Abstract
This article describes an electrochemical immunosensor for rapid determination of Salmonella pullorum and Salmonella gallinarum. The first step in the preparation of the immunosensor involves the electrodeposition of gold nanoparticles used for capturing antibody and enhancing signals. In order to generate a benign microenvironment for the antibody, the ionic liquid (IL) 1-butyl-3-methylimidazolium hexafluorophosphate was used to modify the surface of a screen-printed carbon electrode (SPCE). The single steps of modification were monitored via cyclic voltammetry and electrochemical impedance spectroscopy. Based on these findings, a sandwich immunoassay was worked out for the two Salmonella species by immobilizing the respective unlabeled antibodies on the SPCE. Following exposure to the analytes, secondary antibody (labeled with HRP) is added to form the sandwich. After adding hydrogen peroxide and thionine, the latter is oxidized and its signal measured via CV. A linear response to the Salmonella species is obtained in the 104 to 109 cfu · mL−1 concentration range, and the detection limits are 3.0 × 103 cfu · mL−1 for both species (at an SNR of 3). This assay is sensitive, highly specific, acceptably accurate and reproducible. Given its low detection limit, it represents a promising tool for the detection of S. pullorum, S. gallinarum, and - conceivably - of other food-borne pathogens by exchanging the antibody.

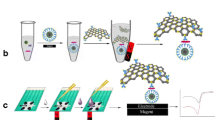
We describe an electrochemical sandwich assay based on a screen-printed carbon electrode, gold nanoparticles and ILs and capable of detecting Salmonella pullorum and Salmonella gallinarum. The preparation is outlined in the Schematic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fowl typhoid (FT), caused by Salmonella gallinarum (S. gallinarum), is an acute or chronic septicemia infectious disease. It primarily transmits by oral or respiratory routes and affects adult poultries or grower groups, its common symptoms are diarrhea, uterine hemorrhage, and spleen [1]. Pullorum disease (PD), caused by Salmonella pullorum (S. pullorum) is an acute systemic disease more common in young birds [2]. The disease can be transmitted vertically and horizontally to others with contaminated poultries that usually results in a high mortality rate. Although FT and PD in many developed countries have been strictly controlled, they often occur in developing countries. FT and PD remain a serious threat for the development of intensive poultry industry. They are also a source of foodborne transmission of disease to humans [3–5]. Therefore, establishing an effective and fast detection method for these two pathogens is required [6]. Multilocus enzyme electrophoresis and sequence analysis showed that S. pullorum and S. Gallinarum have the same antigen O1, O9 and O12, and exhibit high cross-reactivity with each other, so they can be simultaneously detected [7–9].
In our recent work, a direct assay was utilized in a sensitive enzyme immunosensor for pathogenic bacteria [10]. Hu et al. used direct assay to prepare a disposable immunosensor for Enterobacter sakazakii [11]. Zhao et al. also introduced a direct assay for Shigella flexneriIn with a detection limit of 3.1 × 103 cfu · mL−1 [12]. Zhan et al. constructed a kind of disposable immunosensor based on multiwalled carbon nanotube for direct assay for Escherichia coli O157:H7 [13]. However, in direct assay, it is difficult but important that the concentration of enzyme-labeled antibody modified on electrode be controlled precisely in the preparation process. The main problem is that if overdose of enzyme-labeled antibody is modified on the electrode, antibody would not all be covered by antigen, resulting in false negative results. Sandwich assay can be used to construct immunosensor with a better sensitivity and specificity compared to direct assay [14, 15]. A more accurate and reliable (sandwich-based) immunoassay for S. pullorum and S. gallinarum is described here.
Gold nanoparticles (AuNPs) have been applied in immunosensors due to their high specific surface and the ability for immobilizing antibody [16]. Compared with conventional modification method, the electrodeposition of nanoparticles enables AuNPs more evenly and firmly to be deposited on the working electrode. The process is simple and convenient [17].
In order to keep the activity and stability of antibody, to facilitate the immobilization of biocomponents and to promote the practical application of the assay, four materials (β-cyclodextrin, sodium alginate, chitosan and ILs) were dropped on the AuNPs/SPCE and compared to each other. After modified material was selected, we manufactured an immunosensor based on sandwich assay, and the sensitivity and accuracy of the immunosensors were measured. Then we selected the best construction method to optimize experimental conditions.
Materials and methods
Reagents and apparatus
Bacteria were employed in this work included S. pullorum and S. gallinarum (CMCC 50770) as the target bacteria, and Escherichia coli (E. coli, ATCC 8739), Staphylococcus aureus (S. aureus, ATCC 27217), Enterobacter Sakazakii (E.sakazakii, ATCC 29544), Bacillus subtilis (B. subtilis, ACCC 11060). Phosphate buffered saline (PBS, 0.01 M, pH 7.4) is used as control. Bacteria were purchased from China Center of Industrial Culture Collection (CICC, http://www.china-cicc.org/) and conserved in the laboratory of the authors. Anti- S. pullorum and S. gallinarum and HRP-labeled anti- S. pullorum and S. gallinarum were obtained from the China Institute of Veterinary Drug Control (Beijing, China). Chloroauric acid was obtained from Hangzhou Chemical Reagent Co., Ltd. (Hangzhou, China, http://www.hzhxsj.com.cn/). 1-Butyl-3-methylimidazolium hexafluorophosphate (ILs) were obtained from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (Lanzhou, China, http://www.ionicliquid.org/). Thionine (Thi) was obtained from Shanghai Zhongtai Chemical Reagent Co., Ltd. (Shanghai, China,). All other reagents were of analytical grade and the water used was doubly distilled.
The CHI 760C electrochemical workstation was provided by Shanghai ChenHua Instruments, Inc. (Shanghai, China, http://www.sangon.com). Screen-printed carbon electrode (SPCE) was developed by Rong Bin Biotechnology Co., Ltd. (Nanjing, China). As shown in photograph 1, the SPCE consisted of a working electrode, a counter electrode and a reference electrode. The diameter of disk-shaped working electrode was 0.2 cm, and the working electrode and counter electrode were made of a carbon ink whereas the reference electrode was made of silver, which were all printed on an plastic support. The nanostructures of electrode were characterized by a SU-8010 field emission scanning electron microscope (FE-SEM, Hitachi, Japan, http://www.hitachi-hightech.com/jp/). All electrochemical experiments were performed at 22 ± 2 °C.
Current density plots of different modified electrodes. a Bare SPCE, b AuNPs/SPCE, c Anti-S. pullorum and S. gallinarum / AuNPs / SPCE, d ILs/Anti-S. pullorum and S. gallinarum/ AuNPs / SPCE, (e) BSA /ILs/Anti-S. pullorum and S. gallinarum/ AuNPs / SPCE, f Immunoelectrode incubated with S. pullorum and S. gallinarum (109 CFU · mL−1), and g immunoelectrode incubated with HRP-anti-S. pullorum and S. gallinarum
Nyquist plots of EIS: bare electrode a; AuNPs electrodeposited electrode b; Anti-S. pullorum and S. gallinarum/AuNPs/SPCE c ILs/Anti-S. pullorum and S. gallinarum/AuNPs/SPCE d BSA/ILs/Anti-S. pullorum and S. gallinarum/Au NPs/SPCE e; S. pullorum and S. gallinarum/BSA/ILs/Anti-S. pullorum and S. gallinarum/AuNPs/SPCE f HRP-anti-S. pullorum and S. gallinarum/S. pullorum and S. gallinarum/ BSA/ILs/Anti-S. pullorum and S. gallinarum/AuNPs/SPCE g; all in 0.1 mM KCl containing 5.0 mM [Fe(CN6)]3- / 4-
a The specificity of immunosensor for S. pullorum and S. gallinarum. The immunosensor incubated with S. pullorum and S.gallinarum, E. sakazakii, B. subtilis, S. aureus, Vp, E. coli, and S. pullorum Nagative serum, PBS (0.01 M, pH 7.4) with the best reaction conditions, respectively. b S. pullorum and S. gallinarum bacterial suspension containing contaminating microorganism such as S. aureus, E. coli, E. sakazakii, B. subtilis
Preparation of four modified substances
Four substances were dropped on the IgG/AuNPs/SPCE working electrode as follows. The 2.5 % (v/v) ILs was prepared by mixing ILs with double distilled water with the help of sonication. The 2.0 % (w/v) β-cyclodextrin solution was prepared by dissolving β-cyclodextrin into double distilled water, stirred for a few minutes and put into a water bath (60 °C) for 1 h. The 0.25 % (w/v) sodium alginate solution was prepared by dissolving sodium alginate into double distilled water with the help of sonication. 0.2 % (w/v) chitosan was prepared by dissolving chitosan into sodium acetate solution (1 %, w/v).
Preparation of electrochemical immunosensors
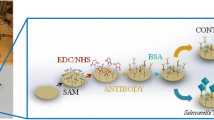
The AuNPs (25 nm) deposited on SPCE were prepared according to the previous report [10]. The electrochemical reduction was performed in a dispersion containing 25 mg · L−1 HAuCl4 with a magnetic stirring and N2 bubbling with SPCE by CV. The scan potential was performed between −1.5 and 0.5 V at a rate of 25 mV · s−1. Then the electrode was rinsed with double-distilled water and dried with blowing N2 at room temperature (25 ± 0.5 °C).
3 μL anti-S. Pullorum and S. gallinarum (isolated from rabbit serum, 1:100 dilution by 0.01 M PBS, pH = 7.4) was coated evenly onto the AuNPs/SPCE surface, and stored at 4 °C for 12 h in a sterile sealed wet box. Then 3 μL 2.5 % ILs was dropped on the working electrode, after drying at room temperature (25 ± 0.5 °C), the immunosensor was washed gently with PBS to remove excess antibody which was not combined with the IgG/AuNPs/SPCE. At last the resulting electrode was incubated in BSA solution (w/w, 0.25 %) at 4 °C for 1 h in order to block the non-specific binding sites. After the modified electrode was washed carefully with PBS, immunosensor was stored at 4 °C when not in use. The obtained modified electrode, denoted as ILs/IgG/AuNPs.
Electrochemical measurements
The preparation of the immunosensor and mechanism of detection of S. Pullorum and S. gallinarum were displayed in Scheme 1. The S. Pullorum and S. gallinarum was detected according to the following procedure: 3 μL of S. Pullorum and S. gallinarum solution (the details of preparation of S. Pullorum and S. gallinarum solution are given in the Electronic Supporting Material) was dropped onto the previously modified electrode, incubated at 30 ± 0.5 °C for 40 min and rinsed carefully with PBS to remove unbound bacterial antigen. Then 3 μL HRP-anti-S. Pullorum and S. gallinarum were dropped, incubated in the same conditions and rinsed with PBS. The above modified electrode was immersed in 0.1 mol · L−1 acetate buffer (pH = 6.5) containing 1.0 mmol · L−1 Thi and 0.8 mmol · L−1 H2O2. CV was acquired with a CHI 760C at a scan rate of 0.1 V · s−1 between −0.6 and −0.1 V. The detection of S. Pullorum and S. gallinarum was performed by measuring the reduction peak current shift (ΔIp) of CV before and after the immune reaction. Before the immunoreaction, the current response was recorded as I1. Due to the Horseradish peroxidase (HRP) accelerating the decomposition of hydrogen peroxide, the current response of the immunosensor increased after the immunoreactions and was recorded as I2. Therefore, changes of immunesensor current value (ΔIpc) was expressed as ΔIpc = I2 - I1. All experimental solutions were deairated by nitrogen for at least 10 min before measuring. All experimental solutions were deaerated by nitrogen for at least 10 min, and a nitrogen atmosphere was kept during the whole electrochemical measurements. Three successive CV scans were performed for each measurement, the last cycle was recorded.
Schematic diagram of the modification process of electrochemical immunosensor and measure mechanism: (a) Electrodeposition of AuNPs on bare SPCE; (b) Anti- S. pullorum and S. gallinarum and ILs were dropped in order;(c) ILs/ Anti- S. pullorum and S. gallinarum /SPCE was blocked with 0.25 % BSA solution; (d) BSA/ILs/ Anti- S. pullorum and S. gallinarum /SPCE incubated with S. pullorum and S. gallinarum (109 CFU · mL−1); (e) The immunosensor incubated with HRP-anti-S. pullorum and S. gallinarum; (f) The principle of electrochemical detection; (g) The change of signal before and after incubation with HRP-anti-S. pullorum and S. gallinarum
Results and discussion
Selection of modified materials and assays
β-Cyclodextrin, sodium alginate, chitosan and ILs are frequently used to improve the performance of immunosensor, due to their excellent adhesion and not harmful for the antibodies to ensure the immobilization of biocomponents and promote the practical application of the prepared AuNPs/SPCE [18–21]. But what kind of material combines AuNPs is better is still not studied. Hence, four substances were researched. Dropped 3 μL ILs, β-cyclodextrin, sodium alginate and chitosan solution on four IgG/AuNPs/SPCEs, respectively. These four different modified electrodes were all used to detect S. Pullorum and S. gallinarum according to the steps described in experimental part. The ΔIp of CV after the immune reaction were all recorded. The result showed in Fig. 1, the ΔIpc of modified electrodes with ILs increases much higher than that of β-cyclodextrin, sodium alginate and chitosan solution modified electrodes. β-cyclodextrin, sodium alginate and chitosan are good film-forming substances, their electron transfer ability is not as strong as ILs, the ΔIp increases lightly compared with the blank experiment after adding S. Pullorum and S. gallinarum and HRP-anti-S. Pullorum and S. gallinarum. It means under ILs modified conditions, the activity of antibody against S. Pullorum and S. gallinarum is highest, the final Horseradish peroxidase loading is best. This was because ILs provided a friendly microenvironment for protein (e.g. antibody and enzyme), reduced the influence of external factors (such as the change of temperature for protein), and maintained biological activity of antibody and enzyme [22–25]. Meanwhile, it significantly increased the rate of electron transfer toward electrode surface [26]. So ILs were chosen as the best protective agent for the SPCE.
Electrochemical characterization of the stepwise modified electrodes
The function of the AuNPs layer
The morphology of bare SPCE and AuNPs/SPCE were characterized using FE-SEM. As shown in Fig. 2a, bare SPCE is covered by smooth and uniform nanoparticles with diameter of about 50 nm. Fig. 2b shows AuNPs with diameter of about 25 nm are successfully electrodeposited on the working electrode. AuNPs were introduced into the fabrication of the immunosensor in order to adsorb antibodies, enhance the electrochemical signals and ensure the sensitivity of the test results. Figure 3 clearly shows that curve b has significant reduction peaks indicating AuNPs deposited on the surface of working electrode. The thin layer of AuNPs deposited on SPCE resulted in an improved performance; its signal (curve d) is much bigger than that of carbon (curve c). Thus, AuNPs are a remarkable material in the fabrication of sensors, due to its good biological compatibility, high electrical conductivity and large specific surface.
Electrochemical characteristics
CV was used to investigate the effect of each component on the electrode through the redox behavior of a reversible redox couple after each assembly step, and their curves were recorded in 1.0 mM Thi and converted into current density. Figure 4 shows a pair of reversible redox peaks of Thi at the bare SPCE (curve a). After SPCE electrodepositing in HAuCl4, the peak currents of the redox peaks (curve b) significantly increase. It means AuNPs have been successfully electrodeposited on the working electrode, which increase the surface area of working electrode and electron transfer speed. The redox currents (curve c, d and e) decrease gradually when anti-S. Pullorum and S. gallinarum, ILs and BSA dropped on the AuNPs/SPCE in certain order, which indicated that anti-S. Pullorum and S. gallinarum, ILs and BSA coated onto the electrode surface by AuNPs. In this work, ILs was used to prolong the activity of antibody. The S. Pullorum and S. gallinarum (109 CFU · mL−1) was firmly captured to the electrode surface through the specific binding affinity between the antigen and antibody, and formed a electronic barriers which hindered electron transfer toward the electrode surface, resulting in the reduction of peak current (curve f). When HRP-anti-S. Pullorum and S. gallinarum was dropped, the reduction peak current value (curve g) greatly increased, implying the enzyme-labeled antibody was bound onto the electrode surface through the immune response, and the HRP catalyzed reduction of H2O2 with the assistance of an electron mediator, which promoted electron transfer between the enzyme and the electrode. The immunosensor response is based on the following redox process:
EIS characterization
Electrochemical impedance spectroscopy (EIS) was employed to monitor the interface properties of the carbon electrode surface during stepwise modifications [27–29]. Different stages of the modified electrode were characterized in the test base solution containing 0.1 mM KCl and 5.0 mM [Fe(CN6)]3- / 4-. As seen from Fig. 5, the Ret of AuNPs/SPCE (curve b, 1.06 × 104 ± 1622 Ω) significantly decreases compared with bare electrode (curve a, 4.97 × 104 ± 4675 Ω), due to the gold nanoparticles not only have a large specific surface area, but also own a highly efficient electron transport property and electro-catalytic activity. So the gold nanoparticles greatly reduced the resistance to accelerate the rate of electron transfer. When anti-S. pullorum and S. gallinarum was self-assembled onto the AuNPs/SPCE, a larger semicircle (curve c, 1.33 × 104 ± 1909 Ω) can be observed, indicating the adsorption of antibody is successful and the Ret greatly increases. Similar situations also occurred when the immunosensors incubated with BSA, S. pullorum and S. gallinarum and HRP-anti-S. pullorum and S. gallinarum (curve d, e, f and g), respectively. With the increasing of material modification, the Ret of electrodes further increase, because the combination between antibody and antigen formed a barrier, and the barrier impeded electron transfer. This result suggested every step of the modification were successful. A very small increase can be seen after ILs being modified, implying ILs exhibited high conductivity and improved the performance of electrochemical immunosensor [26, 30].
Optimization of the experimental conditions
Experimental conditions were optimized. Respective data and figures are given in the Electronic Supplementary Material. From Fig. S2 A it can be observed that the reduction peak current of the immunosensor reaches the maximum value when the pH is 6.5, but decreases when pH continue to increase, resulting in lower current value. Consequently, the optimal pH of 6.5 was chosen in later studies. The concentration of H2O2 also played a very essential role in the detection of S. pullorum and S. gallinarum. With the increasing of H2O2 concentration from 0.1 to 0.8 mmol · L−1, the immunosensor response displays an upward trend, but starts to decrease when H2O2 concentration >0.8 mmol · L−1 (as shown in Fig. S2 B). Therefore, 0.8 mmol · L−1 was the most optimum H2O2 concentration for measurements.
The binding of antigen-antibody can be influenced by the incubation temperature and incubation time. As shown in Fig. S2 C, the reduction peak current increases with increasing incubation temperature from 22 to 30 °C, but decreases as the temperature increases further. Fig. S2 D shows that ΔIpc sharply increases with the increase of incubation time from 10 min to 40 min and then trends to a constant value, which suggests that 40 min is enough for the immune reaction after S. pullorum and S. gallinarum dropping to anti-S. pullorum and S. gallinarum. Therefore, the optimal incubation temperature and incubation time was 30 °C and 40 min. Fig. S2 E and F display a similar situation after dropping HRP-anti-S. pullorum and S. gallinarum, hence, an incubation temperature of 30 °C and time of 40 min was selected for the immunoassay.
Calibration curve of the immunosensor
Under these optimal conditions different concentrations of S. pullorum and S. gallinarum (from 101 to 1010 CFU · mL−1) were detected. As Fig. 6 shows, ΔIpc increases with increasing concentrations of S. pullorum and S. gallinarum. The more S. pullorum and S. gallinarum was adsorbed on the electrode the more HRP-labeled antibodies were adsorbed on the surface of electrode. The increase amount of HRP-labeled antibody results in more H2O2 is catalyzed, therefore ΔIpc increases. The plot of ΔIpc versus the logarithm of S. pullorum and S. gallinarum concentration shows a linear relationship in the concentration range from 104 to 109 CFU · mL−1, and the linear regression equations is ΔIpc (μA) = 0.4785x - 0.884, R2 = 0.9926. The limit of detection (LOD), which is defined as three times the standard deviation of the blank sample measurements, is estimated to be 3.0 × 103 CFU · mL−1 (Fig. 6a inset). And the CV curves of increasing concentrations of S. pullorum and S. gallinarum were showed in Fig. 6b. As Table 1 shows, this sensor performance has a potential in reducing detection limit and more convenient as compared to other systems for bacteria detection.
Specificity, reproducibility and stability of the immunosensor
The specificity and interference are very important for immunosensor to distinguish the target bacteria from other foodborne pathogens in samples. To prove the specificity of the constructed immunosensor, experiments were conducted using E. sakazakii, E. Coli, S. Aureus, B. Subtilis, B. Cereus, and S. pullorum and S.gallinarum, and all of the bacteria solution concentrations were 109 CFU · mL−1, PBS was used as blank control. The results displayed in Fig. 7a, the current increase induced by S. pullorum and S. gallinarum (3.352 ± 0.0872) is significantly larger than the current increase induced by other bacteria and PBS with 0.3966 ± 0.1141, suggesting the immunosensor has a high specificity for S. pullorum and S. gallinarum. The specificity of immunosensor was attributed to the highly specific antigen-antibody immunoreaction.
In order to investigate the influence of other bacteria on the detection of S. pullorum and S. gallinarum, the immunosensor were dropped with mixed bacteria solution which S. pullorum and S. gallinarum bacterial suspension containing microorganism such as S. aureus, E. coli, E. sakazakii, B. subtilis, and compared ΔIpc. The results in Fig. 7b shows that the ΔIpc causes by S. pullorum and S. gallinarum solutions with and without contaminating microorganisms just has inconspicuous change, suggesting that ΔIpc was caused by the interaction between the antibody and specific antigen not by non-specific adsorption of other microorganism. Therefore, the modified sensors towards S. pullorum and S. gallinarum owned highly specificity.
A long-term storage stability of the prepared immunosensor was also measured. 21 immunosensors were stored at 4 °C when they were not in use, and intermittently measured every 5 days with three immunosensors, they retained 93.8 % of their initial signal after a storage period of 30 days. Similar experiments were done to measure the storage stability of the prepared immunosensor without ILs, and 85.4 % of the initial signal remained after 30 days. The reason why the response of the immunosensor with ILs decreased much slower might be the fact that ILs formed a friendly microenvironment to maintain the activity and stability of antibody. Therefore, the modified sensors towards S. pullorum and S. gallinarum owned good stability.
The reproducibility of the immunosensor was investigated by independently monitoring the reduction peak current values of five modified electrodes under same experimental conditions. And the relative standard deviation (RSD) obtained at the concentration of 109 CFU · mL−1 was 9.07 %. Therefore, the modified sensors towards S. pullorum and S. gallinarum owned satisfying reproducibility.
Detection of S. pullorum and S. gallinarum in real samples
In order to verify the application of the newly developed immunosensor in real sample detection, a series of food samples: eggs, chicken meat were bought from market and analysed. The S. pullorum and S. gallinarum in real samples were respectively tested with the immunosensor and the standard culture method (China National Food Safety Standard GB/T 17999.8-2008). We found that all of the food samples were not affected by S. pullorum and S. gallinarum. A blind method was used and performed by two teams. The detail steps were as follows: one team randomly added a proper dose of S. pullorum and S. gallinarum into the negative samples and mixed it with other samples. Another team used the newly developed sensors and the standard culture method in the assays. The two teams were not allowed to interact during the whole process. The results are shown in Table S1, where the numbers correspond to true positive or negative results detected by the corresponding methods. Accuracy is defined as the agreement between results obtained by the developed method and the reference standard method for identical samples. By comparing the results of the electrochemical immunosensor with the standard method, the true positive rate was 100 % and true negative rates were 87.5 and 80 % in chicken and egg samples, respectively. The sandwich sensor shows good agreement with the standard method, indicating that there is an acceptable accuracy and reliability of the immunosensor. The immunosensor holds great promise as a reliable tool for the detection of S. pullorum and S. gallinarum in real samples.
Conclusions
An electrochemical immunosensor based on the sandwich principle has been successfully constructed for detection of S. pullorum and S. gallinarumin in this work. Different modified materials were investigated and compared in terms of sensitivity. ILs have good conductibility and experiments have demonstrated ILs can remarkably improve the performance of immunosensor. The biosensor shows wide linear range, low detection limit and high specificity, and can be used for detection of S. pullorum and S. gallinarumin in real samples. Importantly, the sandwich assay strategy can remarkably improve the performance of immunosensor provide a sensing platform for detection of S. pullorum and S. gallinarumin and the whole analytical process can be finished in 24 h. Through these experiments we developed an immunosensor with simple, rapid and economical characteristics; this immunosensor strategy can be used to develop other biosensors for pathogenic bacteria and would become a useful tool for pathogenic microorganism screening in clinical diagnostics, food safety and environmental monitoring.
References
Hong SS (2013) Therapeutic effects of bacteriophages against salmonella gallinarum infection in chickens. J Microbiol Biotechnol 23:1478–1483
Barrow PA, Neto OCF (2011) Pullorum disease and fowl typhoid—new thoughts on old diseases: a review. Avian Pathol 40:1–13
Batista DFA, De Freitas Neto OC, Lopes PD et al (2013) Polymerase chain reaction assay based on ratA gene allows differentiation between salmonella enterica subsp. Enterica serovar gallinarum biovars gallinarum and pullorum. J Vet Diagn Investig 25:259–262
Van Immerseel F, Studholme DJ, Eeckhaut V et al (2013) Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 31:4940–4945
Soria MC, Soria MA, Bueno DJ et al (2013) Comparison of 3 culture methods and PCR assays for salmonella gallinarum and salmonella pullorum detection in poultry feed. Poult Sci 92:1505–1515
Roda A, Mirasoli M, Roda B et al (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178:7–28
Baumler AJ, Tsolis RM, Ficht TA et al. (1998) Evolution of host adaptation in Salmonella enterica. Infect Immun 66
Hu CM, Dou WC, Zhao GY (2014) Enzyme immunosensor based on gold nanoparticles electroposition and streptavidin-biotin system for detection of S. Pullorum & S. Gallinarum. Electrochim Acta 117:239–245
Bäumler AJ, Hargis BM, Tsolis RM (2000) Tracing the origins of Salmonella outbreaks. Science 287:50–52
Wang D, Dou WC, Zhao GY et al (2014) Immunosensor based on electrodeposition of gold-nanoparticles and ionic liquid composite for detection of Salmonella pullorum. J Microbiol Methods 106:110–118
Hu X, Dou WC, Fu LL et al (2013) A disposable immunosensor for Enterobacter sakazakii based on an electrochemically reduced graphene oxide-modified electrode. Anal Biochem 434:218–220
Zhao GY, Zhan XJ, Dou WC (2011) A disposable immunosensor for Shigella flexneri based on multiwalled carbon nanotube/sodium alginate composite electrode. Anal Biochem 408:53–58
Zhan XJ, Tang WL, Dou WC et al (2013) Dispoable immunosensor for Escherichia Coli O157:H7 based on a multi-walled carbon nanobon nanotube sodium alginate nanocomposite film modified screen-printed carbon electrode. Anal Lett 46:2690–2704
Toh SY, Citartan M, Gopinath SC et al (2015) Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens Bioelectron 64:392–403
Centi S, Messina G, Tombelli S et al (2008) Different approaches for the detection of thrombin by an electrochemical aptamer-based assay coupled to magnetic beads. Biosens Bioelectron 23:1602–1609
Omidfar K, Zarei H, Gholizadeh F et al (2012) A high-sensitivity electrochemical immunosensor based on mobile crystalline material-41-polyvinyl alcohol nanocomposite and colloidal gold nanoparticles. Anal Biochem 421:649–656
Regiart M, Pereira SV, Spotorno VG et al (2013) Nanostructured voltammetric sensor for ultra-trace anabolic drug determination in food safety field. Sensors Actuators B Chem 188:1241–1249
Wang XJ, Li XJ, Luo CN et al (2014) Ultrasensitive molecularly imprinted electrochemical sensor based on magnetism graphene oxide/beta-cyclodextrin/Au nanoparticles composites for chrysoidine analysis. Electrochim Acta 130:519–525
Derkus B, Emregul E, Emregul KC et al (2014) Alginate and alginate-titanium dioxide nanocomposite as electrode materials for anti-myelin basic protein immunosensing. Sensors Actuators B Chem 192:294–302
Chen X, Qin P, Li J et al (2014) Impedance immunosensor for bovine interleukin-4 using an electrode modified with reduced graphene oxide and chitosan. Microchim Acta 182:369–376
Li Y, Liu X, Zeng X et al (2009) Simultaneous determination of ultra-trace lead and cadmium at a hydroxyapatite-modified carbon ionic liquid electrode by square-wave stripping voltammetry. Sensors Actuators B Chem 139:604–610
Attri P, Jha I, Choi EH et al (2014) Variation in the structural changes of myoglobin in the presence of several protic ionic liquid. Int J Biol Macromol 69:114–123
Satoshi Shimano HZ, Itaru H (2007) Preparation of nanohybrid solid-state electrolytes with liquidlike mobilities by solidifying ionic liquids with silica particles. Am Chem Soc 19:6
Li R, Xia Q, Li Z et al (2013) Electrochemical immunosensor for ultrasensitive detection of microcystin-LR based on graphene-gold nanocomposite/functional conducting polymer/gold nanoparticle/ionic liquid composite film with electrodeposition. Biosens Bioelectron 44:235–240
Kumar A, Rani A, Venkatesu P et al (2014) Quantitative evaluation of the ability of ionic liquids to offset the cold-induced unfolding of proteins. Phys Chem Chem Phys: PCCP 16:15806–15810
Du P, Liu S, Wu P et al (2007) Preparation and characterization of room temperature ionic liquid/single-walled carbon nanotube nanocomposites and their application to the direct electrochemistry of heme-containing proteins/enzymes. Electrochim Acta 52:6534–6547
Feng R, Zhang Y, Yu HQ et al (2013) Nanoporous PtCo-based ultrasensitive enzyme-free immunosensor for zeranol detection. Biosens Bioelectron 42:367–372
Fang Y-S, Chen S-Y, Huang X-J et al (2014) Simple approach for ultrasensitive electrochemical immunoassay of clostridium difficile toxin B detection. Biosens Bioelectron 53:238–244
Guo AP, Li YY, Cao et al (2015) An electrochemical immunosensor for ultrasensitive detection of carbohydrate antigen 199 based on Au@CuxOS yolk-shell nanostructures with porous shells as labels. Biosens Bioelectron 63
Fan HX, Zhang Y, Wu D et al (2013) Construction of label-free electrochemical immunosensor on mesoporous carbon nanospheres for breast cancer susceptibility gene. Anal Chim Acta 770:62–67
Dong J, Zhao H, Xu M et al (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem 141:1980–1986
Olsen EV, Pathirana ST, Samoylov AM et al (2003) Specific and selective biosensor for Salmonella and its detection in the environment. J Microbiol Meth 53:273–285
Nguyen P-D, Tran TB, Nguyen DTX et al (2014) Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of Salmonella typhimurium. Sensors Actuators B Chem 197:314–320
Si SH, Lia X, Fung YS, Zhu DR (2001) Rapid detection of Salmonella enteritidis by piezoelectric immunosensor. Microchem J 68:7
Babacan PP S, Letcher S, Rand A (2002) Piezoelectric flow injection analysis biosensor for the detection of salmonella typhimurium. Instit Food Technol 67:13
Wong YY, Ng SP, Ng MH et al (2002) Immunosensor for the differentiation and detection of salmonella species based on a quartz crystal microbalance. Biosens Bioelectron 17:676–684
Acknowledgments
This project was supported by the Food Science and Engineering the most important discipline of Zhejiang province (JYTSP20141062). The Talent training provincial superior paper funded project (1110JY1412001P). Postgraduate Scientific and Technological Innovation Project of Zhejiang Gongshang University (3100XJ1514146) and Plans for college students in Zhejiang Province science and technology innovation activities (acrobatic tender grass talent programme) project (1110JQ4212048G). Project supported by the fund of the National Natural Science Fund (30571623). Analysis and testing projects of Zhejiang public innovation platform (2015C37023).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 850 kb)
Rights and permissions
About this article
Cite this article
Fei, J., Dou, W. & Zhao, G. A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta 182, 2267–2275 (2015). https://doi.org/10.1007/s00604-015-1573-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1573-x