Abstract
This study is aimed at creating a number of derivatives of natural amino acids based on dioctylamine and diethanolamine diesters with potential antibacterial activity. Simple and universal schemes of the synthesis allow them to be used for obtaining a series of samples in the preparative quantities necessary for the implementation of physiochemical and biochemical studies. The synthesized sample based on glycyldioctylamide shows a promising level of antimicrobial activity (MIC) against Gram-positive and Gram-negative bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are many antibiotics with various molecular structures and antimicrobial agents effective against particular bacterial species; however, the resistance of new bacterial strains to the well-known antibacterial agents is increasing steadily every year [1]. This has been a long-standing problem that requires a continuous search for new pharmaceutical substances—a new generation of antibiotics which do not trigger the rapid development of resistance in different species of microorganisms.
The achievements in the fields of biotechnology, genetic engineering, and synthetic chemistry have opened up new possibilities in the search of therapeutic methods to replace antibiotic therapy. Currently, it is the widespread application of bacteriophages and antibodies. Other promising strategies involving probiotics, cationic detergents and antimicrobial peptides (AMPs) are at different stages of development [2]. Most of the well-known antibiotics target the cellular processes of bacteria and thereby can be inefficient against some mutations, the effects of enzymes, and other intracellular modifications. AMPs were developed as molecules targeting bacterial cell membranes and thus are considered as the new promising tools for overcoming these difficulties [3]. The membrane used as the target provides the advantage of peptide agents over the common antibiotics, as resistance to these agents develops slowly, if at all. Some of them are being used in clinical practice [4] and some are undergoing clinical trials [5].
However, despite the high biological activity of most AMP representatives, only a few of them are used in medicine because of the high hemolytic effect on mammalian cells [6].
Numerous studies in this filed have led to the conclusion that the toxicity and antibacterial properties of molecules can be influenced by modifying not only the chemical composition but also the molecular architecture [7]. By maintaining a constant chemical composition, it is possible to achieve the effective and safe hydrophobicity of the molecule by varying the composition and positional arrangement of the hydrophobic fragments in the structure of the compound. It has been shown that a certain threshold hydrophobicity is required for significant activity against bacterial cells [8]. However, such an increase in hydrophobicity cannot be uncontrolled as it leads to a significant increase in toxicity, which prevents it from being used in clinical studies.
Another promising field of research attracting the attention of scientists is the application of amphiphilic peptidomimetics or lipopeptides [9]. They can have a high level of antimicrobial activity with minor side effects, be simple to synthesize, and offer an opportunity to manipulate their composition [10]. Natural antibiotics were the base for the development of synthetic membrane-active substances now demonstrating an impressive potential for a wide range of modifications, which makes them promising as future antibacterial agents [11, 12].
In general, the developed compounds of low-molecular-weight peptidomimetics have a uniform structure: one or two hydrophobic aliphatic chains, amino acid regions as a hydrophilic head group, and a spacer connecting the two domains. Such an amphiphilic structure allows them to interact with negatively charged bacterial membranes [13, 14]. Amino acids as hydrophilic domains also influence the bioavailability of lipopeptides. They depend on factors such as the number of positively charged groups, as well as the amino acid structure and configuration. The molecules with the peptide component represented by L‑lysine or L‑phenylalanine residues demonstrate the maximum level of antimicrobial activity [15, 16].
The structure, length, and degree of saturation of the hydrophobic domain also influence the antibacterial properties of the compounds. The studies have shown an interrelationship between the length of the hydrophobic block of the amphiphilic compound and the minimum inhibitory concentration (MIC) necessary for the suppression of microbial growth [17]. Lipophilic domains are usually the saturated and unsaturated aliphatic chains or, sometimes, cationic amphiphiles containing either aromatic compounds or steroid derivates such as cholesterol [18].
The studies performed in model lipid bilayers suggest that antibacterial peptides can form pores in the plasma membrane resulting in the uncontrolled permeability of a bacterial cell [19–21]. The small bactericidal agents function mainly due to the active interaction and depolarization of bacterial cell walls [22]. It eventually leads to the leakage of cytoplasmic material and the lysis of cells [23].
This study is aimed at developing the schemes of production and synthesis of two series of new cationic peptidomimetics based on aliphatic amine derivatives differing in the structure of the amino acid in the polar group and in its positive charge volume for further assessment of the interdependence between the structure and the antibacterial activity.
EXPERIMENTAL
The 1H-NMR spectra were recorded in a deuterated solvent with a 400-MHz Bruker WM-400 pulse NMR spectrometer. The internal standard was hexamethyldisiloxane. Thin layer chromatography (TLC) was performed on Sorbfil plates (Krasnodar). Preparative thin layer chromatography (PTLC) was performed on Sigma Aldrich TLC standard grade silica gel (Germany). The spots of compounds with an amino group were detected during the TLC (heating to 50°C in a 5% ninhydrin solution).
N-(tert-butoxycarbonyl)-glycine (2a). The 4 M NaOH solution (up to pH 8) and 1.31 g (5.99 mmol) of di-tert-butyl pyrocarbonate in 10 mL of THF were added dropwise to the solution of 0.3 g (3.99 mmol) of glycine in 25 mL of distilled water and stirred at room temperature for 3 h. After completion of the reaction, the solvent was removed under vacuum. Then the resultant substance was dissolved in 50 mL of distilled water, acidified with a citric acid 20% solution to pH 3, extracted with ethyl acetate (3 × 50 mL), and dried over sodium sulfate. The solvent was evaporated in a rotary evaporator and 0.58 g (83.2%) of product 2a was obtained. 1H-NMR spectrum (DMSO, δ, ppm): 1.39 (s, 9H, CCH3), 3.95 (d, 2H, CH2), 7.32 (d, 1H, NH).
N-(tert-butoxycarbonyl)-β-L-alanine (2b). Boc-(β-Ala)-OH was obtained similarly: 0.57 g of product 2b (89.4%) was obtained from 0.3 g (3.37 mmol) of β‑L-Ala. The 1H-NMR spectrum (CDCl3, δ, ppm): 1.43 (s, 9H, CCH3), 2.50 (t, 2H, NHCH2CH2), 3.29 (m, 2H, NHCH2CH2), 6.72 (d, 1H, NH).
N-(tert-butoxycarbonyl)-γ-L-aminobutyric acid (2c). Boc-(GABA)-OH was obtained similarly: 0.48 g of product 2c (81.6%) was obtained from 0.3 g (2.91 mmol) of GABA. 1H-NMR spectrum (CDCl3, δ, ppm): 1.43 (s, 9H, CCH3), 1.74 (m, 2H, NHCH2CH2CH2), 2.26 (t, 2H, NHCH2CH2CH2), 3.24 (t, 2H, NHCH2CH2CH2), 6.58 (d, 1H, NH).
Nα,Nδ-bis (tert-butoxycarbonyl)-L-lysine (8a). BocOrn(Boc)-OH was obtained similarly: 0.55 g (73.2%) of product 8a was obtained from 0.3 g (2.27 mmol) of L-Orn ⋅ HCl. The 1H-NMR spectrum (CDCl3, δ, ppm): 1.42 (s, 18H, CCH3), 1.65 (dd, 2H, CHCH2CH2), 1.75 (m, 2H, CHCH2CH2CH2), 3.11 (m, 2H, CHCH2CH2CH2), 4.31 (s, 1H, CH), 6.49 (d, 1H, δ-NH), 6.65 (d, 1H, α-NN).
Nα,Nε-bis(tert-butoxycarbonyl)-L-lysine (8b). BocLys(Boc)-OH was obtained similarly: 0.56 g (79.4%) of product 8b was obtained from 0.3 g (2.05 mmol) of L‑LysHCl. 1H-NMR spectrum (CDCl3, δ, ppm): 1.41 (s, 18H, CCH3), 1.52 (m, 4H, CHCH2CH2CH2CH2); 1.60 (d, 2H, CHCH2CH2CH2CH2); 3.06 (d, 2H, CHCH2CH2CH2CH2); 4.30 (s, 1H, CH); 6.03 (s, H, ε-NH); 6.69 (s, 1H, α-NH).
N,N-di-n-octylamine (4). The mixture of 1 g (7.74 mmol) of n-octylamine, 1.49 (7.74 mmol) of 1‑bromoctane, and 1.07 (7.74 mmol) of potassium carbonate in 8 mL of THF was stirred at 80°C for 12 h. After completion of the reaction, the solvent was removed under vacuum and the mass was dissolved in 25 mL of chloroform, washed with distilled water (3 × 25 mL), and dried over anhydrous Na2SO4. The product was isolated by column chromatography in the petroleum ether/ethyl acetate/triethylamine system (10:1:0.05, v/v). 1H-NMR spectrum (DMSO, δ, ppm): 0.88 (t, 6H, CH3), 1.35 (m, 20H, CH2), 1.58 (m, 4H, NHCH2CH2), 2.62 (dd, 4H, NHCH2CH2), 2.79 (d, 1H, NH).
Trifluoroacetate glycyl-di-n-octylamide (6а). A 0.07 g (0.289 mmol) of di-n-octylamine 4 solution in 5 mL of anhydrous methylene chloride was added under stirring to a solution of 0.15 g (0.433 mmol) of BocGly-OH 2a and 0.071g (0.577 mmol) of DMAP in 5 mL of anhydrous methylene chloride cooled to 0°C. Then, 0.12 g (0.577 mmol) of DCC was added to the reaction mass under intensive stirring and the mixture was left to stand for 2 h. The reaction was monitored by TLC. After completion of the reaction, the precipitated dicyclohexylurea was filtered, the reaction mass was washed with water to pH 7, and dried over Na2SO4. The product was isolated by preparative thin layer chromatography in the chloroform/methanol system (9 : 1, v/v). 0.092 g (80%) of product 5a was obtained. The protective group was removed from the technical product with 0.26 mL (3.46 mmol) of trifluoroacetic acid in 1 mL of anhydrous methylene chloride under stirring. Then the solvent with the excess acid was removed under vacuum and 0.088 g (92.8%) of trifluoroacetate salt 6a was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.88 (t, 6H, CH3), 1.36–1.27 (m, 20H, CH2), 1.62 (m, 4H, β‑CH2), 3.44 (dd, 4H, α-CH2), 3.91 (t, 2H, NH2CH2), 6.02 (d, 2H, NH2).
Trifluoroacetate β-L-alanyl-di-n-octylamide (6b). The reaction for obtaining 5b was performed similarly: 0.19 g (78%) of product 5b was obtained from 0.15 g (0.793 mmol) of 2b. After the removal of the protective group, 0.18 g (91%) of trifluoroacetate salt 6b was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.87 (t, 6H, CH3), 1.33–1.26 (m, 20H, CH2), 1.64 (m, 4H, β-CH2), 2.82 (m, 2H, NH2CH2CH2), 3.18 (t, 2H, NH2CH2CH2), 3.49 (dd, 4H, α-CH2), 4.59 (d, 2H, NH2).
Trifluoroacetate 4-aminobutyl-di-n-octylamide (6c). The reaction for obtaining 5c was performed similarly: 0.17 g (75%) of product 5c was obtained from 0.15 g (0.738 mmol) of 2c. After removal of the protective group, 0.175 g (96%) of trifluoroacetate salt 6c was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.87 (t, 6H, CH3), 1.29 (m, 20H, CH2), 1.61 (m, 4H, β‑CH2), 1.97 (m, 2H, NH2CH2CH2CH2), 2.35 (m, 2H, NH2CH2CH2CH2), 2.56 (d, 2H, NH2), 3.04 (t, 2H, NH2CH2CH2CH2), 3.44 (dd, 4H, α-CH2).
Trifluoroacetate L-ornityl-di-n-octylamide (10a). The reaction for obtaining 9a was performed similarly: 0.11 g (69%) of product 9a was obtained from 0.15 g (0.451 mmol) of 8a. After removal of the protective groups, 0.107 g (93.1%) of trifluoroacetate salt 10a was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.87 (t, 3H, CH3), 1.27 (m, 20H, CH2), 1.57 (dd, 2H, CHCH2CH2CH2), 1.66 (m, 4H, β-CH2), 1.84 (m, 2H, CHCH2CH2CH2), 1.99 (d, 2H, δ-NH2), 2.86 (m, 2H, CHCH2CH2CH2), 3.43 (d, 4H, α-CH2), 3.73 (s, 1H, CH), 7.48 (d, 2H, α-NH2).
Trifluoroacetate L-lysyl-di-n-octylamide (10b). The reaction for obtaining 9b was performed similarly: 0.114 g (71%) of product 9b was obtained from 0.15 g (0.433 mmol) of 8b. After the removal of the protective groups, 0.112 g (93.1%) of trifluoroacetate salt 10b was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.80 (t, 6H, CH3), 1.31 (m, 20H, CH2), 1.46 (dd, 4H, CHCH2CH2CH2CH2), 1.61 (m, 4H, β-CH2), 1.71 (m, 2H, CHCH2CH2CH2CH2), 1.91 (d, 2H, ε-NH2), 2.61 (m, 2H, CHCH2CH2CH2CH2), 3.45 (m, 4H, α‑CH2), 3.63 (s, 1H, CH), 7.51 (d, 2H, α-NH2).
N-tert-butoxycarbonyl-di-ethanolamine (12). A 4 M NaOH solution in 25 mL of distilled water (to pH 8) and 3.12 g (14.26 mmol) of the di-tert-butyl-pyrocarbonate in 45 mL of THF was added dropwise to 1 g (9.52 mmol) of diethanolamine solution 11 in 15 mL of THF. The reaction mixture was stirred at room temperature for 3 h with the maintenance of pH 8. The solvent was removed under vacuum. The reaction product was extracted with chloroform (3 × 75 mL) and dried over sodium sulfate. The solvent was evaporated in a rotary evaporator. The yield of product 12 was 1.84 g (93.7%). 1H-NMR spectrum (CDCl3, δ, ppm): 1.48 (s, 9H, CH3), 3.31 (t, 4H, NHCH2CH2), 3.86 (t, 4H, NHCH2CH2).
N-tert-butoxycarbonyl-O,O'-dioctanoyl-diethanolamine (13). We added 3.57 g (29.2 mmol) of DMAP, a solution of 9.02 g (43.8 mmol) of DCC in 100 mL of methylene chloride, and 1.5 g (7.3 mmol) of product 12 in 35 mL of methylene chloride under stirring to 4.21 g (29.2 mmol) of the octanoic acid solution in 50 mL of anhydrous methylene chloride cooled to 0°C. The mixture was kept for 24 h under intensive stirring. The reaction was monitored by TLC. The precipitated dicyclohexylurea was filtered. The product was isolated by column chromatography in the toluene/ethyl acetate system (1 : 5, v/v) and 4.2 g (92%) of product 13 was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.80 (t, 6H, CH3), 1.22 (m, 20H, CH2CH3), 1.36 (s, 9H, CCH3), 1.54 (s, 4H, C(O)OCH2CH2), 2.22 (t, 4H, C(O)OCH2CH2), 3.41 (t, 4H, NHCH2CH2), 4.10 (t, 4H, NHCH2CH2).
O,O'-dioctanoyl-diethanolamine (14). We dissolved 2 g (3.2 mmol) of compound 13 in 40 mL of anhydrous methylene chloride; then a mixture of 20 mL of trifluoroacetic acid in 20 mL of anhydrous methylene chloride was added under stirring. After 1 h, the reaction mixture was evaporated in a rotary evaporator; the residue was dissolved in 40 mL of chloroform and washed with a 10% aqueous solution of sodium bicarbonate (3 × 40 mL) and water to pH 7, then it was dried over sodium sulfate and evaporated. The yield of product 14 was 1.2 g (70.6%). 1H-NMR spectrum (CDCl3, δ, ppm): 0.89 (t, 6H, CH3), 1.24 (m, 20H, CH2CH3), 1.62 (t, 4H, C(O)OCH2CH2), 2.34 (t, 4H, C(O)OCH2CH2), 2.97 (t, 4H, NHCH2CH2), 4.21 (t, 4H, NHCH2CH2), 4.51 (s, 1H, NH).
Trifluoroacetate glycyl-O,O'-dioctanoyl-diethanolamine (15a). We added 0.071 g (0.577 mmol) of DMAP, 0.12 g (0.577 mmol) of the DCC solution in 10 mL of methylene chloride and 0.139 g (0.389 mmol) of product 14 in 35 mL of methylene chloride under stirring to 0.15 g (0.433 mmol) of the BocGly-OH 2a solution in 5 mL of anhydrous methylene chloride cooled to 0°C. The mixture was kept for 24 h under intensive stirring. The reaction was monitored by TLC. The precipitated dicyclohexylurea was filtered; the reaction mixture was washed with water to pH 7 and dried over Na2SO4. The product was isolated by preparative thin layer chromatography in the chloroform/methanol system (20 : 1, v/v). 0.167 g (75%) of product 15a was obtained. The protective group was removed from the technical product with 0.37 g (4.87 mmol) of trifluoroacetic acid in 10 mL of anhydrous methylene chloride under stirring; the solvent with the excess acid was removed under vacuum, and the quantitative yield of trifluoroacetate salt was obtained. 1H-NMR spectrum (CDCl3, δ, ppm): 0.89 (t, 6H, CH3), 1.28 (m, 16H, CH2CH3), 1.56 (t, 4H, C(O)OCH2CH2), 2.34 (t, 4H, C(O)OCH2CH2), 3.61 (t, 4H, NHCH2CH2), 3.88 (s, 2H, NH2CH2), 4.27 (m, 4H, NHCH2CH2), 5.99 (s, 2H, NH2).
Trifluoroacetate β-L-alanine-O,O'-dioctanoyl-diethanolamine (15b). The reaction was performed similarly. We obtained 0.29 g (71%) of product 15b from 0.15 g (0.793 mmol) of 2b. The 1H-NMR spectrum (CDCl3, δ, ppm): 0.86 (t, 6H, CH3), 1.28 (m, 16H, CH2CH3), 1.55 (m, 4H, C(O) OCH2CH2), 2.38 (m, 4H, C(O)OCH2CH2), 2.74 (s, 2H, NH2CH2CH2), 3.06 (t, 2H, NH2CH2CH2), 3.57 (dd, 4H, NHCH2CH2), 4.19 (m, 4H, NHCH2CH2), 4.41 (s, 2H, NH2).
RESULTS AND DISCUSSION
The hydrophilic–lipophilic balance (HLB) value is one of the most important characteristics of potential peptidomimetics. The HLB values and, consequently, the structures of the hydrophobic and hydrophilic blocks of the target compounds represent the possibility of electrostatic and hydrogenous interactions between therapeutic molecules and bacterial cell wall components; and they also lay the foundation of the fine details of the mechanism of antimicrobial activity. The first stage of the study was the theoretical calculation of HLB using the ACD/Labs, LogP software [24, 25] for the planned cationic amphiphiles differing in the length of aliphatic chains in the hydrophobic block, as well as in the hydrocarbon chain length and the number of charged groups in the structure of the amino acid in the hydrophilic head group, which made it possible to select the most promising samples for further studies. The calculated HLB values of the studied library of structures are within the range of probable antibacterial activity and vary from 5.43 to 6.34. The data obtained were the base for developing the schemes of production and synthesis of compounds 6 (a–c), 10 (a, b), and 15 (a, b) (Table 1).
In this study, it has been proposed to use dioctylamine to form the hydrophobic block of compounds 6 (a–c) and 10 (a, b). At the same time, the polar group is formed by the derivatives of glycine 6a, beta-L-alanine 6b, and GABA 6c (scheme 1), as well as L‑ornithine 10a or L-lysine 10b used as the control [26] (scheme 2). Dioctylamine 4 was obtained by the reaction between octylamine 3 and 1-bromoctane with heating to 80°C in the presence of K2CO3 [27].

Scheme 1.

Scheme 2.
Amino acids 1 (a–c) and 7 (a, b) were treated with di-tert-butyl-pyrocarbonate (Boc2O) and 4 M NaOH in THF as a solvent for 1 h and then stirred at room temperature for 3 h [28]. The resultant compounds were dissolved in water, saturated with a sodium chloride solution, acidified with a citric acid 20% solution to pH 3, extracted with ethyl acetate and dried with sodium sulfate. Thus, the protected amino acids 2 (a–c) and 8 (a, b) were obtained with the yields of 83.2, 89.4, 81.6, 73.2, and 79.4%, respectively.
Compounds 5 (a–c) and 9 (a, b) were obtained by the carbodiimide method with the involvement of N,N′-dicyclohexylcarbodiimide (DCC) and 4-dimethyl-aminopyridine (DMAP)) [29]. For this purpose, the solution of the amino component 4 was added under stirring to the solution of the amino acid and DMAP mixture in anhydrous methylene chloride. The mixture was cooled to 0°C; the DCC solution was added to anhydrous methylene chloride and stirred for 2 h. After the completion of the reaction, the precipitated dicyclohexylurea was filtered. The Boc-protection was removed with trifluoroacetate acid in methylene chloride (1 : 1 v/v) to obtain cationic amphiphiles 6 (a–c) and 10 (a, b), respectively. The structures of the products were verified by the 1H-NMR spectroscopy data.
According to Scheme 3, the hydrophobic region is formed alternatively based on the aliphatic derivative of diethylamine (14) obtained in the reaction with octanoic acid in the presence of DCC and DMAP. For this purpose, first the Boc-derivative of diethanolamine (12) was formed and then, after the hydrocarbon radical addition reaction, the protection was removed with trifluoroacetic acid. The target amphiphiles 15 (a, b) were obtained in the conjugation reaction between the hydrophilic block 2 (a, b) and the hydrophobic domain 14 using DCC in the presence of DMAP. After the removal of the protective groups, the yield of cationic amphiphiles 15a and 15b was 75 and 71%, respectively. The structures of the compounds were verified by the 1H-NMR spectroscopy data.
The advantage of the developed and implemented schemes of the synthesis of cationic amphiphiles based on the derivates of aliphatic amines consists in the simplicity and universality of the proposed approach, which can be used to produce several series of purposefully modified samples in the preparative amounts necessary for the subsequent biochemical studies.
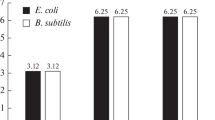
The preliminary assessment of the antibacterial effects of the synthesized compounds performed at the Gause Institute of New Antibiotics and at the National Research Center for Hematology demonstrated a satisfactory level of activity against several bacterial strains. The sample based on glycyl dioctylamide showed a promising level with the minimum inhibitory concentration (MIC) values for Gram-positive (Staphylococcus aureus, Bacillus subtilis) and Gram-negative (Escherichia coli) microorganisms of 2, 50, and 5 μg/mL, respectively. The detailed results of this research will be presented in the next publication.

Scheme 3.
REFERENCES
Ghosh, C., Manjunath, G.B., Akkapeddi, P., Yarlagadda, V., Hoque Uppu, J.D., Konai, M.M., and Haldar, J., J. Med. Chem., 2014, vol. 57, no. 4, p. 1428. https://doi.org/10.1021/jm401680a
Fjell, C.D., Hiss, J.A., Hancock, R.E., and Schneider, G., Nat. Rev. Drug Discovery, 2011, vol. 11, no. 1, p. 37. https://doi.org/10.1038/nrd3591
Yount, N.Y. and Yeaman, M.R., Proc. Natl. Acad. Sci. U. S. A., 2004, vol. 101, no. 19, p. 7363. https://doi.org/10.1073/pnas.0401567101
Pirri, G., Giuliani, A., Nicoletto, S.F., Pizzuto, L., and Rinaldia, C., Cent. Eur. J. Biol., 2009, vol. 4, p. 258. https://doi.org/10.2478/s11535-009-0031-3
Fjell, C.D., Hiss, J.A., Hancock, R.E.W., and Schneider, G., Nat. Rev. Drug Discovery, 2012, vol. 11, p. 37.
Faber, C., Stallmann, H., Lyaruu, D., Joosten, U., Von Eiff, C., Van Nieuw Amerongen, A., and Wuisman P.I., Antimicrob. Agents Chemother., 2005, vol. 49, p. 2438. https://doi.org/10.1128/AAC.49.6.2438-2444.2005
Jung, D., Rozek, A., Okon, M., and Hancock, R.E., Chem. Biol., 2004, vol. 11, no. 7, p. 949. https://doi.org/10.1016/j.chembiol.2004.04.020
Li, J., Nation, R.L., Turnidge, J.D., Milne, R.W., Coulthard, K., Rayner, C.R., and Paterson, D.L., Lancet Infect Dis., 2006, vol. 6, no. 9, p. 589. https://doi.org/10.1016/S1473-3099(06)70580-1
Niu, Y., Wang, M., Cao, Y., Nimmagadda, A., Hu, J., Wu, Y., and Ye, X.-S., J. Med. Chem., 2018, vol. 61, no. 7, p. 2865. https://doi.org/10.1039/C7CC07285F
Haug, B.E., Stensen, W., Kalaaji, M., Rekdal, O., and Svendsen, J.S., J. Med. Chem., 2008, vol. 51, no. 14, p. 4306. https://doi.org/10.1021/jm701600a
Radzishevsky, I.S., Rotem, S., Bourdetsky, D., Navon-Venezia, S., Carmeli, Y., and Mor, A., Nat. Biotechnol., 2007, vol. 25, p. 657. https://doi.org/10.3390/ijms12095971
Zou, H., Koh, J.J., Li, J., Qiu, S., Aung, T.T., Lin, H., Lakshminarayananr, R., Dai, X., Cao, D., Liu, S., and Beuermanr, W., J. Med. Chem., 2013, vol. 56, p. 2359. https://doi.org/10.1021/jm301683j
Hurdle, J.G., O’Neill, A.J., Chopra, I., and Lee, R.E., Nat. Rev. Microbiol., 2011, vol. 9, no. 1, p. 62. https://doi.org/10.1038/nrmicro2474
Hoque, J., Konai, M.M., Sequeira, S.S., Samaddar, S., and Haldar, J., J. Med. Chem., 2016, vol. 59, no. 23, p. 10750. https://doi.org/10.1021/acs.jmedchem.6b01435
Konai, M.M. and Haldar, J., Bioconjugate Chem., 2017, vol. 28, no. 4, p. 1194. https://doi.org/10.1021/acs.bioconjchem.7b00055
Konai, M.M., Ghosh, C., Yarlagadda, V., Samaddar, S., and Haldar, J., J. Med. Chem., 2014, vol. 57, no. 22, p. 9409. https://doi.org/10.1021/jm5013566
Ghosh, C., Sarkar, P., Samaddar, S., Uppua, D., and Haldar, J., Chem. Commun., 2017, vol. 53, p. 8427. https://doi.org/10.1039/C7CC04206J
Sheng, R., Zhuang, X., Wang, Z., Cao, A., Lin, K., and Zhu. J., Nanomaterials, 2016, vol. 6, no. 69, p. 223. https://doi.org/10.3390/nano6040069
Brogden, K.A., Nat. Rev. Microbiol., 2005, vol. 3, no. 3, p. 238. https://doi.org/10.1038/nrmicro1098
Pouny, Y., Rapaport, D., Mor, A., Nicolas, P., and Shai, Y., Biochemistry, 1992, vol. 31, no. 49, p. 12416. https://doi.org/10.1021/bi00164a017
Baumann, G. and Mueller, P., J. Supramol. Struct., 1974, vol. 2, nos. 5–6, p. 538. https://doi.org/10.1002/jss.400020504
Zhang, E., Bai, P.-Y., Cui, D.-Y., Chu, W.-C., Hua, Y.-G., and Liu, Q., Eur. J. Med. Chem., 2018, vol. 143, p. 1489. https://doi.org/10.1016/j.ejmech.2017.10.044
Jennings, M.C., Minbiole, K.P.C., and Wuest, W.M., ACS Infect. Dis., 2015, vol. 1, no. 7, p. 288. https://doi.org/10.1021/acsinfecdis.5b00047
Szymanowski, J. and Hiron, C.G., J. Chem. Technol. Biotechnol., 1984, p. 218.
Denieva, Z.G., Romanova, N.A., Bodrova, T.G., Budanova, U.A., and Sebyakin, Yu.L., Moscow Univ. Chem. Bull. (Engl. Transl.), 2019, vol. 74, no. 6, p. 300. https://doi.org/10.3103/S0027131419060087
Zhang, E., Bai, P.-Y., Cui, D.-E., Chu, W.-C., Hua, Y.-G., Liu, Q., and Liu, H.-M., Eur. J. Med. Chem., 2018, vol. 143, no. 17, p. 1489. https://doi.org/10.1016/j.ejmech.2017.10.044
Meka, R.R., Godeshala, S., Marepally, S., Thorat, K., Rachamalla, H., Dhayani, A., Hiwale, A., Banerjee, R., Chaudhuria, A., and Vemula, V., RSC Adv., 2016, vol. 6, p. 77841. https://doi.org/10.1039/C6RA07256A
Marusova (Soloveva), V.V., Zagitova, R.I., Budanova, U.A., and Sebyakin, Yu.L., Moscow Univ. Chem. Bull. (Engl. Transl.), 2018, vol. 73, no. 2, p. 74. https://doi.org/10.3103/S0027131418020098
Denieva, Z.G., Budanova, U.A., and Sebyakin, Yu.L., Mendeleev Commun., 2019, vol. 29, p. 32. https://doi.org/10.1016/j.mencom.2019.01.009
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 20-04-00672).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by E. Makeeva
About this article
Cite this article
Filatova, S.M., Denieva, Z.G., Budanova, U.A. et al. Synthesis of Low-Molecular-Weight Antibacterial Peptide Mimetics Based on Dialkyl- and Diacylamines. Moscow Univ. Chem. Bull. 75, 320–327 (2020). https://doi.org/10.3103/S0027131420060048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131420060048