunetti, L., Orlando, G., Ferrante, C., Chiavaroli, A., and Vacca, M., Peptide YY (3–36) inhibits dopamine and norepinephrine release in the hypothalamus, Eur. J. Pharmacol., 2005, vol. 519, nos. 1–2, pp. 48–51.
Motorykina, E.S., Khirazova, E.E., Maslova, M.V., Maklakova, A.S., Graf, A.V., Bayzhymanov, A.A., Kurko, O.D., Zamyatina, L.A., Andreyeva, L.A., Sokolova, N.A., Myasoyedov, N.F., and Kamenskii, A.A., Changes in feeding and drinking motivations and glucose content in male rats after single or chronic administration of obestatin or its fragment (1–4), Dokl. Biol. Sci., 2015, vol. 460, pp. 1–4.
Samson, W.K., Yosten, G.L.C., Chang, J.K., Ferguson, A.V., and White, M.M., Obestatin inhibits vasopressin secretion: Evidence for a physiological action in the control of fluid homeostasis, J. Endocrinol., 2008, vol. 196, no. 3, pp. 559–564.
Agnew, A., Calderwood, D., Chevallier, O.P., Greer, B., Grieve, D.J., and Green, B.D., Chronic treatment with a stable obestatin analog significantly alters plasma triglyceride levels but fails to influence food intake; fluid intake; body weight; or body composition in rats, Peptides, 2011, vol. 32, no. 4, pp. 755–762.
Bliss, E.S. and Whiteside, E., The gut-brain axis, the human gut microbiota and their integration in the development of obesity, Front. Physiol., 2018, vol. 9.
Fujimiya, M., Ataka, K., Asakawa, A., Chen, C.Y., Kato, I., and Inui, A., Regulation of gastroduodenal motility: Acyl ghrelin, des-acyl ghrelin and obestatin and hypothalamic peptides, Digestion, 2012, vol. 85, no. 2, pp. 90–94.
Chen, C.Y., Lee, W.J., Chong, K., Lee, S.D., and Liao, Y.D., Impact of intracerebroventricular obestatin on plasma acyl ghrelin, des-acyl ghrelin and nesfatin-1 levels, and on gastric emptying in rats, Mol. Med. Rep., 2012, vol. 6, no. 1, pp. 191–196.
Bassil, A.K., Häglund, Y., Brown, J., Rudholm, T., Hellström, P.M., Näslund, E., Lee, K., and Sanger, G.J., Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract, Br. J. Pharmacol., 2007, vol. 150, no. 1, pp. 58–64.
Grande, C., Gesmundo, I., Settanni, F., Taliano, M., Gallo, D., Gargantini, E., Ghigo, E., and Granata, R., Obestatin enhances in vitro generation of pancreatic islets through regulation of developmental pathways, PLoS One, 2013, vol. 8, no. 5.
Ren, A.J., Guo, Z.F., Wang, Y.K., Wang, L.G., Wang, W.Z., Lin, L., Zheng, X., and Yuan, W.J., Inhibitory effect of obestatin on glucose-induced insulin secretion in rats, Biochem. Biophys. Res. Commun., 2008, vol. 369, no. 3, pp. 969–972.
Gao, X.Y., Kuang, H.Y., Liu, X.M., Wang, X.Y., Pan, Y.H., and Ma, X.X., Decreased obestatin in plasma in metabolically obese, normal-weight men with normal glucose tolerance, Diabetes Res. Clin. Pract., 2008, vol. 79, no. 1, pp. e5–e6.
Fujimiya, M., Asakawa, A., Ataka, K., Kato, I., and Inui, A., Different effects of ghrelin, des-acyl ghrelin and obestatin on gastroduodenal motility in conscious rats, World J. Gastroenterol., 2008, vol. 14, no. 41, pp. 6318–6326.
Morley, J.E., Farr, S.A., Sell, R.L., Hileman, S.M., and Banks, W.A., Nitric oxide is a central component in neuropeptide regulation of appetite, Peptides, 2011, vol. 32, no. 4, pp. 776–780.
Agnew, A.J., Robinson, E., McVicar, C.M., Harvey, A.P., Ali, I.H.A., Lindsay, J.E., McDonald, D.M., Green, B.D., and Grieve, D.J., The gastrointestinal peptide obestatin induces vascular relaxation via specific activation of endothelium-dependent NO signalling, Br. J. Pharmacol., 2012, vol. 166, no. 1, pp. 327–338.
Penna, C., Tullio, F., Femmino, S., Rocca, C., Angelone, T., Cerra, M.C., Gallo, M.P., Gesmundo, I., Fanciulli, A., Brizzi, M.F., Pagliaro, P., Alloatti, G., and Granata, R., Obestatin regulates cardiovascular function and promotes cardioprotection through the nitric oxide pathway, J. Cell. Mol. Med., 2017, vol. 21, no. 12, pp. 3670–3678.
Vergote, V., Baert, B., Vandermeulen, E., Peremans, K., van Bree, H., Slegers, G., Burvenich, C., and De Spiegeleer, B., LC-UV/MS characterization and DOE optimization of the iodinated peptide obestatin, J. Pharm. Biomed. Anal., 2008, vol. 46, no. 1, pp. 127–136.
Khirazova, E.E., Maslova, M.V., Motorykina, E.S., Frid, D.A., Graf, A.V., Maklakova, A.S., Sokolova, N.A., and Kamenskii, A.A., Effects of single intranasal administration of obestatin fragments on the body weight and feeding and drinking behaviors, Dokl. Biol. Sci., 2013, vol. 453, no. 1, pp. 336–337.
Subasinghage, A.P., Green, B.D., Flatt, P.R., Irwin, N., and Hewage, C.M., Metabolic and structural properties of human obestatin {1-23} and two fragment peptides, Peptides, 2010, vol. 31, no. 9, pp. 1697–1705.
Motorykina, E.S., Khirazova, E.E., Maslova, M.V., Graf, A.V., Maklakova, A.S., Bayzhymanov, A.A., Kurko, O.D., Andreyeva, L.A., Sokolova, N.A., Myasoyedov, N.F., and Kamenskii, A.A., Changes in behavior and blood corticosterone level in male and female rats after single administration of obestatin fragment 1-4, Bull. Exp. Biol. Med., 2016, vol. 161, no. 2, pp. 218–220.
Funding
The reported study was funded by Russian Foundation for Basic Research, project number 19-115-50398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
About this article
Cite this article
Graf, A.V., Khirazova, E.E., Maslova, M.V. et al. Obestatin and Its Fragments: A New Approach to the Regulation of Body Weight under Normal and Pathological Conditions. Moscow Univ. Biol.Sci. Bull. 75, 50–64 (2020). https://doi.org/10.3103/S0096392520020042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392520020042