Abstract
Protein kinases represent one of the largest eukaryotic enzyme superfamilies. However, only a few can directly phosphorylate tubulin and contribute to the modulation of the “tubulin code.” The authors previously confirmed the structural and functional homology of the plant protein kinase IREH1 and members of the mammalian MAST kinase family. Their participation in the regulation of the microtubule system in plant and animal cells was also experimentally confirmed. At the same time, the direct contribution of MAST/IRE to the “tubulin code” remains unclear. In the current study, based on bioinformatical and structural biology methods, the possibility of such an interaction was evaluated. The target sites of MAST/IRE-phosphorylation of tubulin were predicted based on similarity to the generalized specific profiles. Two potential MAST/IRE specific sites, conserved in human and Arabidopsis tubulins were selected: Thr73 (80) exists in most isotypes of α-tubulin and Ser115 was found in the majority of human and plant isotypes of β-tubulin. It was predicted that phosphorylation of the first site can affect the assembly of α/β-tubulin heterodimer, and phosphorylation of the second may affect the interaction between neighboring protofilaments of microtubules. The last site Ser433, was found in both γ-tubulin isotypes of A. thaliana, but it was absent in mammals. The external position of Ser433 in plant γ-tubulin allows for suggesting that phosphorylation of this amino acid can affect the structure of the γTuRC complex but it does not affect inner contacts of γTuSC and their interaction in the ring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Microtubules (MT) are key element of the cytoskeleton involved in many important cellular processes, such as cell division, cell polarity, intracellular transport, and movement (Amos and Schlieper, 2005; Desai and Mitchison, 1997; Howard and Hyman, 2003; Lansbergen and Akhmanova, 2006). Tubulin, as a core MT protein, is an important substrate for posttranslational modifications (PTM) (Gadadhar et al., 2017; Janke, 2014; Wloga et al., 2017; Yu et al., 2015). Together with the expression of different tubulin isotypes, diverse PTM form the basis of the “tubulin code” as the main source of MT functional diversity. Changes in charges, local conformations, and volumes in regions of target sites caused by the addition or removal of various chemical groups shape and control the functional specialization and plasticity of MT (Gadadhar et al., 2017; Smertenko et al., 1997). Known PTM of tubulin include phosphorylation, acetylation, polyglutamylation, ubiquitinylation, tyrosylation/detyrosylation, methylation, formation of Δ2-tubulin, tyrosine nitration, polyamination, glycylation, etc. (Blume et al., 2010; Chakraborti et al., 2016; Wloga et al., 2017; Smertenko et al., 1997; Yemets et al., 2011). In particular, phosphorylation is an extremely important modification that integrates various signaling pathways affecting cell division, proliferation, differentiation, movement, gene transcription, metabolism, adaptation to environmental conditions, etc. (Sawyer, 2007).
In plants, phosphorylation is carried out by numerous protein kinases. Thus, the genome of A. thaliana contains more than 1000 protein kinase genes (according to our data, 1021 genes) (Chudinova et al., 2017; Karpov et al., 2017; Wang et al., 2003, 2007; Zulawski et al., 2014). However, only a small part of them is related to the regulation of the cytoskeleton and cell division, and only a few of them can directly participate in tubulin phosphorylation (Hornbeck et al., 2015; Magiera et al., 2014). According to experimental data, for most protein kinases involved in the regulation of the cytoskeleton and cell division, the target proteins, as well as their phosphorylation sites, are quite often poorly defined or unknown (Hornbeck et al., 2015). This applies entirely to the group of MAST—Microtubule-Associated Serine/Threonine Kinases), which belong to the AGC Ser/Thr protein kinase family. In mammals and Drosophila, MAST protein kinases take an active part in regulation of various microtubule structures (interphase microtubules, preprophase band, and mitotic spindle). Higher plants also have protein kinases homologous to MAST, but the structural and functional mechanisms of their interaction with the cytoskeleton remain poorly understood (Bryantseva et al., 2010; Chudinova et al., 2017; Karpov et al., 2009, 2010). We previously confirmed the similarity of the sequences and structures of the plant protein kinase IREH1 (Incomplete Root Hair Elongation 1; At3g17850) and animal protein kinases of the MAST family (Karpov et al., 2010; Chudinova et al., 2017). We also cloned a fragment of grape MAST-like kinase containing the catalytic domain and the full-length cDNA of IREH1 from A. thaliana (Bryantseva et al., 2010; Chudinova et al., 2017). Recombinant GFP-IREH1 protein expressed in mammalian cells revealed colocalization of the plant homolog with the centrosome. At the same time, it was demonstrated that the centrosomal colocalization of plant IREH1 is associated with the N-terminal region of the specified protein kinase (Chudinova et al., 2017). However, at the same time, the sites of phosphorylation of tubulin isotypes by MAST/IRE protein kinases remain unknown.
For plants, one of the most sensitive test systems demonstrating the effect of tubulin phosphorylation on the morpho-physiological characteristics of cells is root hairs (Yemets et al., 2008; Karpov et al., 2019; Oyama et al., 2002). A decrease in the expression levels of the protein kinase IREH1 impaired the growth of Arabidopsis root hairs (Oyama et al., 2002). Normally, root hair growth starts with the expansion of the vacuole, and, subsequently, in the subapical region, near the vacuole, there is an increase in the number of MT, which are positioned in parallel to the longitudinal axis (Grierson et al., 2014). There is no information on how cytoplasmic MT relocalization occurs and how it is related to the regulation of root hair ontogenesis. However, there is an assumption that this may be related to IREH1 since human MAST, homologous to IREH1, regulates the formation of morphologically similar structures, parallel MT in “cuffs” of spermatids (Chudinova et al., 2017; Walden and Millette 1996). In addition, MAST2 activity was recorded in round spermatozoa at the beginning of the formation of “cuffs” and its sharp decrease at the final stages of spermatogenesis was revealed (Walden et al., 1993). In early Drosophila embryos, the MAST-like protein kinase “Drop out” (dop) regulates membrane centers of primary microtubule nucleation by phosphorylattion of dynein molecules (Hain et al., 2014; Pelissier et al., 2003). In turn, we found that plant IREH1 colocalizes with centers of microtubule organization in animal cells and is potentially involved in microtubule regulation (Chudinova et al., 2017). At the same time, the question of whether this regulation is related to the direct phosphorylation of tubulin, and whether plant IRE-like protein kinases belong to a unique group of modulators of the “tubulin code” of plants, remains unexplored. Therefore, in this study, based on the results of bioinformatics and structural biological studies, we determined the most probable sites of MAST/IRE-specific phosphorylation of tubulin isotypes in human and A. thaliana.
MATERIALS AND METHODS
Control sites of MAST-specific phosphorylation. Experimentally confirmed sites known for human Greatwall protein kinase (MASTL, GWL, UniProtKB: Q96GX5): 55-KGQKYFD GDYNMAK-69 identified in cAMP-dependent phosphoprotein 19 (ARP19, UniProtKB: P56211) and 60KGQKYFD
GDYNMAK-69 identified in cAMP-dependent phosphoprotein 19 (ARP19, UniProtKB: P56211) and 60KGQKYFD GDYNMAK-74 identified in alpha-endosulfin (ENSA, UniProtKB: O43768) were used as the main control sites (Fig. 1). As an additional source of information on MAST-specific phosphorylation, the PhosphoNetworks database was used (www.phosphonetworks.org), which contains information obtained using a combination of bioinformatics methods and the protein chip (Hu et al., 2014a, 2014b; Newman et al., 2013).
GDYNMAK-74 identified in alpha-endosulfin (ENSA, UniProtKB: O43768) were used as the main control sites (Fig. 1). As an additional source of information on MAST-specific phosphorylation, the PhosphoNetworks database was used (www.phosphonetworks.org), which contains information obtained using a combination of bioinformatics methods and the protein chip (Hu et al., 2014a, 2014b; Newman et al., 2013).
MAST1-specific human protein phosphorylation sites deposited in PhosphoNetworks, included

MAST2-specific human protein phosphorylation sites deposited in PhosphoNetworks included

Construction of MAST-specific phosphorylation site motifs. Motifs of MAST-specific phosphorylation sites were constructed according to the recommendations of the PROSITE service (https://prosite. expasy.org) (Sigrist et al., 2013). When scanning proteins using the ScanProsite program, the search option in a user-defined collection of motifs and target proteins was applied (Option three: Submit PROTEIN sequences and MOTIFS to scan them against each other) (Sigrist et al., 2013). As protein targets, we used up-to-date revisions of human and Arabidopsis α-, β-, and γ-tubulin isotype sequences deposited in UniProtKB (www.uniprot.org, UniProt Consortium, 2023):
H. sapiens—α-tubulin isotypes:
TBA1A (Q71U36), TBA1B (P68363),
TBA1C (Q9BQE3), TBA3C (P0DPH7),
TBA3E (Q6PEY2), TBA4A (P68366),
TBA8 (Q9NY65) and TBAL3 (A6NHL2);
β-tubulin isotypes:
TBB1 (Q9H4B7), TBB2B (Q9BVA1),
TBB2A (Q13885), TBB4A (P04350),
TBB4B (P68371), TBB5 (P07437),
TBB3 (Q13509), TBB6 (Q9BUF5)
TBB8 (Q3ZCM7);
γ-tubulin isotypes:
TBG1 (P23258) and TBG2 (Q9NRH3).
A. thaliana—α-tubulin isotypes:
TBA1 (P11139), TBA2 (B9DGT7),
TBA3 (Q56WH1), TBA4 (Q0WV25),
TBA5 (B9DHQ0), TBA6 (P29511);
β-tubulin isotypes:
TBB1 (P12411), TBB2 (Q56YW9),
TBB3 (Q9ASR0), TBB4 (P24636),
TBB5 (P29513), TBB6 (P29514),
TBB7 (P29515), TBB8 (P29516),
TBB9 (P29517);
γ-tubulin isotypes:
TBG1 (P38557) and TBG2 (P38558).
Phylogenetic clustering. The clustering of control and potential phosphorylation sites were identified using a profile search performed by aligning the corresponding amino acid sequences by ClustalX (v.2.0.10) using UPGMA clustering (Atteson et al., 1997) and bootstrapping (Efron et al., 1996). Further visualization and dendrogram analysis were performed using MEGA v.11 (www.megasoftware.net) (Tamura et al., 2021) and FigTree (Tree Figure Drawing Tool) version 1.4.4 (http://tree.bio.ed.ac.uk). Predicted motif positions were compared with experimentally confirmed sites from the PhosphoSitePlus database (http://www.phosphosite.org) and saved in Xp ± 7 format (*.fasta) (Hornbeck et al., 2015).
Structural and biological studies. Visualization of structural models, analysis of protein complexes, and topology of phosphorylation sites was performed using the PyMOL v.1.5.0.5 program (www.pymol.org). The structural analysis was based on the use of RCSB Protein Data Bank structures—6BR1, 6BRF, 6BRY, 6BS2 (for α and β-tubulins) (Banerjee et al., 2018)—of the previously constructed SD model of the plant γTuSC complex (Karpov et al., 2017), constructed using protein structure homology modeling servers I-Tasser (Yang et al., 2015) and Swiss-Model (Bienert et al., 2017).
RESULTS
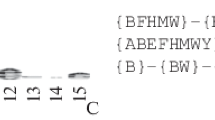
To predict the sites of specific phosphorylation of α-, β-, and γ-tubulins of H. sapiens and A. thaliana, first of all, a generalized motif was constructed based on the sequences of experimentally confirmed sites of protein kinases of the MAST family. The initial selection included experimentally confirmed sites of specific phosphorylation by protein kinase GWL (Greatwall/MASTL), specific phosphorylation sites are known for cAMP-regulated phosphoprotein 19 (ARP19) and alpha-endosulfin (ENSA) (Fig. 1). In addition, sites from the PhosphoNetworks project associated with human MAST1 and MAST2 protein kinases were added to the sample (www.phosphonetworks.org) (Hu et al., 2014a,b; Newman et al., 2013) (see Materials and Methods for details). The full sample included 77 phosphorylation sites: 31 for GWL, 33 for MAST1, and 12 for MAST2. Based on the results of the alignment of the control sites, subject to the restriction of the opening of gaps, we created an appropriate search motif (according to the PROSITE rules): {CH-NRWY}-{NWY}-{CHQWY}-{CWY}-{CFHV}-{CGMWY}-{MQRWY}-[ST]-{CIMWY}-{CFMQWY}-[DEGHKNSTY]-{MPW}-{NPTW}-{CMY}-{FMNRWY}.
At the next stage of research, the specified motif was used to identify promising consensus sites in the complete set of sequences of α-, β-, and γ-tubulin isotypes of H. sapiens and A. thaliana (see Materials and methods). According to the results of the search using the ScanProsite tool, 51 potential sites of MAST-specific phosphorylation of tubulins for H. sapiens and A. thaliana were revealed. The results of a comparative analysis of the identified phosphorylation sites of human tubulins with PhosphoSitePlus data (www.phosphosite.org) confirmed their coincidence with the results of mass spectrometry. Since this motif exhibits a degraded nature, the number of predicted MAST/IRE phosphorylation sites was excessive. In this regard, at the next stage, joint clustering of predicted and experimentally confirmed GWL sites was carried out. Since the sequences of all GWL sites from ARP19 and ENSA proteins turned out to be identical, clustering was performed using one common sequence: KGQKYFD GD YNMAK (55-KGQKYFD
GD YNMAK (55-KGQKYFD GDYNMAK-69 from human ARP19/UniProtKB: P56211 and 60KGQKYFD
GDYNMAK-69 from human ARP19/UniProtKB: P56211 and 60KGQKYFD GDYNMAK-74 from human ENSA/ UniProtKB: O43768) (Fig. 1). For joint clustering, all sampling sites were prepared in the generally accepted form of S/T ± 7 and saved in *.fasta file format. At the same time, during the alignment of the PTM sections, the opening of gaps was prohibited. Thus, clustering in ClustalX based on UPGMA and bootstrapping without conventional sequence alignment was applied (Fig. 2). The constructed tree allowed us to determine the most promising phosphorylation sites of α-, β-, and γ-tubulins, which formed a common clade with the control sequence. For α-, β-tubulins, one site was selected for α-, β-tubulin isotypes. These sites had a common location for most isotypes of α- and β-tubulins of H. sapiens and A. thaliana.
GDYNMAK-74 from human ENSA/ UniProtKB: O43768) (Fig. 1). For joint clustering, all sampling sites were prepared in the generally accepted form of S/T ± 7 and saved in *.fasta file format. At the same time, during the alignment of the PTM sections, the opening of gaps was prohibited. Thus, clustering in ClustalX based on UPGMA and bootstrapping without conventional sequence alignment was applied (Fig. 2). The constructed tree allowed us to determine the most promising phosphorylation sites of α-, β-, and γ-tubulins, which formed a common clade with the control sequence. For α-, β-tubulins, one site was selected for α-, β-tubulin isotypes. These sites had a common location for most isotypes of α- and β-tubulins of H. sapiens and A. thaliana.
Results of joint clustering (bootstrapping by the UPGMA method with gap opening restriction) of the MAST/IRE-specific phosphorylation sites predicted for human and Arabidopsis tubulin isotypes and the experimentally proven conserved site MASTL (GWL—Serine/threonine-protein kinase greatwall) with ARP19 (cAMP-regulated phosphoprotein 19) and ENSA_HUMAN (Alpha-endosulfin).
For α-tubulin, the closest cluster was formed by a group of fragments corresponding to the Thr73 site (Thr81 in human TBAL3 (A6NHL2)). The specified site was revealed in all human α-tubulins (TBA1A, TBA1B, TBA3C, TBA3E, TBA8, and TBAL3) and all α-tubulins of Arabidopsis (TBA1, TBA2, TBA3, TBA4, TBA5, and TBA6) (Fig. 3). Only the human TBA4A isotype was an exception. Based on the results of the analysis of structural models of microtubule fragments of H. sapiens and A. thaliana (Fig. 4a), localization of Thr73 on the internal contact area of α/β-tubulin heterodimers was established (Fig. 4b). Thus, the obtained results indicate that Thr73 phosphorylation can affect the assembly and integrity of tubulin heterodimers in animals and higher plants.
Structural topology analysis of identified MAST/IREH1 sites in mammalian and plant microtubules: (a) topology of Thr73(81) of α-tubulin and Ser115 of β-tubulin in the protofilament of microtubules of mammals (above) and plants (below); (b) location of Thr73 of α-tubulin in intradimeric contact of molecules of α and β-tubulin of the experimentally solved structure of mammals (PDB: 6BR1, left) and plant model (right).
For β-tubulin, the closest cluster was a group of fragments corresponding to the Ser115 site and uniting most β-tubulins of A. thaliana (TBB2, TBB3, TBB4, TBB7, TBB8, and TBB9) and H. sapiens (TBB5, TBB3, TBB4B, TBB2B, and TBB2A) (Fig. 3). At the same time, this site was not identified in isotypes TBB1, TBB5, and TBB6 of A. thaliana and TBB4A, TBB6, TBB8, and TBB1 of H. sapiens. The location of Ser115 on the surface of the protein molecule suggests that its phosphorylation should affect the lateral interactions of neighboring protofilaments (Figs. 4a, 5a).
Structural arrangement of Ser115 in β-tubulin and Ser433 in γ-tubulin. (a) The location of the Ser115 residue on the surface of the β-tubulin molecule indicates that its phosphorylation can affect the interaction between neighboring microtubule protofilaments. (a, b) The position of Ser433 in the γ-tubulin molecule indicates that this residue does not affect the internal contacts of the small γ-tubulin complex (γTuSC) and their polymerization. However, the external localization of the Ser433 residue suggests that, in higher plants, the phosphorylation of this amino acid may affect the interaction of the large complex (γTuRC) with associated proteins.
A separate clade corresponding to the Ser433 site was found in the case of γ-tubulin (Fig. 3). Sites from this clade showed minimal distance to the control (GWL-specific phosphorylation site) and are represented by only two identical sites from γ-tubulin of Arabidopsis: TBG1 and TBG2. At the same time, this site was absent in human γ-tubulins. The results of a Blastp search of this site in the “reviewed” section of UniProtKB showed that the first 100 hits were represented exclusively by 71 γ-tubulin sequences from flowering plants (Magnoliophyta). Thus, if phosphorylation of the S433 residue in A. thaliana will be confirmed experimentally in the future, it will indicate the presence of an IRE-dependent mechanism of posttranslational regulation, which is unique to higher plants.
To analyze the structural localization of Ser433, we supplemented the previously constructed model of the primary center of microtubule nucleation of A. thaliana (a fragment represented by a complex of three γTuSC heterotetramers) (Karpov et al., 2017). To obtain a more complete picture, two α/β-tubulin heterodimers were added to the γTuSC complex. The analysis of the position of the Ser433 site showed that phosphorylation of the specified amino acid residue cannot affect the internal and ring contact interfaces of small γTuSC complex (Figs. 4a, 4b). However, its external localization indicated that phosphorylation of the Ser433 residue may affect the external interactions of the large γTuRC complex of plant primary centers of microtubule nucleation (Yemets et al., 2008).
DISCUSSION
According to the obtained results, two of the identified potential sites of MAST/IRE-specific phosphorylation are conserved in humans and Arabidopsis: Thr73 (80), which is conserved in the most of α-tubulin isotypes, and Ser115, found in most animal and plant β-tubulin isotypes. A third site of potential phosphorylation on Ser433 residue was found in both γ-tubulin isotypes of A. thaliana, but it was absent in mammalian γ-tubulins. It should be noted that Ser433 and its amino acid surroundings are conserved in most members of Magnoliophyta (The UniProt Consortium T, 2023). The highlighted amino acid residues correspond to the canonical motifs of phosphorylation sites, and the structural topology confirms their availability for modification.
The functional value of the Thr73 α-tubulin residue was confirmed by the fact that this residue is localized in the area of the internal interface of the α/β-tubulin dimer (Prota et al., 2014). The position of Thr73 indicates that its phosphorylation can not only affect the structure of the tubulin heterodimer, GTP/GDP exchange site, and the interaction with the cofactor (Mg2+) but also the properties of the interdimer space of the canonical colchicine binding site (Prota et al., 2014; Vela-Corcia et al., 2018). According to PhospoSitePlus (ID: 14582260), phosphorylation of human α-tubulin at residue Thr73 was previously confirmed by mass spectrometry (Hornbeck et al., 2015; Mertins et al., 2016). In addition, the fundamental role of the Thr73 residue was confirmed by experiments on HeLa, Jurkat, and K562 breast tumor cell cultures (Hornbeck et al., 2015; Kettenbach et al., 2011; Mertins et al., 2016; Zhou et al., 2013).
Another site, the Ser115 residue of β-tubulin is located in the lateral contact zone between microtubule protofilaments, and its phosphorylation in mammals was previously confirmed by phosphoproteomic analysis of HeLa cell proteins (Liu et al., 2015). Phosphorylation of the Ser115 residue of β-tubulin in mammals was also detected by mass spectrometry (ID: 4713923) (Hornbeck et al., 2015; Klammer et al., 2012; Mertins et al., 2016), as well as proven by experiments on breast tumor cell cultures, H2009, H2887, HeLa, Jurkat, and MKN-45 (Hornbeck et al., 2015, Kettenbach et al., 2011; Klammer et al., 2012; Mertins et al., 2016). In addition, it was recently shown that Ser115 residue of β-tubulin in A. thaliana can also be phosphorylated by NIMA-related kinase 6 (NEK6) (Takatani et al., 2017).
Previously, we transfected the African green monkey kidney cell line (Vero) with a plasmid construct expressing a chimeric gene GFP-IREH1, and it was shown that the product of its expression is distributed in the cytoplasm, mainly colocalizing with the centrosome. However, further experiments proved the absence of colocalization of IREH1 with centrosomes in cells with destroyed MT and its restoration after the reappearance of MT. Therefore, the binding of the plant protein kinase IREH1 to the animal centrosome depends on MT (Chudinova et al., 2017). This was also confirmed by immunoprecipitation of chimeric pEGFP-IREH1 and pEGFP-C3 gene expression products from HEK293 cell lysates with anti-GFP antibodies. As a result of immunoprecipitation with mammalian γ-tubulin, complex formation was not detected. Thus, the plant protein kinase IREH1 in human cells shows direct or indirect affinity to the microtubule but not to the centrosomal tubulin (Chudinova et al., 2017).
Comparing the data of previous experiments and the current bioinformatics study, we can conclude that the Thr73 (80) residue of α-tubulin and the Ser115 residue of β-tubulin are the most probable target sites of the plant protein kinase IREH1 and the animal protein kinase MAST. Even though it was previously experimentally proven that these sites are part of the “tubulin code” and are phosphorylated in vitro (Hornbeck et al., 2015; Klammer et al., 2012; Mertins et al., 2016), the hypothesis of their association with protein kinases IREH1 and MAST was proposed for the first time. The conservatism of these areas in plant and animal tubulins confirms our theory about not only structural but also certain functional conservatism of plant IREH1 and animal MAST (Bryantseva et al., 2010; Chudinova et al., 2017; Karpov et al., 2009a, 2009b, 2010).
The possibility of direct phosphorylation of plant tubulin by IREH1 should also not be ignored. Although Ser433 is present only in γ-tubulin of plants, it should be taken into account that this site turned out to be the most similar to the experimentally proven sites of the animal protein kinase MASTL (GW—Serine/threonine-protein kinase greatwall) (Figs. 1, 3). However, at this stage, this hypothesis remains somewhat debatable but undoubtedly requires further research with application of experimental methods.
REFERENCES
Amos, L.A. and Schlieper, D., Microtubules and maps, Adv. Protein Chem., 2005, vol. 71, pp. 257–298. https://doi.org/10.1016/S0065-3233(04)71007-4
Atteson, K., The performance of neighbor-joining algorithms of phylogeny reconstruction, in Lecture Notes in Computer Science, 1997, vol. 1276, pp. 101–110. https://doi.org/10.1007/BFb0045077
Banerjee, S., Arnst, K.E., Wang, Y., Kumar, G., Deng, S., Yang, L., Li, G.B., Yang, J., White, S.W., Li, W., and Miller, D.D., Heterocyclic-fused pyrimidines as novel tubulin polymerization inhibitors targeting the colchicine binding site: Structural basis and antitumor efficacy, J. Med. Chem., 2018, vol. 61, no. 4, pp. 104–1718. https://doi.org/10.1021/acs.jmedchem.7b01858
Bienert, S., Waterhouse, A., de Beer, T.A., Tauriello, G., Studer, G., Bordoli, L., and Schwede, T., The SWISS-MODEL Repository – new features and functionality, Nucleic Acids Res., 2017, vol. 45, no. D1, pp. D13–D319. https://doi.org/10.1093/nar/gkw1132
Blume, Ya., Yemets, A., Sheremet, Ya., Nyporko A., Sulimenko, V., Sulimenko, T., and Draber, P., Exposure of beta-tubulin regions defined by antibodies on a Arabidopsis thaliana microtubule protofilament model and in the cells, BMC Plant Biol., 2010, vol. 10, p. 29. https://doi.org/10.1186/1471-2229-10-29
Bryantseva, S.A., Gavryushina, E.S., Yemets, A.I., Karpov, P.A., Blume, Ya.B., Drygin, Yu.F., and Nadezhdina, E.S., MAST2-like protein kinase from grape Vitis vinifera: Cloning of catalytic domain cDNA, Cytol. Genet., 2010, vol. 44, no. 4, pp. 227–232. https://doi.org/10.3103/S0095452710040079
Chakraborti, S., Natarajan, K., Curiel, J., Janke, C., and Liu, J., The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease, Cytoskeleon, 2016, vol. 73, no. 10, pp. 521–550. https://doi.org/10.1002/cm.21290
Chudinova, E.M., Karpov, P.A., Fokin, A.I., Yemets, A.I., Lytvyn, D.I., Nadezhdina, E.S., and Blume, Y.B., MAST-like protein kinase IREH1 from Arabidopsis thaliana co-localizes with the centrosome when expressed in animal cells, Planta, 2017, vol. 246, no. 5, pp. 959–969. https://doi.org/10.1007/s00425-017-2742-4
Desai, A. and Mitchison, T.J., Microtubule polymerization dynamics, Ann. Rev. Cell Dev. Biol., 1997, vol. 13, pp. 83–117. https://doi.org/10.1146/annurev.cellbio.13.1.83
Efron, B., Halloran, E., and Holmes, S., Bootstrap confidence levels for phylogenetic trees, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, no. 23, pp. 13429–13434. https://doi.org/10.1073/pnas.93.23.13429
Gadadhar, S., Bodakuntla, S., Natarajan, K., and Janke, C., The tubulin code at a glance, J. Cell Sci., 2017, vol. 130, pp. 1347–1353. https://doi.org/10.1242/jcs.199471
Grierson, C., Nielsen, E., Ketelaarc, T., and Schiefelbein, J., Root hairs, Arabidopsis Book, 2014, vol. 12, p. e0172. https://doi.org/10.1199/tab0172
Hain, D., Langlands, A., Sonnenberg, H.C., Bailey, C., Bullock, S.L., and Müller, H.A., The Drosophila MAST kinase Drop out is required to initiate membrane compartmentalisation during cellularisation and regulates dynein-based transport, Development, 2014, vol. 141, pp. 2119–2130. https://doi.org/10.1242/dev.104711
Hornbeck, P.V., Zhang, B., Murray, B., Kornhauser, J.M., Latham, V., and Skrzypek, E., PhosphoSitePlus, 2014: Mutations, PTMs and recalibration, Nucleic Acids Res., 2015, vol. 43, no. D1, pp. D512–D520. https://doi.org/10.1093/nar/gku1267
Howard, J. and Hyman, A.A., Dynamics and mechanics of the microtubule plus end, Nature, 2003, vol. 422, no. 6933, pp. 753–758. https://doi.org/10.1038/nature01600
Hu, J., Rho, H.S., Newman, R.H., Hwang, W., Neiswinger, J., Zhu, H., Zhang, J., and Qian, J., Global analysis of phosphorylation networks in humans, Biochim. Biophys. Acta, 2014a, vol. 1844, no. 1, pp. 224–231. https://doi.org/10.1016/j.bbapap.2013.03.009
Hu, J., Rho, H., Newman, R., Zhang, J., Zhu, H., and Qian, J., PhosphoNetworks: A database for human phosphorylation networks, Bioinformatics, 2014b, vol. 30, no. 1, pp. 141–142. https://doi.org/10.1093/bioinformatics/btt627
Janke, C., The tubulin code: Molecular components, readout mechanisms, and functions, J. Cell Biol., 2014, vol. 206, no. 4, pp. 461–472. https://doi.org/10.1083/jcb.201406055
Karpov, P.A., Nadezhdina, E.S., Yemets, A.I., Matusov, V.G., Nyporko, A.Yu., Shashina, N.Yu., and Blume, Ya.B., Bioinformatic search of plant protein kinases involved in the phosphorylation of microtubular proteins and the regulation of the cell cycle, Cytol. Genet., 2009, vol. 43, no. 3, pp. 201–215. https://doi.org/10.3103/S0095452709030104
Karpov, P.A., Nadezhdina, E.S., Yemets, A.I., Matusov, V.G., Nyporko, A.Yu., Shashina, N.Yu., and Blume, Ya.B., Bioinformatic search of plant microtubule-and cell cycle related serine-threonine protein kinases, BMC Genomics, 2010, vol. 11, no. 1, p. S14. https://doi.org/10.1186/1471-2164-11-S1-S14
Karpov, P.A., Raevsky, A.V., Krasnoperova, E.E., Isayenkov, S.V., Yemets, A.I., and Blume, Ya.B., Protein kinase KIN10 from Arabidopsis thaliana as a potential regulator of primary microtubule nucleation centers in plants, Cytol. Genet., 2017, vol. 51, no. 6, pp. 415–421. https://doi.org/10.3103/S0095452717060056
Karpov, P.A., Sheremet, Y.A., Blume, Y.B., and Yemets, A.I., Studying the role of protein kinases CK1 in organization of cortical microtubules in Arabidopsis thaliana root cells, Cytol. Genet., 2019, vol. 53, pp. 441–450. https://doi.org/10.3103/S0095452719060033
Kettenbach, A.N., Schweppe, D.K., Faherty, B.K., Pechenick, D., Pletnev, A.A., and Gerber, S.A., Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells, Sci. Signal., 2011, vol. 4, no. 179, p. rs5. https://doi.org/10.1126/scisignal.2001497
Klammer, M., Kaminski, M., Zedler, A., Oppermann, F., Blencke, S., Marx, S., Müller, S., Tebbe, A., Godl, K., and Schaab, C., Phosphosignature predicts dasatinib response in non-small cell lung cancer, Mol. Cell Proteomics, 2012, vol. 11, no. 9, pp. 651–668. https://doi.org/10.1074/mcp.M111.016410
Lansbergen, G. and Akhmanova, A.S., Microtubule plus end: A hub of cellular activities, Traffic, 2006, vol. 7, no. 5, pp. 499–507. https://doi.org/10.1111/j.1600-0854.2006.00400.x
Liu, N., Xiong, Y., Ren, Y., Zhang, L., He, X., Wang, X., Liu, M., Li, D., Shui, W., and Zhou, J., Proteomic profiling and functional characterization of multiple posttranslational modifications of tubulin, J. Proteome Res., 2015, vol. 14, pp. 3292–3304. https://doi.org/10.1021/acs.jproteome.5b00308
Magiera, M.M. and Janke, C., Post-translational modifications of tubulin, Curr. Biol., 2014, vol. 24, no. 9, pp. R351–R354. https://doi.org/10.1016/j.cub.2014.03.032
Mertins, P., Mani, D.R., Ruggles, K.V., Gillette, M.A., Clauser, K.R., Wang, P., Wang, X., Qiao, J.W., Cao, S., Petrali, F., et al., Proteogenomics connects somatic mutations to signalling in breast cancer, Nature, 2016, vol. 534, no. 7605, pp. 55–62. https://doi.org/10.1038/nature18003
Newman, R.H., Hu, J., Rho, H.S., Xie, Z., Woodard, C., Neiswinger, J., Cooper, C., Shirley, M., Clark, H.M., Hu, S., Hwang, W., Jeong, J.S., Wu, G., Lin, J., Gao, X., Ni, Q., Goel, R., Xia, S., Ji, H., Dalby, K.N., Birnbaum, M.J., Cole, P.A., Knapp, S., Ryaza-nov, A.G., Zack, D.J., Blackshaw, S., Pawson, T., Gingras, A.C., Desiderio, S., Pandey, A., Turk, B.E., Zhang, J., Zhu, H., and Qian, J., Construction of human activity-based phosphorylation networks, Mol. Syst. Biol., 2013, vol. 9, p. 655. https://doi.org/10.1038/msb.2013.12
Oyama, T., Shimura, Y., and Okada, K., The IRE gene encodes a protein kinase homologue and modulates root hair growth in Arabidopsis, Plant J., 2002, vol. 30, pp. 289–299. https://doi.org/10.1046/j.1365-313x.2002.01290.x
Pelissier, A., Chauvin, J.-P., and Lecuit, T., Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis, Curr. Biol., 2003 vol. 13, pp. 1848–1857. https://doi.org/10.1016/j.cub.2003.10.023
Prota, A.E., Danel, F., Bachmann, F., Bargsten, K., Buey, R.M., Pohlmann, J., Reinelt, S., Lane, H., and Steinmetz, M.O., The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization, J. Mol. Biol., 2014, vol. 426, pp. 1848–1860. https://doi.org/10.1016/j.jmb.2014.02.005
Sawyer, T.K., Protein kinases and protein phosphatases in signal transduction pathways, in Comprehensive Medicinal Chemistry II, Elsevier, 2007, vol. 959–992. https://doi.org/10.1016/b0-08-045044-x/00075
Sigrist, C.J.A., de Castro, E., Cerutti, L., Cuche, B.A., Hulo, N., Bridge, A., Bougueleret, L., and Xenarios, I., New and continuing developments at PROSITE, Nucleic Acids Res., 2013, vol. 41, pp. D344–D347. https://doi.org/10.1093/nar/gks1067
Smertenko, A., Blume, Y.B., Viklický, V., Opatrný, Z., and Dráber, P., Posttranslational modifications and multiple isoforms of tubulin in Nicotiana tabacum cells, Planta, 1997, vol. 201, no. 3, pp. 349–358. https://doi.org/10.1007/s004250050077
Takatani, S., Ozawa, S., Yagi, N., Hotta, T., Hashimo-to, T., Takahashi, Y., Takahashi, T., and Motose, H., Directional cell expansion requires NIMA-related kinase 6 (NEK6)-mediated cortical microtubule destabilization, Sci. Rep., 2017, vol. 7, no. 1, p. 7826. https://doi.org/10.1038/s41598-017-08453-5
Tamura, K., Stecher, G., and Kumar, S., MEGA11: Molecular Evolutionary Genetics Analysis Version 11, Mol. Biol. Evol., 2021, vol. 38, no. 7, pp. 3022–3027. https://doi.org/10.1093/molbev/msab0
The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023, Nucleic Acid Res., 2023, vol. 51, no. D1, pp. D523–D531. https://doi.org/10.1093/nar/gkac12
Vela-Corcía, D., Romero, D., de Vicente, A., and Pérez-García, A., Analysis of β-tubulin-carbendazim interaction reveals that binding site for MBC fungicides does not include residues involved in fungicide resistance, Sci. Rep., 2018, vol. 8, no. 1, p. 7161. https://doi.org/10.1038/s41598-018-25335
Walden, P.D. and Cowan, N.J., A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette, Mol. Cell Biol., 1993, vol. 13, pp. 7625–7635. https://doi.org/10.1128/mcb.13.12.7625-7635.1993
Walden, P.D. and Millette, C.F., Increased activity associated with the MAST205 protein kinase complex during mammalian spermiogenesis, Biol. Reprod., 1996, vol. 55, no. 5, pp. 1039–1044. https://doi.org/10.1095/biolreprod55.5.1039
Wang, D., Harper, J.F., and Gribskov, M., Systematic trans-genomic comparison of protein kinases between Arabidopsis and Saccharomyces cerevisiae, Plant Physiol., 2003, vol. 1325, no. 1, pp. 2152–2165. https://doi.org/10.1104/pp.103.021485
Wang, H., Chevalier, D., Larue, C., Ki Cho, S., and Walker, J.C., The protein phosphatases and protein kinases of Arabidopsis thaliana, Arabidopsis Book, 2007, vol. 5, p. e0106. https://doi.org/10.1199/tab.0106
Wloga, D., Joachimiak, E., and Fabczak, H., Tubulin post-translational modifications and microtubule dynamics, Int. J. Mol. Sci., 2017, vol. 18, no. 10, p. 2207. https://doi.org/10.3390/ijms18102207
Yang, J., Yan, R., Roy, A., Xu, D., Poisson, J., and Zhang, Y., The I-TASSER Suite: Protein structure and function prediction, Nat. Methods, 2015, vol. 12, no. 1, pp. 7–8. https://doi.org/10.1038/nmeth.3213
Yemets, A., Sheremet, Y., Vissenberg, K., Van Orden, J., Verbelen, J.P., and Blume, Ya.B., Effects of tyrosine kinase and phosphatase inhibitors on microtubules in Arabidopsis root cells, Cell Biol. Int., 2008, vol. 32, no. 6, pp. 630–637. https://doi.org/10.1016/j.cellbi.2008.01.013
Yemets, A., Stelmakh, O., and Blume, Ya.B., Effects of the herbicide isopropyl-N-phenyl carbamate on microtubules and MTOCs in lines of Nicotiana sylvestris resistant and sensitive to its action, Cell Biol. Int., 2008, vol. 32, no. 6, pp. 623–629. https://doi.org/10.1016/j.cellbi.2008.01.012
Yemets, A.I., Krasylenko, Yu.A., Lytvyn, D.I., Sheremet, Ya.A., and Blume, Ya.B., Nitric oxide signaling via cytoskeleton in plants, Plant Sci., 2011, vol. 181, no. 5, pp. 545–554. https://doi.org/10.1016/j.plantsci.2011.04.017
Yu, I., Garnham, C.P., and Roll-Mecak, A., Writing and reading the tubulin code, J. Biol. Chem., 2015, vol. 290, no. 28, pp. 17163–17172. https://doi.org/10.1074/jbc.R115.637447
Zhou, H., Di Palma, S., Preisinger, C., Peng, M., Polat, A.N., Heck, A.J., and Mohammed, S., Toward a comprehensive characterization of a human cancer cell phosphoproteome, J. Proteome Res., 2013, vol. 12, no. 1, pp. 260–271. https://doi.org/10.1021/pr300630k
Zulawski, M., Schulze, G., Braginets, R., Hartmann, S., and Schulze, W.X., The Arabidopsis Kinome: Phylogeny and evolutionary insights into functional diversification, BMC Genomcs, 2014, vol. 15, no. 1, p. 548. https://doi.org/10.1186/1471-2164-15-548
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human or animal subjects.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Karpov, P.A., Ozheredov, S.P., Steshenko, A.O. et al. Bioinformatical View on the Contribution of MAST/IRE-Dependent Phosphorylation in the Tubulin Code. Cytol. Genet. 58, 202–213 (2024). https://doi.org/10.3103/S0095452724030058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452724030058