Abstract
Cholestasis-associated renal injury, known as cholemic nephropathy (CN), is a severe clinical complication. CN could lead to acute kidney injury. However, there is no specific pharmacological intervention for cholestasis-induced renal injury. Oxidative stress and mitochondrial impairment seem to play a role in the pathogenesis of CN. Betaine (3-methyl glycine; BET) is an amino acid derivative with a wide range of pharmacological actions. Several studies mentioned the effects of BET on oxidative stress in different experimental models. It has also been found that BET positively affected mitochondrial function. The current study aimed to evaluate the effects of BET supplementation on CN in the bile duct ligation (BDL) model of cholestasis. BET (0.25% and 1% w: v in drinking water) was administered to BDL animals for seven consecutive days. Biomarkers of oxidative stress and mitochondrial indices were evaluated in cholestatic animals. Significant elevation in reactive oxygen species formation, lipid peroxidation, and oxidized glutathione (GSSG) was evident in BDL rats. Moreover, reduced glutathione (GSH) levels and renal antioxidant capacity were decreased in cholestatic animals. Mitochondrial depolarization, mitochondrial permeabilization, decreased mitochondrial dehydrogenases activity, and lipid peroxidation was also detected in the kidney of BDL rats. Tubular atrophy, interstitial inflammation, and renal fibrosis were also detected in BDL rats. BET alleviated biomarkers of oxidative stress, improved mitochondrial indices, and decreased renal histopathological alterations in BDL animals. The antioxidant effects and the positive effects of BET on cellular mitochondria seem to play a fundamental role in its nephroprotective role during cholestasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Betaine (BET) is the methylated form of the amino acid glycine. BET is synthesized endogenously by organs such as the liver (Day and Kempson 2016; Preedy 2015). BET synthesis capability is widely variable among various species (Day and Kempson 2016; Preedy 2015; Sidransky and Farber 1960). Humans are mostly dependent on the food sources of BET (Day and Kempson 2016; Preedy 2015; Sidransky and Farber 1960). BET is present at very high concentrations in seafood, beans, and cereals (Ross et al. 2014; Zeisel et al. 2003). Regulation of cellular osmotic pressure is a fundamental physiological role identified for BET (Day and Kempson 2016; Preedy 2015). BET is present in cells exposed to extreme osmotic changes (e.g., enterocytes and kidney cells) (Duffjr and Howell 2015; Ommati et al. 2020; Zhou et al. 2015). On the other hand, several pharmacological properties have been identified for BET (Craig 2004; Heidari et al. 2018d, e). It has been found that BET is an excellent protective agent against liver injury with different etiologies (Craig 2004; Heidari et al. 2018d, e). Several studies also mentioned the nephroprotective properties of BET (Fan et al. 2014; Hagar and Al Malki 2014; Hagar et al. 2015). BET could enhance cellular antioxidant defense mechanisms (Bingül et al. 2016; Erman et al. 2004; Hossain et al. 2012; Ji and Kaplowitz 2003; Rasheed et al. 2014). Hence, the effects of BET on oxidative stress could play a significant role in its cytoprotective mechanisms.
Kidneys are the most affected extrahepatic organs in cholestatic patients (Betjes and Bajema 2006; Erlinger 2014; Fickert et al. 2013; Krones et al. 2015, 2018a, b; Mandorfer and Hecking 2019). Cholestasis-induced renal injury is known as “cholemic nephropathy” (CN) (Betjes and Bajema 2006; Erlinger 2014; Fickert et al. 2013; Krones et al. 2015, 2018a, b; Mandorfer and Hecking 2019). CN could cause renal failure and the need for organ transplantation (Betjes and Bajema 2006; Erlinger 2014; Fickert et al. 2013; Krones et al. 2015, 2018a, b; Mandorfer and Hecking 2019). Hence investigating the mechanisms of renal injury and development of potential therapeutic agents against this complication could have tremendous clinical value. Extrahepatic cholestasis induced by bile duct ligation (BDL) is a reliable experimental tool to induce CN (Fickert et al. 2013). The accumulation of potentially cytotoxic molecules in serum, and finally, the kidney tissue is thought to be responsible for renal damage in CN (Fickert et al. 2013). Although the precise mechanism involved in the pathogenesis of CN is not well understood, several studies mentioned the pivotal role of oxidative stress and mitochondrial impairment in this complication (Heidari et al. 2018b; Martínez-Cecilia et al. 2016; Orellana et al. 2000; Panozzo et al. 1995; Sheen et al. 2010).
This study aimed to evaluate the potential renoprotective properties of BET in an animal model of cholestasis. BDL rats were supplemented with BET (0.25 and 1% w: v in drinking water) for seven consecutive days. Serum and urine markers of organ injury, kidney tissue biomarkers of oxidative stress, mitochondrial indices, and tissue histopathological alterations were monitored.
Materials and methods
Chemicals
N-chloro tosylamide (Chloramine-T), trichloroacetic acid, sodium acetate, citric acid, n-propanol, p-dimethyl amino benzaldehyde, methanol HPLC grade, iodoacetic acid, dithiothreitol, sucrose, 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), thiobarbituric acid, sodium citrate, ethylenediamine tetra-acetic acid (EDTA), phosphoric acid, acetonitrile HPLC grade, and 2amino2-hydroxymethyl-propane-1,3-diol-Hydrochloride (Tris-HCl) were obtained from Merck (Darmstadt, Germany). Betaine (Trimethylglycine), dichlorodihydrofluorescein diacetate (DFC-DA), oxidized glutathione (GSSG), and reduced glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Biomarkers of organ injury, including alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen, creatinine, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), albumin, glucose, and bilirubin, were assessed by commercial kits obtained from Pars Azmoon® (Tehran, Iran). Serum and urine bile acids were measured using the EnzyFluo™ Bile Acid Assay Kit (BioAssay® Systems, Hayward, CA 94,545, USA). All salts used for preparing buffer solutions were of analytical grade and purchased from Merck (Darmstadt, Germany).
Animals
Male mature Sprague-Dawley rats (n = 32; 250–300 g) were obtained from Shiraz University of Medical Sciences, Shiraz, Iran. Animals were housed in plastic cages over wood-chip bedding, in a standard environment (temperature of 23 ± 1ºC and a 12 L: 12D photoschedule along with a ≈ 40% humidity). Animals were allowed free access to RoyanFeed® (Isfahan, Iran) rodents chow diet and tap water. Animals received humane care, and all the experiments were carried out in agreement with the guidelines for the use of experimental animals approved by the ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (#98-01-36-19445).
Surgery and experimental setup
Animals were anesthetized (10 mg/kg of xylazine and 70 mg/kg of ketamine, i.p), a midline incision was made through the linea alba. Then, the common bile duct was localized, doubly ligated, and cut between these two ligatures. The sham operation consisted of laparotomy and bile duct identification and manipulation. Animals were equally allotted into five groups (n = 08/group). Rats were treated as follows: 1) Sham-operated (Vehicle-treated); 2) BDL; 3) BDL + Betaine (0.25% w: v in drinking water); 4) BDL + Betaine (1% w: v in drinking water). Cholestasis-associated cholemic nephropathy was assessed seven days after BDL surgery.
Serum and urine biochemistry
A Mindray BS-200® auto analyzer and kits (Pars Azmun®, Tehran, Iran) were used to measure serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), gamma-glutamyl transpeptidase (γ-GT), bilirubin, glucose, protein, blood urea nitrogen (BUN), and creatinine (Cr). Serum and urine bile acids were measured using the EnzyFluo™ Bile Acid Assay Kit (BioAssay® Systems, Hayward, CA 94,545, USA) based on a fluorometric method.
Kidney histopathology and organ coefficient
For histopathological assessments, samples of kidney were fixed in buffered formalin solution (0.4% w: v sodium phosphate monobasic, 0.64% w: v sodium phosphate dibasic, and 10% v: v formaldehyde in double distilled water, pH = 7.4). Paraffin-embedded sections of prepared tissues (5 µm) were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing. Kidney fibrotic changes were determined by Masson’s trichrome staining in BDL rats. Periodic acid–Schiff (PAS) stain was used to evaluate renal cast formation in BDL rats. A pathologist analyzed samples in a blind fashion. The organs (liver, spleen, and kidney) weight index was measured as Organ weight index = [Wet organ weight (g)/Body weight (g)] × 100.
Reactive oxygen species (ROS) formation
Reactive oxygen species in the kidney were estimated by a previously described method using 2′, 7′dichlorofluorescein diacetate (DCFDA) as a probe (Gupta et al. 2007). Briefly, kidney tissue (200 mg) was homogenized in 5 mL of ice-cooled Tris-HCl buffer (40 mM, pH = 7.4). Samples of the tissue homogenate (100 µl) were mixed with Tris-HCl buffer (1 mL) and DCF-DA (Final concentration 10 µM). Samples were incubated for 15 min at 37ºC (in the dark). Finally, the fluorescence intensity was assessed by a fluorimeter (FLUOstar Omega® multifunctional microplate reader with λexcit = 485 nm and λem = 525 nm (Gupta et al. 2007; Socci et al. 1999).
Lipid peroxidation
The assessment of TBARS (thiobarbituric acid reactive substances) was used as an index of lipid peroxidation in the kidney tissue. Briefly, the reaction mixture composed of 500 µl of tissue homogenate (10% w: v in KCl, 1.15% w: v), thiobarbituric acid (1 mL of 0.375%, w: v solution), and meta-phosphoric acid (3 mL of 1% w: v solution, pH = 2). Samples were mixed and heated (100 °C water bath, 45 min). Afterward, n-butanol (2 mL) was added and samples were vortexed, and centrifuged (17,000 g for 10 min). Finally, the absorbance of developed color in the upper phase was measured at λ = 532 nm (EPOCH plate reader, BioTek® instruments, USA).
Tissue and mitochondrial glutathione content
The reduced (GSH) and oxidized (GSSG) glutathione content in the kidney tissue and isolated mitochondria was measured using an HPLC method, as previously described (Meeks and Harrison 1991). The stationary phase composed of an NH2 column (25 cm, Bischoff chromatography, Leonberg, Germany) (Meeks and Harrison 1991). The mobile phases consisted of buffer A (Acetate buffer: Water; 1:4 v: v) and buffer B (Water: Methanol; 1:4 v: v) and a gradient method with a steady increase of buffer B to 95% in 25 min. The flow rate of the mobile phase was 1 mL/min (Meeks and Harrison 1991). Nanomole levels of GSH and GSSG can be measured by this method (Meeks and Harrison 1991). GSH and GSSG were used as external standards. Tissue samples (200 mg) were homogenized in Tris-HCl buffer (250 mM; pH = 7.4; 4ºC), and TCA (500 µl of 50% w: v solution) was added. Mitochondria samples (500 µl; 1 mg protein/mL) were also treated with 50 µl of TCA (50% w: v). Samples were incubated on ice for 15 min. Afterward, samples were mixed well and centrifuged (17,000 g, 15 min, 4ºC). Then, 1 mL of the supernatant was collected in 5 mL tubes, and 300 µl of the NaOH: NaHCO3 (2 m: 2 m) was added. Then, 100 µl of iodoacetic acid (1.5% w: v in double-distilled water) was added, and samples were incubated in the dark for 1 h (4ºC, in the dark). After the incubation period, 2, 4-dinitrofluorobenzene (500 µl of DNFB; 1.5% w: v in absolute ethanol) was added and incubated in the dark (25ºC, at least for 24 h). Finally, 25 µl of samples were injected into the described HPLC system (Meeks and Harrison 1991; Truong et al. 2006).
Total antioxidant capacity of kidney tissue
Ferric reducing antioxidant power (FRAP) assay measures the formation of a blue-colored ferrous (Fe2+)-tripyridyltriazine compound from the colorless oxidized ferric (Fe3+) form by the action of tissue electron-donating antioxidants (Katalinic et al. 2005). In the current study, the FRAP reagent (freshly prepared) composed of 10 volumes of acetate buffer (300 mmol/L, pH = 3.6), 1 volume of TPTZ (10 mmol/L in 40 mmol/L hydrochloric acid), and 1 volume of ferric chloride (20 mmol/L). Tissue was homogenized in an ice-cooled Tris-HCl buffer (250 mM Tris-HCl, 200 mM sucrose and 5 mM dithiothreitol, pH = 7.4). Then, 100 µl of tissue homogenate was added to 900 µl of the FRAP reagent. The reaction mixture was incubated at 37ºC (5 min, in the dark). Finally, the absorbance of developed color was assessed at λ = 595 nm (Ultrospec 2000® UV spectrophotometer, Pharmacia Biotech, Uppsala, Sweden) (Alía et al. 2003).
Kidney hydroxyproline content
Kidney hydroxyproline content was assessed spectrophotometrically using the Erlich reagent. Briefly, 500 µl of kidney tissue homogenate (20% w: v in phosphate-buffered saline; PBS; pH = 7.4) was digested in 1 mL of 6 N HCl at 120 °C for 12 h. The digested homogenate was cooled and centrifuged (17,000 g, 5 min), and an aliquot of the supernatant (25 µl) was added to 25 µl of citrate–acetate buffer (pH = 6). Afterward, 500 µl of 56 mM chloramines-t-solution was added, and the mixture was left at room temperature for 20 min. Then, 500 µl Ehrlich’s reagent (15 g of p-Dimethyl amino benzaldehyde in n-propanol: perchloric acid; 2:1 v: v) was added, and the mixture was incubated at 65 °C for 15 min. Then, samples were centrifuged (17,000 g, 5 min), and the intensity of developed color was measured spectrophotometrically (λ = 550 nm, EPOCH plate reader, BioTek® instrument, USA) (Hamdy and El-Demerdash 2012).
Renal mitochondria isolation
Rats’ kidneys were washed (NaCl 0.9% w: v, 4ºC) and minced in the ice-cold isolation buffer (2 mM HEPES, 70 mM mannitol, 220 mM sucrose, 0.5 mM EGTA and 0.1% bovine serum albumin, pH = 7.4). Minced tissue was transported into fresh isolation buffer (5 mL buffer: 1 g tissue) and homogenized. Renal mitochondria were isolated by differential centrifugation method as previously described (Fernández-Vizarra et al. 2010). First, the tissue homogenate was centrifuged at 1000 g for 20 min at 4 ºC. Then, the supernatant was centrifuged at 10,000 g (20 min at 4 ºC) to pellet the mitochondrial fraction. The second centrifugation step was repeated at least three times using a fresh isolation buffer medium to enhance mitochondrial yield. Finally, the mitochondrial pellet was re-suspended in a buffer (5 mL buffer/g tissue) containing 70 mM mannitol, 220 mM sucrose, and 2 mM HEPES (pH = 7.4). The mitochondria fractions used to assess mitochondrial permeabilization and mitochondrial depolarization were suspended in mitochondria permeabilization buffer (pH = 7.2; 10 mM HEPES, 125 mM sucrose, 65 mM KCl), and depolarization assay buffer (pH = 7.2; 10 mM KCl, 50 µM EGTA, 220 mM sucrose, 68 mM mannitol, 5 mM KH2PO4, 2 mM MgCl2, and 10 mM HEPES) (Fernández-Vizarra et al. 2010). Samples protein concentrations were determined based on the Bradford method to standardize the obtained data.
Lipid peroxidation in isolated mitochondria
Thiobarbituric acid-reactive substances (TBARS) were used as a method to assess lipid peroxidation. Previous studies mentioned that the presence of sucrose in mitochondria isolation buffer interferes with the lipid peroxidation test in isolated mitochondria preparations (Caro et al. 2012). Therefore, mitochondria preparations were washed once (to remove sucrose) in ice-cooled MOPS-KCl buffer (50 mM MOPS, 100 mM KCl, 4 ºC, pH = 7.4). For this purpose, isolated kidney mitochondria were suspended in 5 mL of the MOPS-KCl buffer and centrifuged (15,000 g, 15 min). The pellet was re-suspended in MOPS–KCl buffer and used for TBARs assay. The mitochondria preparations (1 mg protein/mL) were mixed with 1 mL of a solution containing HCl (0.24 N), thiobarbituric acid (0.375% w: v), trichloroacetic acid (15% w: v), and Trolox (500 µM). Samples were heated for 15 min at 100 ºC in a water bath (Caro et al. 2012). Then 1 mL of n-butanol was added and vortexed (5 min). Samples were centrifuged (17,000 g, 5 min), and the absorbance of the upper phase (n-butanol phase) was measured (λ = 532 nm, EPOCH®, BioTek® Instruments, USA) (Caro et al. 2012).
Mitochondrial membrane potential
Mitochondrial uptake of rhodamine 123 was applied for the evaluation of mitochondrial depolarization (Ahmadian et al. 2017). Rhodamine 123 is accumulated in the mitochondrial matrix by facilitated diffusion. When the mitochondrion is depolarized, there is no facilitated diffusion. Therefore, the amount of rhodamine 123 in the supernatant will be increased. In the current study, the mitochondrial fractions (0.5 mg protein/mL; in the mitochondrial membrane potential assay buffer) were incubated with 10 µl of rhodamine 123 (Final concentration of 10 µM, 30 min, 37ºC, in the dark). Afterward, samples were centrifuged (17,000 g, 2 min, 4ºC), and the fluorescence intensity of the supernatant was assessed by a fluorimeter (FLUOstar Omega®; BMG Labtech, Germany; λexcit = 485 nm and λem = 525 nm) (Ahmadian et al. 2017).
Mitochondrial ATP levels
Based on a previously reported protocol, mitochondrial ATP level was assessed by HPLC (Chen et al. 2007). Briefly, isolated mitochondria (1 mg protein/mL) were treated with 100 µl ice-cooled phosphoric acid (50% w: v, 4 ºC), mixed, and incubated on ice (10 min). Afterward, samples were centrifuged (30 min, 17,000 g, 4 ºC), and the supernatant (100 µl) was treated with 15 µl of ice-cooled 1 m KOH solution. Samples (25 µl) were injected into an HPLC system consisted of an LC-18 column (25 cm µ-Bondapak column). The mobile phase was composed of potassium phosphate buffer (100 mM KH2PO4, pH = 7 adjusted with KOH), tetrabutylammonium hydroxide (1 mM), and acetonitrile (2.5% v: v). The flow rate was 1 mL/min, and the UV detector was set at λ = 254 nm (Chen et al. 2007).
Mitochondrial permeability transition
The changes in optical density at λ = 540 nm was used as a method to estimate mitochondrial swelling and permeabilization (Caro et al. 2012). Briefly, isolated mitochondria (0.5 mg protein/mL) were suspended in swelling buffer (125 mM Sucrose, 10 mM HEPES, 65 mM KCl, pH = 7.2). The absorbance was monitored (25 °C, during 30 min of incubation), using an EPOCH® plate reader (Highland Park, USA). An increase in mitochondrial swelling is connected with a decrease in the absorbance of the samples. The results are reported as maximal mitochondrial swelling amplitude (ΔOD at λ = 540 nm) (Caro et al. 2012).
Statistical methods
Data are given as mean ± SD. The comparison of data sets was performed by the one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons as the post hoc test. Histopathological alteration scores are given as median and quartiles for five random pictures per group. The Kruskal–Wallis tests (followed by the Mann Whitney U test) was used for the statistical analysis of histopathological scores. Values of P < 0.05 were considered statistically significant. GraphPad Prism® 8 was used to visualize data in the current study.
Results
BET was administered in animals drinking water in the current study. Therefore, daily water intake was monitored to assess any changes in this parameter (Fig. 1). No significant difference in daily water intake was detected between BET-treated and other groups (Fig. 1).
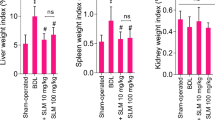
Animals’ organ weight indices were evaluated in the control and cholestatic groups (Fig. 2). Significant hepatomegaly and splenomegaly were evident in the BDL group that confirm the occurrence of cholestasis in this study (Fig. 2). There were no substantial changes in the kidney weight index seven days after BDL surgery (Fig. 2). It was found that BET administration (0.25 and 1%) significantly mitigated signs of hepatomegaly and splenomegaly in BDL rats (Fig. 2).
Serum biochemical measurements revealed a significant elevation in biomarkers of liver and bile duct injury (Fig. 3). On the other hand, BET treatment (0.25 and 1% w: v) significantly decreased serum biomarkers of liver injury in cholestatic rats (Fig. 3). There were no significant differences in biomarkers, such as serum bilirubin, ALP, and γ-GT between BDL and BET-treated groups (Fig. 3). Biomarkers of renal injury were also significantly increased in the serum and urine of BDL animals (Fig. 4). It was found that the BET administration significantly decreased the level of urine and serum biomarkers of renal injury in cholestatic animals (Fig. 4). It is noteworthy to mention that Cr and BUN, as well as urine markers assessed in the current study, are conventional biomarkers for evaluating renal function. Indeed, evaluating biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) or kidney injury molecule-1 (KIM-1) could give a better insight on the level of renal injury and the nephroprotective effects of agents such as BET in cholestasis.
Oxidative stress biomarkers, including ROS formation, lipid peroxidation, and tissue GSSG levels were significantly increased in cholestatic rats (Fig. 5). Moreover, renal tissue antioxidant capacity and GSH reservoirs were decreased considerably in the BDL group (Fig. 5). It was found that BET administration (0.25% and 1% w: v) alleviated biomarkers of oxidative stress in the renal tissue of cholestatic animals (Fig. 5).
Kidney oxidative stress biomarkers in bile duct ligated (BDL) rats. BET: Betaine; ROS: Reactive oxygen species; DCF: Dichlorodihydrofluorescein; GSH: Glutathione; TBARS: Thiobarbituric acid reactive substances. Data are given as mean ± SD (n = 08). # Indicates significantly different as compared with the BDL group (P < 0.05). ns: not significant
Mitochondrial indices were evaluated in the kidney of cholestatic animals (Fig. 6). Significant mitochondrial depolarization and permeabilization, as well as decreased mitochondrial dehydrogenases activity and depleted mitochondrial ATP, were evident in mitochondria isolated from the kidney of BDL animals (Fig. 6). On the other hand, mitochondrial markers of oxidative stress, such as lipid peroxidation and GSSG, were significantly increased in the BDL group (Fig. 7). Moreover, mitochondrial GSH and GSH/GSSG ratio were significantly decreased in cholestatic animals (Fig. 7). It was found that BET supplementation significantly improved renal mitochondrial function in cholestatic animals (Fig. 6). Moreover, BET significantly decreased biomarkers of oxidative stress in the kidney mitochondria of cholestatic animals (Fig. 7). The effects of BET on mitochondrial indices were not dose-dependent in the current study (Figs. 6 and 7).
Renal tissue histopathological alterations in BDL animals were evident as interstitial inflammation and tubular atrophy and degeneration (Fig. 8; Table 1). Significant collagen deposition and fibrotic lesions were also detected in the kidney of BDL rats (Trichrome Masson staining, Fig. 8). However, there was no significant elevation in the level of renal hydroxyl proline in the current study (Fig. 8). Tubular cast formation was another histopathological alteration in the renal tissue of cholestatic animals (Fig. 9; PAS stain). It was found that BET administration (0.25% and 1% w: v) significantly mitigated renal tissue histopathological changes and cast formation in BDL animals (Figs. 8 and 9, and Table 1).
Effect of betaine (BET) supplementation on bile acids-induced nephropathy in cholestatic rats. BDL: Bile duct ligated. Significant renal tissue histopathological changes, including tubular atrophy and degeneration and interstitial inflammation, were evident in the kidney of BDL rats (Table 1). It was found that the BET administration significantly alleviated histopathological alterations in cholestasis-induced renal injury (Table 1)
Kidney tissue fibrosis and hydroxyproline content in bile duct ligated (BDL) rats. Trichrome staining. BET: Betaine. Significant tissue fibrosis (blue-colored in the Trichrome stain) was detected in the kidney of cholestatic animals. It was found that BET supplementation mitigated cholestasis-associated renal fibrosis in BDL rats. Data for kidney collagen deposition (% blue area in trichrome staining) are represented as mean ± SD (n = 08).# Indicates significantly different as compared with the BDL group (P < 0.05). ns: not significant
Effect of betaine (BET) supplementation on bile cast formation (Red arrow) in the kidney of cholestatic animals. BDL: Bile duct ligated. Significant tubular cast formation was detected in BDL animals (red arrow). It was found that the BET administration significantly decreased tubular casts in the cholestatic animals. Data for bile cast numbers are given as mean ± SD (n = 08). # Indicates significantly different as compared with the BDL group (P < 0.05)
Discussion
Several diseases, as well as drugs and xenobiotics, could induce cholestasis in humans (Gossard and Talwalkar 2014; Rodríguez-Garay 2003). Cholestasis is associated with significant tissue injury in the liver (Gossard and Talwalkar 2014; Rodríguez-Garay 2003). On the other hand, it has been found that other organs, rather than the liver, could be influenced by cholestasis (Forton et al. 2004; Patil and Mayo 2008; Sheen et al. 2010; Wright et al. 2007). Kidneys are the most affected extrahepatic organs in cholestasis (Aniort et al. 2017; Betjes and Bajema 2006; Erlinger 2014; Krones et al. 2015; Mandorfer and Hecking 2019). Cholestasis-induced kidney damage is known as cholemic nephropathy (CN) (Holt et al. 1999; Krones et al. 2015, 2018a, b). Exposure of the kidney tissue to a high concentration of cytotoxic molecules such as bilirubin and hydrophobic bile salts are proposed to be involved in the pathogenesis of CN (Chávez-Iñiguez et al. 2019; Krones et al. 2018a, b).
Although the exact mechanism(s) of renal injury in CN is not well-known, several studies mentioned the fundamental role of oxidative stress and its associated complications in this disease (Heidari et al. 2018b; Heidari and Niknahad 2019; Ishida et al. 2016). Oxidative stress-induced tissue injury is a well-known phenomenon associated with cholestasis (Fang et al. 2019; Grattagliano et al. 2017; Heidari et al. 2017; Smid et al. 2016). It has been found that change in glutathione redox status is an essential event in the kidney of cholestatic animals (Martínez-Cecilia et al. 2016). On the other hand, critical targets such as proteins and lipids are affected by cholestasis. A significant decrease in tissue antioxidants capacity, lipid peroxidation, and protein carbonylation have been documented in previous studies (Heidari et al. 2017, 2018a, d; Ommati et al. 2019). Mitochondrial impairment also seems to be an essential factor involved in the pathogenesis of renal injury in CN (Heidari 2019; Heidari et al. 2019). Mitochondrial damage is directly associated with oxidative stress (Brookes et al. 2004). Moreover, the release of cell death mediators from this organelle could lead to cell death and organ injury (Brookes et al. 2004; Ott et al. 2007).
It was found that BET effectively prevented the decrease in renal GSH stores and improved GSH/GSSG ratio as an essential index of oxidative stress (Figs. 5 and 7). The positive effects of BET on renal GSH reservoirs might be indirectly associated with its role in GHS precursors synthesis (Zhou et al. 2015). BET acts as an essential cellular source of the amino acid cysteine (Zhou et al. 2015). Cysteine is an amino acid used for the synthesis of GSH. Hence, BET could act as a cysteine supply for the synthesis of cellular GSH. In our study, we found that the levels of GSH were significantly higher in the kidney of BET supplemented cholestatic animals (Figure). Hence, the effect of BET on the cellular antioxidant system could play a significant role in counteracting cholestasis-associated oxidative stress.
In the current study, the oxidative damage of renal lipids was evident in cholestatic animals (Fig. 5). The decreased detoxification capacity of the kidney tissue could lead to a massive oxidative stress response. As mentioned, the effect of BET on vital antioxidant molecules could play a fundamental role in preventing oxidative stress-induced cellular damage. On the other hand, it has been found that BET intermediary metabolites such as s-adenosyl methionine (SAM) could act as a direct, reactive species scavenger. The level of ROS, as well as lipid peroxidation and protein damage, was significantly lower in BDL animals supplemented with BET (Fig. 5). The exact role of BET metabolites in its cytoprotective mechanisms warranted further investigations in this field.
Although the exact source of ROS is not known during cholestasis, inflammatory cells or cellular mitochondria might be the primary sources for reactive species in the liver and kidney of cholestatic animals (Copple et al. 2010; Gujral et al. 2003; Heidari et al. 2019; Krones et al. 2017; Tag et al. 2015a, b; Woolbright and Jaeschke 2012). Interestingly, the positive effects of BET on mitochondrial function and mitochondria-facilitated ROS formation has been repeatedly mentioned (Heidari et al. 2018b, d, e; Lee 2015). The anti-inflammatory properties of BET have also been mentioned in previous investigations (Go et al. 2005; Yi and Kim 2012). Based on these data, the effects of BET on mitochondrial function and inflammatory response might play an essential role in the protective effects of this compound in the kidney of cholestatic animals. Despite the source of ROS, the data obtained from this study revealed that BET supplementation effectively decreased oxidative stress and tissue histopathological changes during cholestasis. Indeed, further studies are warranted to precisely determine the role of inflammatory and fibrotic biomarkers, genes, and proteins such as F480, MCP-1, COL 1A1, or TGF-β in the pathogenesis of cholestasis-induced renal injury and determining the potential protective role of BET on these parameters.
Various studies revealed that BET administration could enhance mitochondrial function, enhance cellular ATP levels, and prevent mitochondria-mediated cell death (Graf et al. 2002; Heidari et al. 2018b, c; Lee 2015). In the current study, we found that the BET administration significantly improved mitochondrial indices in the kidney of cholestatic animals (Fig. 6). Moreover, BET decreased mitochondrial biomarkers of oxidative stress (Fig. 7). Based on these data, the positive effects of BET on cellular mitochondria might play a fundamental role in its effects against cholestasis-induced renal injury.
The effects of BET on the liver function could have a direct role in renal function. As shown in Fig. 3, BET could significantly decrease the serum level of liver injury biomarkers (Fig. 3). This could mention the hepatoprotective role of BET in cholestatic rats. Recently, we found that BET could significantly alleviate oxidative stress biomarkers and improve hepatic mitochondrial indices (Heidari et al. 2018e). On the other hand, betaine treatment didn’t affect serum bilirubin and bile acid levels because of the permanent secretion of these cytotoxic molecules in the BDL model (Fig. 3). Hence, these cytotoxic molecules continuously reach the kidney and damage this organ. Based on these data, a large part of the nephroprotective effects of BET in the current model could be mediated through the direct impact of this compound against cytotoxic molecules.
BET is a very safe agent, and no adverse effects have been described even at very high daily doses of this compound (9–15 g/day) (Craig 2004). Hence, BET could be readily administered against oxidative stress with different etiologies in humans. The results of our study in BDL animals strongly supported the use of BET as a nephroprotective agent in cholestasis-associated renal complications. These data suggest that BET could be a promising therapy for cirrhosis-associated CN, particularly at its early stages. Indeed, future investigations are needed to reveal other mechanisms of nephroprotective effects of BET precisely, and extrapolating these data to human cases of cholestasis-induced renal injury.
Abbreviations
- ΔΨ:
-
Mitochondrial membrane potential
- ATP:
-
Adenosine tri-phosphate
- BDL:
-
Bile duct ligation
- BET:
-
Betaine
- CN:
-
Cholemic nephropathy
- HPLC:
-
High-performance liquid chromatography
- TBARS:
-
Thiobarbituric acid reactive substances
References
Ahmadian E, Babaei H, Mohajjel Nayebi A, Eftekhari A, Eghbal MA (2017) Mechanistic approach for toxic effects of bupropion in primary rat hepatocytes. Drug Res 67:217–222. https://doi.org/10.1055/s-0042-123034
Alía M, Horcajo C, Bravo L, Goya L (2003) Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 23:1251–1267. https://doi.org/10.1016/S0271-5317(03)00131-3
Aniort J, Poyet A, Kemeny J-L, Philipponnet C, Heng A-E (2017) Bile Cast Nephropathy Caused by Obstructive Cholestasis. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 69:143–146. https://doi.org/10.1053/j.ajkd.2016.08.023
Betjes MGH, Bajema I (2006) The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. Journal of Nephrology 19:229–233
Bingül İ, Aydın AF, Başaran-Küçükgergin C, Doğan-Ekici I, Çoban J, Doğru-Abbasoğlu S, Uysal M (2016) High-fat diet plus carbon tetrachloride-induced liver fibrosis is alleviated by betaine treatment in rats. Int Immunopharmacol 39:199–207. https://doi.org/10.1016/j.intimp.2016.07.028
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physio-Cell Physiol 287:C817–C833. https://doi.org/10.1152/ajpcell.00139.2004
Caro AA, Adlong LW, Crocker SJ, Gardner MW, Luikart EF, Gron LU (2012) Effect of garlic-derived organosulfur compounds on mitochondrial function and integrity in isolated mouse liver mitochondria. Toxicol Lett 214:166–174. https://doi.org/10.1016/j.toxlet.2012.08.017
Chávez-Iñiguez JS, Meza-Ríos A, Santos-Garcia A, García-García G, Armendariz-Borunda J (2019) Cholemic nephropathy: Hyperbilirubinemia and its impact on renal function. J Ren Hepatic Disord 3:33–39. https://doi.org/10.15586/jrenhep.2019.52
Chen Y, Xing D, Wang W, Ding Y, Du L (2007) Development of an ion-pair HPLC method for investigation of energy charge changes in cerebral ischemia of mice and hypoxia of Neuro‐2a cell line. Biomed Chromatogr 21:628–634. https://doi.org/10.1002/bmc.798
Copple BL, Jaeschke H, Klaassen CD (2010) Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis 30:195–204. https://doi.org/10.1055/s-0030-1253228
Craig SAS (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549. https://doi.org/10.1093/ajcn/80.3.539
Day CR, Kempson SA (2016) Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta 1860:1098–1106. https://doi.org/10.1016/j.bbagen.2016.02.001
Duffjr MR, Howell EE (2015) Chemical interactions of betaine with folate and other biomolecules. Betaine: Chemistry, Analysis, Function and Effects:62. https://doi.org/10.1039/9781782628446-00062
Erlinger S (2014) Bile acids in cholestasis: bad for the liver, not so good for the kidney. Clin Res Hepatol Gastroenterol 38:392–394. https://doi.org/10.1016/j.clinre.2014.03.003
Erman F, Balkan J, Çevikbaş U, Koçak-Toker N, Uysal M (2004) Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids 27:199–205. https://doi.org/10.1007/s00726-004-0105-5
Fan C-Y, Wang M-X, Ge C-X, Wang X, Li J-M, Kong L-D (2014) Betaine supplementation protects against high-fructose-induced renal injury in rats. J Nutr Biochem 25:353–362. https://doi.org/10.1016/j.jnutbio.2013.11.010
Fang J et al (2019) Polydatin protects against acute cholestatic liver injury in mice via the inhibition of oxidative stress and endoplasmic reticulum stress. 55:175–183. https://doi.org/10.1016/j.jff.2019.02.029
Fernández-Vizarra E, Ferrín G, Pérez-Martos A, Fernández-Silva P, Zeviani M, Enríquez JA (2010) Isolation of mitochondria for biogenetical studies: An update. Mitochondrion 10:253–262. https://doi.org/10.1016/j.mito.2009.12.148
Fickert P et al (2013) Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology 58:2056–2069. https://doi.org/10.1002/hep.26599
Forton DM et al (2004) Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut 53:587–592. https://doi.org/10.1136/gut.2003.016766
Go EK, Jung KJ, Kim JY, Yu BP, Chung HY (2005) Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-κB via nuclear factor-inducing kinase/IκB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci 60:1252–1264. https://doi.org/10.1093/gerona/60.10.1252
Gossard AA, Talwalkar JA (2014) Cholestatic liver disease. Med Clin N Am 98:73–85. https://doi.org/10.1016/j.mcna.2013.09.002
Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Häussinger D (2002) Prevention of bile acid–induced apoptosis by betaine in rat liver. Hepatology 36:829–839. https://doi.org/10.1053/jhep.2002.35536
Grattagliano I, Oliveira PJ, Vergani L, Portincasa P (2017) Oxidative and nitrosative stress in chronic cholestasis. In: Liver Pathophysiology. Elsevier, Amsterdam, pp 225–237. https://doi.org/10.1016/B978-0-12-804274-8.00017-5
Gujral JS, Farhood A, Bajt ML, Jaeschke H (2003) Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct–ligated mice. Hepatology 38:355–363. https://doi.org/10.1053/jhep.2003.50341
Gupta R, Dubey DK, Kannan GM, Flora SJS (2007) Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int 31:44–56. https://doi.org/10.1016/j.cellbi.2006.09.007
Hagar H, Al Malki W (2014) Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol 37:803–811. https://doi.org/10.1016/j.etap.2014.02.013
Hagar H, El Medany A, Salam R, El Medany G, Nayal OA (2015) Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol 67:133–141. https://doi.org/10.1016/j.etp.2014.11.001
Hamdy N, El-Demerdash E (2012) New therapeutic aspect for carvedilol: Antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol 261:292–299. https://doi.org/10.1016/j.taap.2012.04.012
Heidari R et al (2018e) Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed Pharmacother 103:75–86. https://doi.org/10.1016/j.biopha.2018.04.010
Heidari R (2019) The footprints of mitochondrial impairment and cellular energy crisis in the pathogenesis of xenobiotics-induced nephrotoxicity, serum electrolytes imbalance, and Fanconi’s syndrome: A comprehensive review. Toxicology 423:1–31. https://doi.org/10.1016/j.tox.2019.05.002
Heidari R et al (2019) Mitochondrial dysfunction as a mechanism involved in the pathogenesis of cirrhosis-associated cholemic nephropathy. Biomed Pharmacother 109:271–280. https://doi.org/10.1016/j.biopha.2018.10.104
Heidari R, Niknahad H (2019) The role and study of mitochondrial impairment and oxidative stress in cholestasis. In: Vinken M (ed) Experimental Cholestasis Research. Methods in Molecular Biology. Springer New York, New York, pp 117–132. https://doi.org/10.1007/978-1-4939-9420-5_8
Heidari R, Moezi L, Asadi B, Ommati MM, Azarpira N (2017) Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis. PharmaNutrition 5:109–117. https://doi.org/10.1016/j.phanu.2017.07.001
Heidari R, Ahmadi A, Mohammadi H, Ommati MM, Azarpira N, Niknahad H (2018a) Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed Pharmacother 107:834–840. https://doi.org/10.1016/j.biopha.2018.08.050
Heidari R, Ghanbarinejad V, Mohammadi H, Ahmadi A, Esfandiari A, Azarpira N, Niknahad H (2018b) Dithiothreitol supplementation mitigates hepatic and renal injury in bile duct ligated mice: Potential application in the treatment of cholestasis-associated complications. Biomed Pharmacother 99:1022–1032. https://doi.org/10.1016/j.biopha.2018.01.018
Heidari R, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Niknahad H (2018c) Mitochondria protecting amino acids: Application against a wide range of mitochondria-linked complications. PharmaNutrition 6:180–190. https://doi.org/10.1016/j.phanu.2018.09.001
Heidari R, Jafari F, Khodaei F, Shirazi Yeganeh B, Niknahad H (2018d) Mechanism of valproic acid-induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. Nephrology 23:351–361. https://doi.org/10.1111/nep.13012
Holt S, Marley R, Fernando B, Harry D, Anand R, Goodier D, Moore K (1999) Acute cholestasis-induced renal failure: effects of antioxidants and ligands for the thromboxane A2 receptor. Kidney Int 55:271–277. https://doi.org/10.1046/j.1523-1755.1999.00252.x
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Journal of Botany. https://doi.org/10.1155/2012/872875
Ishida T, Kotani H, Miyao M, Kawai C, Jemail L, Abiru H, Tamaki K (2016) Renal impairment with sublethal tubular cell injury in a chronic liver disease mouse model. PloS One 11. https://doi.org/10.1371/journal.pone.0146871
Ji C, Kaplowitz N (2003) Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124:1488–1499. https://doi.org/10.1016/S0016-5085(03)00276-2
Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2,2’-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol Toxicol Pharmacol: CBP 140:47–52. https://doi.org/10.1016/j.cca.2005.01.005
Krones E et al (2017) NorUrsodeoxycholic acid ameliorates cholemic nephropathy in bile duct ligated mice. J Hepatol 67:110–119. https://doi.org/10.1016/j.jhep.2017.02.019
Krones E, Wagner M, Eller K, Rosenkranz AR, Trauner M, Fickert P (2015) Bile acid-induced cholemic nephropathy. Dig Dis 33:367–375. https://doi.org/10.1159/000371689
Krones E, Pollheimer MJ, Rosenkranz AR, Fickert P (2018) Cholemic nephropathy–historical notes and novel perspectives. . Biochim Biophys Acta (BBA) - Mol Basis Dis 1864:1356–1366. https://doi.org/10.1016/j.bbadis.2017.08.028
Krones E, Pollheimer MJ, Rosenkranz AR, Fickert P (2018) Cholemic nephropathy–historical notes and novel perspectives. . Biochim Biophys Acta (BBA) - Mol Basis Dis 1864:1356–1366. https://doi.org/10.1016/j.bbadis.2017.08.028
Lee I (2015) Betaine is a positive regulator of mitochondrial respiration. Biochem Biophys Res Commun 456:621–625. https://doi.org/10.1016/j.bbrc.2014.12.005
Mandorfer M, Hecking M (2019) The Renaissance of cholemic nephropathy: A likely underestimated cause of renal dysfunction in liver disease. Hepatology 69:1858–1860. https://doi.org/10.1002/hep.30558
Martínez-Cecilia D et al (2016) Oxidative stress influence on renal dysfunction in patients with obstructive jaundice: A case and control prospective study. Redox Biol 8:160–164. https://doi.org/10.1016/j.redox.2015.12.009
Meeks RG, Harrison S (1991) Hepatotoxicology. CRC Press, New York. https://doi.org/10.1002/hep.1840160532
Ommati MM et al (2019) Carnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanisms. Biol Trace Elem Res 187:151–162. https://doi.org/10.1007/s12011-018-1358-2
Ommati MM et al (2020) Betaine supplementation mitigates intestinal damage and decreases serum bacterial endotoxin in cirrhotic rats. PharmaNutrition 12:100179. https://doi.org/10.1016/j.phanu.2020.100179
Orellana M, Rodrigo R, Thielemann L, Guajardo V (2000) Bile duct ligation and oxidative stress in the rat: effects in liver and kidney. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 126:105–111. https://doi.org/10.1016/S0742-8413(00)00102-X
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922. https://doi.org/10.1007/s10495-007-0756-2
Panozzo MP, Basso D, Balint L, Zaninotto M, Bonvicini P, Plebani M (1995) Renal functional alterations in extrahepatic cholestasis: Can oxidative stress be involved? Eur Surg Res 27:332–339. https://doi.org/10.1159/000129417
Patil A, Mayo MJ (2008) Complications of cholestasis. In: Md KDL, Md JAT (eds) Cholestatic Liver Disease. Clinical Gastroenterology. Humana Press, Totowa, pp 155–169. https://doi.org/10.1007/978-1-59745-118-5_9
Preedy V (2015) Betaine: Chemistry, analysis, function and effects. Royal Society of Chemistry, London
Rasheed R, Ashraf MA, Hussain I, Haider MZ, Kanwal U, Iqbal M (2014) Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz J Bot 37:399–406. https://doi.org/10.1007/s40415-014-0089-7
Rodríguez-Garay EA (2003) Cholestasis: human disease and experimental animal models. Ann Hepatol 2:150–158. https://doi.org/10.1016/S1665-2681(19)32126-X
Ross AB, Zangger A, Guiraud SP (2014) Cereal foods are the major source of betaine in the Western diet–analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem 145:859–865. https://doi.org/10.1016/j.foodchem.2013.08.122
Sheen J-M, Huang L-T, Hsieh C-S, Chen C-C, Wang J-Y, Tain Y-L (2010) Bile duct ligation in developing rats: temporal progression of liver, kidney, and brain damage. J Pediatr Surg 45:1650–1658. https://doi.org/10.1016/j.jpedsurg.2009.12.019
Sidransky H, Farber E (1960) Liver choline oxidase activity in man and in several species of animals. Arch Biochem Biophys 87:129–133. https://doi.org/10.1016/0003-9861(60)90133-8
Smid V et al (2016) Changes in liver ganglioside metabolism in obstructive cholestasis-the role of oxidative stress. Folia Biol 62:148
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155:109–117. https://doi.org/10.1006/exnr.1998.6969
Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R (2015) Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Visualized Exp: JoVE. https://doi.org/10.3791/52438
Tag CG, Weiskirchen S, Hittatiya K, Tacke F, Tolba RH, Weiskirchen R (2015b) Induction of experimental obstructive cholestasis in mice. Lab Anim 49:70–80. https://doi.org/10.1177/0023677214567748
Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ (2006) Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38:733–744. https://doi.org/10.1080/03602530600959607
Woolbright BL, Jaeschke H (2012) Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol 18:4985–4993. https://doi.org/10.3748/wjg.v18.i36.4985
Wright G et al (2007) Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45:1517–1526. https://doi.org/10.1002/hep.21599
Yi E-Y, Kim Y-J (2012) Betaine inhibits in vitro and in vivo angiogenesis through suppression of the NF-κB and Akt signaling pathways. Int J Oncol 41:1879–1885. https://doi.org/10.3892/ijo.2012.1616
Zeisel SH, Mar M-H, Howe JC, Holden JM (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307. https://doi.org/10.1093/jn/133.5.1302
Zhou Y, Kempson SA, Danbolt NC (2015) CHAPTER 6: Betaine Uptake with a Focus on the Betaine/GABA Transporter. In: Betaine. pp 76–89. https://doi.org/10.1039/9781782628446-00076
Acknowledgements
This investigation was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences (Grant No.: 98-01-36-19445), Shiraz, Iran, by the last author (Dr. Reza Heidari); and Shanxi Agricultural University (SXAU, Grant No.: 2018YJ33 (National Natural Science Foundation) and K271999031 (Outstanding Doctors Volunteering to Work in Shanxi Province), Shanxi, China, by the first author (Dr. Mohammad Mehdi Ommati). Authors thank the Pharmaceutical Sciences Research Center (PSRC) of Shiraz University of Medical Sciences and Life Sciences Department of SXAU for providing technical facilities to carry out this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None to be declared.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ommati, M.M., Farshad, O., Azarpira, N. et al. Betaine alleviates cholestasis-associated renal injury by mitigating oxidative stress and enhancing mitochondrial function. Biologia 76, 351–365 (2021). https://doi.org/10.2478/s11756-020-00576-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00576-x