Abstract
A series of (E)-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline derivatives have been synthesized from 2-(piperidin-1-yl)quinoline-3-carbaldehyde with substituted anilines and these were characterized on the basis of spectral data such as IR, H–NMR, mass spectrometry and X-ray single crystal diffraction analysis. The title compounds were evaluated for their in vitro anticancer activity against various human cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heterocyclic compounds play an important role in the designing new class of drug derivatives. Among them the Quinoline derivatives have displayed potent anticancer activities with targeting different active sites [1–3] and these are also shows wide variety of pharmacological activities such as antibacterial [4], antimicrobial [5], antiallergenic [6], anti-inflammatory [7] antitumor [8], anti-tuberculosis [9], anti-malarial [10], anti-obesity [11], antihypertensive [12], tyrokinase platelet-derived growth factor receptor tyrosinekinase (PDGF-RTK) [13], and phosphodiesterase 10A(PDE10A) inhibiting agents [14]. On the other hand, piperidine derivatives are shows verity of biological activities including as antimicrobial [15], anticonvulsant [16], antidepressant (Paroxetine), and attention-deficit hyperactivity disorder (ADHD) drugs. Furthermore, The Schiff bases are one of the important chemical entities in pharmaceutical fields due to a broad spectrum of biological activities including as antimicrobial [17], antitubercular [18], anticancer [19], antioxidant [20]. In the view of importance of quinolines and their derivatives, we have prompted us to take synthesized a series of (E)-3-(2-arylvinyl)-2-(piperidin-1-yl)quinolines and evaluated their anticancer activity (Scheme 1).

EXPERIMENTAL
Melting points were determined in open capillary tubes and are uncorrected. The purity of the compounds was checked by TLC using precoated silica gel plates 60254 (Merck). IR (KBr) spectra were recorded on a Shimadzu FT-IR-8400s spectrophotometer. 1H NMR spectrums were recorded on Bruker Advance II 400 MHz spectrometer using tetramethylsilane as an internal standard. Mass spectra were recorded on a GCMS-QP 1000 EX mass spectrometer.
RESULTS AND DISCUSSION
The synthesis of (E)-N-((2-(piperidin-1-yl)quino-lin-3-yl)methylene)anilines (Va–j) was shown in Scheme 1. The 2-chloroquinoline-3-carbaldehyde (II) was synthesized by the reaction of acetanilide with Vilsmeier-Haack reagent (DMF/POCl3). The compound (II) was reacted with piperidine in the presence of potassium carbonate to yield 2-(piperidin-1-yl)quinoline-3-carbaldehyde (III) intermediate. The compound (III) reacted with substituted aromatic amines (IVa–j) to afford Schiff bases (Va-j) in excellent yield. The structures of the compounds (Va–j) were established by using spectral data such as IR, 1H–NMR and mass spectroscopy.In IR spectrum the compounds shows strong absorption at 1620–1628 cm–1 corresponds to CH=N group. In the 1H–NMR spectrum of compounds shows a singlet resonated between δ 8.7–8.9 ppmindicating the presence proton of CH=N. In the mass spectrum of all the compounds showed their m/z as M + H peak. The spectral analysis confirmed synthesis of titled compounds.
Experimental Section for SXRD
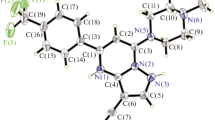
X-ray reflections were collected on Bruker D8 QUEST, CCD diffractometer equipped with a graphite mono-chromator and MoKα fine-focus sealed tube (λ = 0.71073 Å).Intensities for absorption were corrected using SADABS. Structures were solved and refined using SHELXL-2018 with anisotropic displacement parameters for non-H atoms. Hydrogen atoms were experimentally located through the Fourier difference electron density maps in all crystal structures. All C–H atoms were geometrically fixed using HFIX command in SHELX-TL program of Bruker-AXS. Crystallographic cif file are available at www.ccdc.cam.ac.uk/data with ref no. CCDC1876405 (Fig. 1, Table 1).
Anticancer Activity
The newly synthesized (E)-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)anilines (Va–j) were screened for their anticancer activity against four different human cancer cell lines including A-549 (Lung), A-375 (Melanoma), HT-29 (Colon4) and MCF-7 (Breast) by MTT method. The 1 × 104 cells/well were seeded in 200 mL DMEM, supplemented with 10% FBS in each well of 96-well micro culture plates and incubated for 24 h at 37°C in CO2 incubator. The compound (Va–j) were diluted to the desired concentrations in culture medium, were added to the wells with respective vehicle control. After 48 h of incubation, 10 mL MTT (5 mg/mL) was added to each well and the plates were further incubated for 4 h. Then the supernatant from each well was carefully removed, formed crystals were dissolved in 100 mL of DMSO and absorbance at 540 nm wavelength was recorded. The resulted values were expressed as the compound concentration (μmol/L) that causes 50% inhibition of cell growth (IC50), are shown in Table 2. The Combretasta-tin-A4 used as positive control. Among them, the compounds Vc, Vd, Ve and Vi were demonstrated almost near to positive control.
General Method for the Synthesis of Schiff’s Bases (Va-j)
To a mixture of 2-(piperidin-1-yl)quinoline-3-carbaldehyde (1 mmol) (III) and substituted aniline (1 mmol) (IVa–j) in methanol, and 2-3 drops of glacial acetic acid was added to it, then the mixture was refluxed for 2–3 h. The progress of reaction monitored by TLC, after completion of the reaction the solvent evaporated under reduced pressure and cooled. The product obtained was filtered, washed with water, dried and recrystallized from methanol to afford pure crystalline products (Va–j).
Spectral Data
(Va) (E)-2-Methyl-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: M. p. 89–91°C; Yield: 94%; IR spectrum, v, cm–1: 1251, 1502, 1577, 1612 and 1627; 1H NMR, δ, ppm: 1.67–1.81 (m, 6H, 3CH2), 2.43 (s, 3H, CH3), 3.39–3.41 (t, 4H, CH2), 6.93–6.95 (d, 1H, Ar–H), 7.14–7.27 (m, 3H, Ar–H), 7.35–7.39 (dd, 1H, Ar–H), 7.61–7.65 (m, 1H, Ar–H), 7.80–7.87 (m, 2H, Ar–H), 8.55 (s, 1H, –Ar–H), 8.74 (s, 1H, HC=N); HRMS (ESI): m/z [M + H]+ calcd for C22H24N3: 330.1970; found: 330.2012.
(Vb) (E)-2-Chloro-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: M. p. 117–119°C; Yield: 94%; IR spectrum, v, cm–1: 1260, 1508, 1580, 1607 and 1624; 1H NMR, δ, ppm: 1.67–1.79 (m, 6H, CH), 3.39–3.42 (t, 4H, CH2), 7.03–7.05 (dd, 1H, Ar–H), 7.15–7.19 (m, 1H, Ar–H), 7.29–7.39 (m, 2H, Ar–H), 7.46–7.48 (dd, 1H, Ar–H),7.62–7.66 (m, 1H, Ar–H), 7.81–7.87 (m, 2H, Ar–H), 8.55 (s, 1H, –Ar–H), 8.78 (s, 1H, HC=N); HRMS (ESI): m/z [M + H]+ calcd for C22H24ClN: 350.1424; found: 350.1469. HRMS (ESI): m/z [M + H]+ calcd for C22H24ClN2: 350.1424; found: 350.1469.
(Vc) (E)-N-((2-(Piperidin-1-yl)quinolin-3-yl) methylene)aniline: M. p. 94–96°C; Yield: 90%; IR spectrum, v, cm–1: 1269, 1506, 1585, 1612 and 1625; 1H NMR, δ, ppm: 1.66–1.81 (m, 6H, 3CH2), 3.37–3.40 (t, 4H, 2N–CH2), 7.07–7.10 (dd, 2H, Ar–H), 7.16–7.19 (m, 2H, Ar–H), 7.28–7.33 (m, 2H, Ar–H), 7.66–7.69 (m, 1H, Ar–H), 7.77–7.85 (m, 3H, Ar–H), 8.64 (s, 1H, CH2–CO), 8.77 (s, 1H, CH=N); HRMS (ESI): m/z [M + H]+ calcd for C21H22N3: 316.1814; found: 316.1856.
(Vd) (E)-2-chloro-4-nitro-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: 106–108°C; Yield: 89%; IR spectrum, v, cm–1: 1255, 1505, 1591, 1608 and 1624; 1H NMR, δ, ppm: 1.68–1.77 (m, 6H, 3CH2), 3.39–3.42 (t, 4H, CH2), 7.10–7.12 (d, 1H, Ar–H), 7.38–7.42 (dd, 1H, Ar–H), 7.66–7.70 (m, 2H, Ar–H), 7.82–7.88 (dd, 2H, Ar–H), 8.19–8.22 (dd, 1H, Ar–H), 8.38–8.39 (d, 1H, Ar–H), 8.54 (s, 1H, –Ar–H), 8.79 (s, 1H, HC=N); HRMS (ESI): m/z [M+H]+ calcd for C21H20ClN4O2: 395.1275; found: 395.1303.
(Ve) (E)-4-Nitro-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: 121–123°C; Yield: 92%; IR spectrum, v, cm–1: 1269, 1506, 1585, 1612 and 1625; 1H NMR, δ, ppm: 1.69–1.80 (m, 6H, 3CH2), 3.38–3.40 (t, 4H, CH2), 7.29–7.31 (d, 2H, Ar–H), 7.37–7.41 (dd, 1H, Ar–H), 7.65–7.69 (m, 1H, Ar–H), 7.80–7.88 (dd, 2H, Ar–H), 8.30–8.33 (d, 2H, Ar–H), 8.62 (s, 1H, –Ar–H), 8.75 (s, 1H, HC=N); HRMS (ESI): m/z [M + H]+ calcd for C21H21N4O2: 361.1665; found: 361.1711.
(Vf) (E)-4-Bromo-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: 1105–107°C; Yield: 95%; IR spectrum, v, cm–1: 1263, 1500, 1590, 1607 and 1621; 1H NMR, δ, ppm: 1.66–1.80 (m, 6H, 3CH2), 3.39–3.42 (t, 3H, CH2), 7.00–7.03 (dd, 1H, Ar–H), 7.08–7.11 (m, 1H, Ar–H), 7.34–7.39 (m, 2H, Ar–H), 7.62–7.67 (m, 2H, Ar–H), 7.81–7.87 (dd, 2H, Ar–H), 8.51 (s, 1H, Ar–H), 8.79 (s, 1H, HC=N); HRMS (ESI): m/z [M + H]+ calcd for C22H24BrN3: 395.0997; found: 395.1311.
(Vg) (E)-2-(((2-(Piperidin-1-yl)quinolin-3-yl) methylene)amino)phenol: 125–127°C; Yield: 92%; IR spectrum, v, cm–1: 1258, 1501, 1581, 1611 and 1625; 1H NMR, δ, ppm: 1.66–1.82 (m, 6H, 3CH2), 3.36–3.39 (t, 4H, CH2), 6.92–6.96 (m, 1H, Ar–H), 7.04–7.07 (dd, 1H, Ar–H), 7.21–7.23 (m, 1H, Ar–H), 7.34–7.40 (m, 3H, Ar–H), 7.62–7.66 (m, 1H, Ar–H), 7.79–7.87 (dd, 2H, Ar–H), 8.70 (s, 1H, Ar–H), 8.89 (s, 1H, HC=N); HRMS (ESI): m/z [M + H]+ calcd for C21H22N3O: 332.1763; found: 332.1806.
(Vh) (E)-4-Methoxy-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)aniline: 113–115°C; Yield: 94%; IR spectrum, v, cm–1: 1253, 1502, 1583, 1610 and 1624; 1H NMR, δ, ppm: 1.67–1.80 (m, 6H, 3CH2), 3.37–3.39 (t, 4H, CH2), 3.85 (s, 3H, OCH3), 6.96–6.99 (d, 2H, Ar–H), 7.28–7.37 (m, 3H, Ar–H), 7.59–7.63 (m, 1H, Ar–H), 7.77–7.86 (dd, 2H, Ar–H), 8.66 (s, 1H, Ar–H), 8.70 (s, 1H, HC=N); HRMS (ESI): m/z [M+H]+ calcd for C22H24N3O: 346.1919; found: 346.1965.
(Vi) (E)-2-Nitro-N-((2-(piperidin-1-yl)quino-lin-3-yl)methylene)aniline: 102–104°C; Yield: 92%; IR spectrum, v, cm–1: 1250, 1511, 1592, 1602 and 1629; 1H NMR, δ, ppm: 1.69–1.81 (m, 6H, 3CH2), 3.39–3.42 (t, 4H, 2N–CH2), 7.21–7.25 (m, 2H, Ar–H), 7.35–7.38 (dd, 1H, Ar–H), 7.66–7.70 (m, 1H, Ar–H), 7.78–7.91 (m, 2H, Ar–H), 8.17–8.19 (m, 1H, Ar–H), 8.66 (s, 1H, –Ar–H), 8.78 (s, 1H, HC=N); HRMS (ESI): m/z [M+H]+ calcd for C21H21N4O2: 361.1665; found: 361.1711.
(Vj) (E)-4-(((2-(Piperidin-1-yl)quinolin-3-yl)methylene)amino)phenol: 131–133°C; Yield: 94%; IR spectrum, v, cm–1: 1269, 1506, 1585, 1612 and 1625; 1H NMR, δ, ppm: 1.66–1.81 (m, 6H, 3CH2), 3.37–3.40 (t, 4H, 2N–CH2), 3.83 (s, 1H, OH), 6.87–6.91 (dd, 2H, Ar–H), 7.19–7.30 (m, 2H, J = 16.4 Hz, Ar–H), 7.34–7.42 (m, 2H, Ar–H), 7.60–7.64 (m, 1H, Ar–H), 7.78–7.91 (m, 3H, Ar–H), 8.65 (s, 1H, CH2–CO), 8.70 (s, 1H, CH=N); HRMS (ESI): m/z [M + H]+ calcd for C21H22N3O: 332.1763; found: 332.1806.
CONCLUSIONS
In conclusion, a novel series of (E)-N-((2-(piperidin-1-yl)quinolin-3-yl)methylene)anilines have been successfully synthesized and characterized with different analytical data. The compounds anticancer evaluation resulted that the compounds Vc, Vd, Ve and Vi were demonstrated almost near to positive control.
REFERENCES
Gopal, M., Shenoy, S., and Doddamani, L.S., J. Photochem. Photobiol., 2003, vol. 72, p. 69.
Kim, Y.H., Shin, K.J., Lee, T.G., Kim, E., Lee, M.S., Ryu, S.H., and Suh, P.G., Biochem. Pharmacol., 2005, vol. 69, p. 1333.
Zhao, Y.L., Chen, Y.L., Chang, F.S., and Tzeng, C.C., Eur. J. Med. Chem., 2005, vol. 40, p. 792.
Bodke, Y.D., Shankerrao, S., Kenchappa, R., et al., Russ. J. Gen. Chem., 2017, vol. 87, p. 1843.
Ashok, D., Elsanoosi, M., Alanab, B.F.H., et al., Russ. J. Gen. Chem., 2016, vol. 86, p. 1120.
Althuis, T.H., Khadin, S.B., Czuba, L.J., Moore, P.F., and Hess, H.J., J. Med. Chem., 1980, vol. 23, p. 262.
Achar, K.C.S., Hosamani, K.M., and Seetharama reddy, H.R., Eur. J. Med. Chem., 2010, vol. 45, p. 204.
Okten, S., Cakmak, O., Erenler, R., Yuce, O., and Andtekin, S., Turk. J. Chem., 2013, vol. 37, p. 896.
Lilienkampf, A., Mao, J., Wan, B., Wang, Y., Franzblau, S.G., and Kozikowski, A.P., J. Med. Chem., 2009, vol. 52, p. 2109.
Xhamla Nqoro, Naki Tobeka, and Aderibigbe, B.A., Molecules, 2017, vol. 22, p. 2268.
Spek, A.L., J. Appl. Crystallogr., 2003, vol. 36, p. 7.
Muruganantham, N., Sivakumar, R., Anbalagan, N., Gunasekaran, V., and Leonard, J.T., Biol. Pharm. Bull., 2004, vol. 27, p. 1683.
Maguire, M.P., Sheets, K.R., McVety, K., Spada, A.P., and Zilberstein, A., J. Med. Chem., 1994, vol. 37, p. 2129.
Rzasa, R.M., Frohn, M.J., Andrews, K.L., Chmait, S., Chen, N., et al., Bioorg. Med. Chem., 2014, vol. 22, p. 6570.
Ozdemir, A., Lett. Drug Des. Discovery, 2013, vol. 10, p. 44.
Aytemir, D.M. and Calis, U., Arch. Pharm., 2010, vol. 343, p. 173.
Zahan, M. and Islam, M.S., Russ. J. Gen. Chem., 2015, vol. 85, p. 979.
Aboul-Fadl, T., Mohammed, F.A., and Hassan, E.A., Arch. Pharm. Res., 2003, vol. 26, p. 778.
Ghammamy, S. and Sedaghat, S., Russ. J. Gen. Chem., 2013, vol. 83, p. 722.
Senthilkumar, G.S., Sankarganesh, M., Rajesh, J., et al., Russ. J. Gen. Chem., 2017, vol. 87, p. 2654.
ACKNOWLEDGMENTS
The authors are thankful to the Head, Department of Chemistry for providing laboratory facilities. The authors are also thankful to the Director, Central Facilities for Research and Development (CFRD), Osmania University for providing IR and NMR spectral analysis.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Subhash, D., Bhaskar, K. Synthesis, Molecular Structure and Anticancer Activity of (E)-N-((2-Piperidin-1-yl)Quinolin-3-yl)Methylene)Aniline Derivatives. Moscow Univ. Chem. Bull. 76, 169–172 (2021). https://doi.org/10.3103/S0027131421020115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131421020115