Abstract
Several studies have shown the toxic effect of zinc oxide nanoparticles (NZnO) on various cancer cells. This study aimed to assess the cellular death mechanism of the NZnO in MCF-7 human breast cancer cells. The cells were pretreated with zVAD (apoptosis inhibitor), 3-MA (an autophagy inhibitor), and Nec (a necroptosis inhibitor) for two hours, followed by their exposure to NZnO for 48 h. In the presence of Nec and 3-MA, NZnO significantly reduced proliferation and viability of MCF-7 cells while the apoptotic index and Bax/Bcl-2 ratio was significantly increased, compared to the only NZnO-treated cells. In the only NZnO-treated cells expression of Caspases-3 and Caspase-8 were up-regulated. NZnO with Nec and 3-MA could significantly increase expression of Caspase-3 and Caspase-9, but not Caspase-8 in the MCF-7 cells. NZnO in presence of zVAD and 3-MA significantly increased expression of RIPK1, RIPK3, and MLKL genes, compared to the only NZnO-treated cells. Furthermore, viability and proliferation of MCF-7 cells in NZnO with the apoptosis and autophagy inhbitors were lower than the other experimental groups. NZnO with apoptosis and necroptosis inhibitors could significantly increase cell viability, cell proliferation, and relative expression of autophagy-related genes such as LC3-II, Beclin-1, and ATG5, compared to the only NZnO-treated. According to the results of this study, the high toxicity of NZnO toward breast cancer cells mainly depends on activation of necroptosis and suppression of autophagy processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most diagnosed cancers worldwide. Radiotherapy and chemotherapy have several side effects on healthy cells, and the cancer cells are often drug resistant (Haggar and Boushey 2009; Hayat et al. 2007). Previous studies showed the toxicity of nanomaterials against human cancer cells (Bae et al. 2011; Khan et al. 2012; Ahamed et al. 2011). Among the various nanomaterials, special attention has been paid to zinc oxide nanoparticles (NZnO) due to their specific applications in cosmetic products, sunscreen, sensors, solar cells and antibacterial agents (Kołodziejczak-Radzimska and Jesionowski 2014; Sirelkhatim et al. 2015). Zinc as a trace element extensively exists in all tissues, such as the skin, muscle, brain, and bone (Jiang and Pi 2018). NZnO is generally considered safe material by the FDA (Food and Drug Administration). However, high doses of NZnO can induce cytotoxicity in rodents.
NZnO-induced cancer cell death and the mechanism of this process has remained unsolved.
There are three major cell death pathways including apoptosis, autophagy, and necrosis. Other cell death models such as necroptosis, Caspase-independent apoptosis, pyroptosis, paraptosis have also been described (Ricci and Zong 2006).
Several studies have shown the apoptotic effect of NZnO on various cancer cell lines (Wahab et al. 2014; Bisht and Rayamajhi 2016) while little research has been done on its autophagic or necroptotic functions.
Apoptosis is morphologically characterized by chromatin condensation, nuclear fragmentation, cell shrinkage and formation of apoptotic bodies (Eisenberg-Lerner et al. 2009). In addition, apoptosis is regulated in part by Bcl-2 (B cell lymphoma 2) genes that promote cell survival and pro-apoptotic protein such as Bax (Bcl-2–associated X protein). The activation of apoptosis partially depends on the balance between the expression of Bax and Bcl-2 (Cory and Adams 2002).
In autophagy, the nucleus is maintained intact, and autophagy vacuoles (autophagosomes) accumulate inside the cytoplasm. In the process of autophagosome formation, microtubule-associated protein-1 light chain 3 B (LC3B-I) converts to a lipidated form, termed LC3B-II, by a complex of ATG5 (Autophagy related 5), ATG12, and ATG16 proteins. The autophagosomes are degenerated by fusing with lysosomes. Autophagy enhances cell survival by removing damaged organelles and aggregated proteins. However, autophagic cell death can be induced by the excessive accumulation of autophagosomes (Reggiori and Klionsky 2005; Ye et al. 2012; Kalla et al. 2014). On the other hand, mTOR (mammalian target of rapamycin) regulates autophagy by activating several autophagy-related proteins, including Beclin-1, ATG1, ATG5, and ATG7 (He and Klionsky 2009; Marquez and Xu 2012).
Necroptosis, a programmed form of necrosis, is mediated by RIPK3 (receptor interacting protein kinase-3), RIPK1 (receptor interacting protein kinase-1) and MLKL (mixed lineage kinase domain-like pseudokinase) genes. The necroptosis process is often inhibited in the cancerous cells (Li et al. 2012; Su et al. 2015). The expression of necroptotic genes markedly reduces in breast cancer cells, compared to normal cells (Moriwaki et al. 2015).
The major problem of cancer therapy is the ability of cancer cells to evade apoptosis, which results in treatment resistance. Therefore, developing new therapeutic agents to stimulate other cell death pathways is of paramount importance (Cekay et al. 2017; Chen et al. 2014). This study aimed to investigate the impact of NZnO on different cell death mechanisms, including apoptosis, necroptosis, and autophagy in MCF-7 cell line.
Materials and methods
Chemicals
Dulbecco’s Modified Eagle’s medium (DMEM) purchased from Invitrogen company (USA). Anti-LC3-II and FITC-conjugated anti-mouse secondary antibodies were received from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Moreover, RNeasy Mini kit, cDNA synthesis kit, SYBR Green Master Mix, and all primers purchased from Qiagen Company (Qiagen, USA). All other materials acquired from the Sigma Aldrich (Sigma, St Louis, MO, USA).
Experimental design
The human MCF-7 (human breast cancer cells) and MCF-10A (a non-tumorigenic human mammary epithelial cell line) cell lines were purchased from National Center for Genetic and Biological Reserves in Iran and cultured in DMEM/F12 medium supplemented with 10% FBS, streptomycin (100 U/mL) and penicillin (100 mg/mL). The cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C and categorized into 5 groups as follows:
-
I.
Control: received only media
-
II.
Without inhibitors: exposed to 10 µmol/ mL NZnO.
-
III.
zVAD (N-benzyloxycarbonyl-Valyl-Alanyl-Aspartyl-fluoromethylketone, an apoptosis inhibitor) + 3-MA (3-Methyladenine, an autophagy inhibitor): exposed to 10 μmol/mL zVAD plus 10 μmol/mL 3-MA 2 h prior to treatment of NZnO.
-
IV.
Nec (Necrostatin, a necroptosis inhibitor) + 3-MA: exposed to 3 μmol/mL Nec plus 10 μmol/mL 3-MA 2 h prior to treatment of NZnO.
-
V.
zVAD + Nec: exposed to 10 μmol/mL zVAD plus 3 μmol/mL Nec 2 h prior to treatment of NZnO.
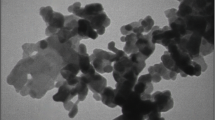
The NZnO was purchased from the Sigma Company and characterized before use. A dynamic light scattering approach of Zetasizer-Nano-ZSP (Malvern, UK) was used to analyze the mean particle size distribution, polydispersity index (PDI) and zeta potential. In addition, morphology and size of the NZnO were evaluated using an atomic force microscope (AFM). The most nanoparticles had a spherical morphology (Fig. 1), and the mean particle size was below 70 nm (Table 1). The zeta potential value was high enough to make the nanoparticles repel each other, and prevent particle aggregation. The PDI value showed an excellent homogeneous NZnO size distribution.
The dose and exposure time of NZnO were selected based on 50% inhibitory concentration (IC50) value (Table 2). NZnO was dissolved in 1% Dimethyl sulfoxide (DMSO) and then diluted in culture medium. The prepared NZnO was sonicated just before use. To evaluate the safety of the DMSO the MCF-7 and MCF-10A cells were treated with NZnO (10 μmol/mL) or 1% DMSO for 48 h, and then MTT assay was performed. DMSO had no significant effect on the viability of the MCF-7 and MCF-10A cells (Table 3).
Cell viability
Cytotoxicity of NZnO was evaluated applying the MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide) [Sigma, USA] assay. Briefly, MCF-7 and MCF-10A (1 × 104 cells/well) cells were cultured in 96-well plates. After treatment, MTT solution at a concentration of 5 mg/mL was added to each well, followed by the incubation of the plate at 37 °C for 4 h. After removing the supernatants, 100 μL of DMSO was added to each well. Using a microplate reader (BioRad, CA), absorbance at 570 nm was measured. The results of the MTT assay were expressed as the percentage of corresponding mean values in control cells. To determine the toxic dose of NZnO on MCF-7 cells, IC50 values were measured by MTT assay, as previously described (Park et al. 2012).
Colony formation assay
The anti-proliferative effect of NZnO was assessed using a colony formation assay. Briefly, 5000 cells were seeded into 6-well plates and treated with NZnO for 48 h in the presence or absence of the apoptosis, autophagy and necroptosis inhibitors. Afterward, the cells were washed and further incubated with complete medium (DMEM + 10% FBS + 1% pen/strep) for 10 days. Following that, the cells were stained with 0.1% crystal violet in phosphate-buffered saline (PBS), and the colonies were counted under a light microscope (Olympus, Japan) [Franken et al. 2006].
DAPI staining
We seeded 500 cells into 6-well culture dishes. After treatment, the cells were fixed in 4 % paraformaldehyde for five minutes at room temperature, then washed with PBS and incubated with DAPI (4′, 6-diamidino-2-phenylindole) solution (2 mg/ mL in PBS) for 10 min. In this method, the cells with an apoptotic condensed chromatin show more brightly stained nuclei. Fluorescence images were captured using a fluorescence microscope (Olympus, Japan). The nucleus with chromatin condensation was considered as apoptotic cells and the percentage of apoptotic cells (apoptotic index) was calculated (Chazotte 2010; Cummings et al. 2004; Mikes et al. 2009; Qi et al. 2019).
Annexin V-FITC/Propidium iodide apoptosis assay
MCF-7 cells were placed in a six-well culture plate and treated with NZnO in the presence or absence of the inhibitors for 48 h as the experimental groups. On the other hand, the control group encompassed untreated cells. An Annexin V-FITC/propidium iodide (PI) assay kit (V13242, Invitrogen, USA) was applied to determine the normal, apoptotic and necrotic cells. The samples were analyzed under a fluorescence microscope (Olympus, Japan). In this process, FITC and PI-negative, FITC-positive and PI-negative, FITC-positive and PI-positive, and FITC-negative and PI-positive were considered viable, early apoptotic, late apoptotic and necrotic cells, respectively. The percentage of each feature was calculated.
Quantitative real-time RT-PCR
Using the RNeasy Mini kit, RNA was isolated from the harvested cells according to the manufacturer’s instructions. In addition, cDNA was produced from the extracted RNAs applying the cDNA synthesis kit based on the manufacturer’s instructions. Approximately two μL of cDNA was amplified in each 25 μL PCR reaction mix containing 12.5 μL of 2x SYBR Green Master Mix, and 0.2 μL of each 10 pmol forward and reverse primers. The sequences of primers are provided in Table 4. PCR amplification was done in 40 cycles using the following program: 95 °C for 10 s (initial denaturation), 95 °C for 15 s (denaturation), and 60 °C for 20 s (annealing and extension). Data analysis was carried out using the 2-ΔΔCT method.
Immunocytochemistry
After treatment, the MCF-7 cells were fixed for 15 min in 4 % paraformaldehyde at 4 °C and washed in PBS. Anti-LC3-II antibody (sc-16755, Santa Cruz, USA) at 1/100 dilution was added to cells and incubated overnight at 4 °C. After washing, FITC-conjugated anti-mouse secondary antibody (sc-2356, Santa Cruz, USA) was exploited to incubate the cells for 50 min at room temperature. Using a fluorescence microscope, fluorescence images were captured (Olympus, Japan), where LC3-II positive cells showed a light green appearance.
Statistical analysis
Data analysis was performed in SPSS (version 21.0, Chicago, IL, USA) using one-way analysis of variance (ANOVA), followed by post-hoc pairwise comparison applying the Bonferroni procedure. Furthermore, the P value of less than 0.05 was considered statistically significant.
Results
Cell viability and colony numbers
According to the results, viability percentage and colony numbers were significantly decreased in the MCF-7 cells treated with NZnO compared to the control group (P = 0.005). Viability percentage and colony numbers of MCF-7 cells were significantly reduced in NZnO with autophagy and apoptosis inhibitors, compared to control (p = 0.000) and NZnO groups (p = 0.003). There was a significant increase in the viability percentage and colony numbers of NZnO with 3-MA and Nec-treated cells, compared with cells only treated with NZnO (p = 0.034). NZnO with apoptosis and necroptosis inhibitors induced a significant increase in the viability percentage and colony numbers, compared to the only NZnO-treated cells (p = 0.046) [Figs. 2 and 3]. NZnO had no significant impact on the viability and colony numbers of the MCF-10A cells (Table 5).
Morphology and percentage of viability of the MCF-7 cells (scale bars: 500 μm). All assays were performed in triplicate, and the mean ± standard deviations are shown (n = 6). *p < 0.05, ** p < 0.01, *** p < 0.001, # p < 0.01, ## p < 0.001; * and # symbols indicate comparison to the control and NZnO without inhibitors groups, respectively. zVAD: apoptosis inhibitor; 3-MA: autophagy inhibitor; Nec: necroptosis inhibitor
DAPI staining
In the only NZnO-treated cells, a significant increase in the apoptotic index in comparison with the control group was observed. Compared to the control group, the apoptotic index was significantly higher in the NZnO with autophagy and apoptosis inhibitors (3-MA and zVAD) group. However, comparison of the apoptotic index with only NZnO-treated cells showed a significant reduction in this regard (p < 0.006). Furthermore, the apoptotic index was significantly elevated in the NZnO with 3-MA and Nec group, compared with the control (p = 0.000) and the only NZnO-treated groups (p = 0.008). NZnO with apoptosis and necroptosis inhibitors (zVAD and Nec) caused a significant increase in the apoptosis index compared to the control group (p = 0.042). Nevertheless, the apoptotic index was significantly decreased compared to the only NZnO-treated cells (p = 0.003) [Fig. 4]. The apoptotic index of MCF-10A cells was not affected by the NZnO with or without inhibitors (Table 5).
Annexin V-FITC/Propidium iodide apoptosis assay
A small number of apoptotic and necrotic cells were observed in the control group. In addition, NZnO significantly increased the percentage of early apoptosis, late apoptosis and necrosis in the MCF-7 cells, compared to the control group (p = 0.000). NZnO with zVAD and Nec (apoptosis and necroptosis inhibitors) significantly decreased late and early apoptosis, compared to the absence of the inhibitors (p = 0.000). NZnO in the presence of apoptosis and autophagy inhibitors significantly decreased late and early apoptosis (p = 0.000), whereas the necrosis percentage was significantly increased (p < 0.028), compared to the absence of these inhibitors. In addition, NZnO caused a significant increase in the late and early apoptosis in the presence of 3-MA and Nec (autophagy and necroptosis inhibitors), compared to the without these inhibitors. In all Nec-treated cells, necrosis was significantly reduced, compared to the without this inhibitor (Fig. 5). NZnO at the concentration of 10 μmol/mL with or without inhibitors had no significant impact on apoptosis and necrosis percentages of the MCF-10A cells (Table 5).
Immunoflorecent microscopy of Annexin/PI staining (scale bars: 5 μm). EA: early apoptosis (Cell membrane is strongly stained with FITC), LA: late apoptosis (Cell membrane is strongly stained with FITC and Nucleus is stained by PI), N: Necrosis (Nucleus have red stain), L: Live (Cells have slightly green stain). Values are expressed as mean ± SD (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001; * and # symbols indicate comparison to control and NZnO without inhibitors groups, respectively
Quantitative real-time RT-PCR
Compared to the only NZnO-treated cells, there was a significant increase in the expression of Bax/Bcl-2 ratio, Caspase-3 and Caspase-9 in the treatment of NZnO with Nec and 3-MA (p = 0.007). Expression of Bax/Bcl-2 ratio, Caspase-3, and Caspase-9 were significantly decreased (p < 0.008), in the presence of zVAD and Nec, compared with the absence of mentioned inhibitors (Fig. 6).
In the NZnO with zVAD and 3-MA group, there was a significant increase in the expression of RIPK1, RIPK3 and MLKL genes compared to the only NZnO-treated cells (p < 0.003). In the 3-MA-treated cells, low expression of Beclin-1 and ATG5 genes was observed. According to the results, the expression of Beclin-1 and ATG5 genes was significantly increased in the NZnO with zVAD and Nec group (p < 0.006), whereas the expression of mTOR was significantly decreased (p < 0.004), compared with the only NZnO-treated cells (Fig. 6). NZnO with or without inhibitors had no significant impact on gene expression in the MCF-10A cells (Table 6).
Immunocytochemistry
According to the results, there was a significant reduction in the percentage of LC3-II positive cells in the only NZnO-treated cells (p < 0.006). In addition, 3-MA-treated cells contained no autophagosome. In the NZnO with Nec and zVAD, the percentage of LC3-II positive cells was significantly reduced, compared to the control group (p < 0.002). Meanwhile, the percentage of LC3-II immunostained cells was significantly increased, compared to the only NZnO-treated cells (p < 0.007) [Fig. 7]. NZnO in the MCF-10A cells had no significant impact on the percentage of LC3-II positive cells (Table 5).
Immunoflorecent microscopy of the MCF-7 cells and percentage of LC3-II positive cells. Bright green staining indicates LC3-II positive cells (scale bars: 5 μm). Values are expressed as mean ± SD. *p < 0.05, **p < 0.01, #p < 0.01; * and # symbols indicate comparison to the control and NZnO without inhibitors groups, respectively
Discussion
The current research demonstrated the cytotoxicity of NZnO in MCF-7 cells. Previous studies showed that NZnO has cytotoxic impacts on MCF-7, A549 (Lung Cancer cell) and ovarian cancer cells (Selvakumari et al. 2015; Bai et al. 2017). NZnO in combination with other anticancer agent have also cytotoxicity activity against cancer cells (Malaikozhundan et al. 2017; Iswarya et al. 2017). In the sudy of Malaikozhundan et al. Pongamia pinnata coated NZnO had anticancer activity against MCF-7 cells.
Owing to their very small size, nanoparticles can enter into the cells and interact with intracellular biomolecules. In addition, nanoparticles can agglomerate in the cytoplasm and destroy cell organelles and DNA. Furthermore, they are able to stimulate various cell death signaling pathways (Chang et al. 2006; Limbach et al. 2005).
In the present study, we have demonstrated that NZnO is effective in reducing cell numbers of MCF-7 cells through growth suppression and inducing cell death. NZnO could obviously inhibit the proliferation of fibrosarcoma HT1080 cells (Arakha et al. 2017).
Cell survival and apoptosis are often applied to examine the efficacy of anti-cancer agents. Anticancer drugs mostly kill the dividing cells by the activation of the apoptosis process (Sun et al. 2014). In the present study, inhibitors of necroptosis and autophagy were used to evaluate the role of apoptosis in NZnO-induced cytotoxicity. When necroptosis and autophagy were inhibited, NZnO significantly reduced the proliferation and viability of MCF-7 cells. As shown by DAPI staining and flow cytometry, apoptosis was elevated in NZnO-treated cells in the presence of these inhibitors. Therefore, necroptosis or autophagy may partially suppress apoptosis induced by NZnO. The increased expression of Bax/Bcl-2 ratio confirmed the induction of apoptosis by NZnO in MCF-7 cells.
Generally, Bcl-2 is expressed in various cancer cells and induces resistance to radiotherapy and chemotherapy. It has been demonstrated that inhibiting Bcl-2 expression increases the efficacy of drug treatment by inducing apoptosis (Williams and Cook 2015; Hata et al. 2015). Expression of Bax is closely related to the development of various malignant tumors, which is significantly reduced in some of them (Katkoori et al. 2010).
In a previous study, NZnO could induce apoptosis in MCF-7 cells by downregulation antiapoptotic genes and upregulation of apoptotic-related genes such as Bax (Moghaddam et al. 2017). In research by Wahab et al. (2014), it was demonstrated that NZnO induced apoptosis by the relative upregulation of the Bax, p53, and Caspase-3 genes in the MCF-7 and HepG2 cancer cells. Upregulation of Caspase-3 and Caspase-9 indicated that intrinsic apoptotic pathway is involved in the NZnO-induced cytotoxicity in the MCF-7 cells. In agreement with this finding, the intrinsic apoptotic pathway was involved in apoptosis induced by NZnO in the MCF-7 cells in the study of Kavithaa et al. (2016).
In the current study, the zVAD, as a Caspase inhibitor, did not fully block early and late apoptosis in MCF-7 cells. As a result, NZnO can induce apoptosis in MCF-7 cells by both Caspase-dependent and Caspase-independent pathways. Kadhem et al. (2019) reported that NZnO induces apoptosis via Caspase-8 in human breast cancer cells. Chung et al. (2015) showed that NZnO has Caspase-mediated apoptotic impacts on Liver cancer cells.
It is well known that when apoptosis inhibits, necroptosis pathway is activated. When necroptosis inhibited, NZnO significantly increased the viability and proliferation of MCF-7 cells. Therefore, necroptosis may have more inhibitory effects on viability and proliferation of MCF-7 cells compared the apoptosis. In the present study, NZnO up-regulated RIPK1 in the MCF-7 cells. RIPK1 expression is reduced in colon cancer (Moriwaki et al. 2015). Conversely, the expression of RIPK1 elevated in glioblastoma tissues and lung cancer (Park et al. 2009; Wang et al. 2013) The different pattern of RIPK1 expression in the various cancers is a consequence of its multifunctional roles in apoptosis or survival of the cancer cells (Moriwaki et al. 2015).
As mentioned in results, Nec, as a RIPK1 inhibitor, considerably induced apoptosis in the NZnO-treated cells. It has been reported that Nec is a Caspase activator, and hence may enhance apoptotic impacts of NZnO on the MCF-7 cells. In agreement with our results, Han et al. (2012) reported that Nec-1 enhances apoptosis in Leukemia Cells. In a previous study, necroptosis was induced by Shikonin with a significant rise in the expression of RIPK1 and RIPK3 (Shahsavari et al. 2018).
According to the results of this study, there was a high expression level of RIPK3 and MLKL genes in NZnO-treated cells. Koo et al. (2015) found that down-regulation of RIP3 was related to cancer development in breast cancer patients. Anthracyclines and oxaliplatin could active necroptosis in cancer cell lines via RIP3 and MLKL upregulation (Yang et al. 2016). Low expression of MLKL is associated with poor prognosis in various cancers such as ovarian, gastric and pancreatic cancers (Ruan et al. 2015; He et al. 2013; Ertao et al. 2016; Colbert et al. 2013).
Resibufogenin could inhibit the growth of colorectal cancer cells through RIP3-mediated necroptosis (Han et al. 2018). Expression of RIP3 is commonly silenced in cancers, making most cancer cells unable to undergo necroptosis (Koo et al. 2015)
The significant increase in the expression of necroptotic-related genes of MCF-7 cells exposed to zVAD indicated that NZnO may reinforce the necroptotic cell death in the presence of Caspase inhibitors. High expression levels of RIPK1 and RIPK3 were reported in MCF-7 cells exposed to Shikonin plus zVAD (Shahsavari et al. 2018).
The data obtained from this study clearly demonstrated that NZnO activated both apoptosis and necroptosis processes. Several recent studies have shown that apoptosis and necroptosis can be induced at the same time in the cancer cells (Polito et al. 2013; Lin et al. 2016). Flowcytometry results were also showed that NZnO could effectively induce both necrosis and apoptosis at the same time. Apoptosis and necrosis are mediated by distinct but overlapping pathways involving mitochondria / endoplasmic reticulum (Whelan et al. 2012). In this regard, Liu et al. (2013) showed that gold nanoparticles induced apoptosis and necrosis in lung cancer cells. Melatonin was involved in the necrosis and apoptosis of the pancreatic cancer cell line (SW-1990) by modulating the Bax / Bcl-2 ratio (Roya et al. 2014). Whelan et al. (2012) found that deletion of Bax significantly reduces necrotic injury during myocardial infarction. Therefore, NZnO may stimulate both apoptotic and necrotic pathways by modulating of Bax / Bcl-2 ratio in the MCF-7 cells.
The MCF-7 cells were treated by zVAD and Nec to determine the role of autophagy. The increasing viability and proliferation of MCF-7 cells in the presence of these inhibitors in comparison with the presence of 3-MA (autophagy inhibitor) indicated autophagy is a protective mechanism for MCF-7 cells and promotes cell survival. To confirm the role of autophagy, LC3-II was detected using immunocytochemistry and the mRNA expression of ATG5, mTOR, and Beclin-1 was also evaluated. Interestingly, there was no significant increase in the expression of LC3-II in the presence of apoptosis and necroptosis inhibitors. Moreover, the downregulation of Beclin-1 and ATG-5, and the upregulation of mTOR genes indicated that NZnO can potentially suppress autophagy process.
In a previous study, NZnO decreased the number of autophagosomes and autophagy marker proteins, such as LC3-II, Beclin 1, and ATG5. Meanwhile, mTOR significantly increased in the NZnO-exposed macrophages (Zhang et al. 2017). In addition, Wang et al. demonstrated that NZnO impaired autophagy in A549 lung epithelial cells. Interestingly, we found that the ratio of Bax/Bcl-2 expression significantly increased in the presence of autophagy inhibitor. In research by Kanematsu et al. (2010), it has been shown that autophagy inhibition enhanced sulforaphane-induced apoptosis in human breast cancer cells. According to Yao et al. (2012) and Huang et al. (2014), inhibition of autophagy by 3-MA led to increased cell death in human breast cancer cells. In contrast, Bai et al. (2017) showed that NZnO induced both apoptosis and autophagy in human ovarian cancer cells.
In this study, 3-MA could also effectively stimulate necroptotic cell death in MCF-7 cells. In agreement with this finding, Echeverry et al. (2015) showed that autophagy prevention enhanced necroptotic cell death in malignant pleural mesothelioma cells.
The results of the current study collectively have indicates that NZnO activates the two major cell death pathways including apoptosis and necroptosis in the MCF-7 cells. Other cell death pathways were not assessed in this study. It is possibility that NZnO stimulate other pathways of cell death in MCF-7 cells. Song et al. (2013) have reported that NZnO induces Pyroptosis (a type of cell death) in lung cancer cells (A549 cells).
In this study, the healthy MCF-10A cells were not affected by the NZnO. Thus, NZnO has high potential as adjuvant therapy for clinical application in breast cancer. In the previous studies, NZnO induced apoptosis and reduced survival of various cancer cells (Wang et al. 2017; Taccola et al. 2011), while it had not any cytotoxic impacts on non-cancerous cells (Taccola et al. 2011; Chandrasekaran and Pandurangan 2016) NZnO has more toxicity against C2C12 myoblastoma cancer cells in comparison with non-tumoral 3 T3-L1 adipocytes (Chandrasekaran and Pandurangan 2016).
Conclusions
The present study demonstrated that NZnO (10 μmol/ mL) effectively suppressed the viability and proliferation of MCF-7 cells by activation of necroptosis and apoptosis, and suppression of autophagy signaling pathways. Furthermore, necroptosis had more preventive effects on the growth of the MCF-7 cells, compared to apoptosis. In our knowledge, this is the first report on the necroptotic effects of NZnO on cancer cells. The present study provides potential strategies for developing treatments against apoptosis-resistant cancers. It is recommended that future studies be conducted to increase our knowledge about other cell death signaling pathways in NZnO-treated cancer cells.
Abbreviations
- 3-MA:
-
3-Methyladenine
- ATG5:
-
(Autophagy related 5)
- AFM:
-
Atomic force microscope
- Bax:
-
Bcl-2–associated X protein
- Bcl-2:
-
B cell lymphoma 2
- DAPI:
-
4′, 6-diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s Modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- LC3-II:
-
light chain 3-II
- MLKL:
-
mixed lineage kinase domain-like pseudokinase
- MTT:
-
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide
- Nec:
-
Necrostatin
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PI:
-
Propidium iodide
- RIPK1:
-
receptor interacting protein kinase-1
- RIPK3:
-
receptor interacting protein kinase-3
- zVAD:
-
N-benzyloxycarbonyl-Valyl-Alanyl-Aspartyl-fluoromethylketone; Z-VAD.fmk
References
Ahamed M, Akhtar MJ, Raja M, Ahmad I, Siddiqui MKJ, Al-Salhi MS, Alrokayan SA (2011) ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomed Nanotechnol Biol Med 7:904–913. https://doi.org/10.1016/j.nano.2011.04.011
Arakha M, Roy J, Nayak PS, Mallick B, Jha S (2017) Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy-mediated apoptotic cell death. Free Radic Biol Med 110:42–53. https://doi.org/10.1016/j.freeradbiomed.2017.05.015
Bae KH, Chung HJ, Park TG (2011) Nanomaterials for cancer therapy and imaging. Mol Cells 31:295–302. https://doi.org/10.1007/s10059-011-0051-5
Bai DP, Zhang XF, Zhang GL, Huang YF, Gurunathan S (2017) Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomedicine 12:6521–6535. https://doi.org/10.2147/IJN.S140071
Bisht G, Rayamajhi S (2016) ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine 3:9. https://doi.org/10.5772/63437
Cekay MJ, Roesler S, Frank T, Knuth AK, Fulda S (2017) Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines. Cancer Lett 410:228–237. https://doi.org/10.1016/j.canlet.2017.09.002
Chandrasekaran M, Pandurangan M (2016) In vitro selective anti-proliferative effect of zinc oxide nanoparticles against co-cultured C2C12 myoblastoma cancer and 3T3-L1 normal cells. Biol Trace Elem Res 172:148–154. https://doi.org/10.1007/s12011-015-0562-6
Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R (2006) Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small 12:1412–1417. https://doi.org/10.1002/smll.200600218
Chazotte B (2010) Labeling nuclear DNA using DAPI. In: Yuste (ed) Imaging: a laboratory manual. CSHL Press, Cold Spring Harbor
Chen Q et al (2014) Apoptosis, necrosis, and autophagy in mouse intestinal damage after 15-Gy whole body irradiation. Cell Biochem Funct 32:647–656. https://doi.org/10.1002/cbf.3068
Chung IM, Rahuman AA, Marimuthu S, Kirthi AV, Anbarasan K, Rajakumar G (2015) An investigation of the cytotoxicity and caspase-mediated apoptotic effect of green synthesized zinc oxide nanoparticles using Eclipta prostrata on human liver carcinoma cells. Nanomaterials (Basel) 5:1317–1330. https://doi.org/10.3390/nano5031317
Colbert LE, Fisher SB, Hall WA, Saka B, Shelton JW et al (2013) Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer 119:3148–3155. https://doi.org/10.1002/cncr.28144
Cory S, Adams M (2002) The Bcl 2 family: regulators of the cellular life or death switch. Nat Rev Cancer 2:647–656. https://doi.org/10.1038/nrc883
Cummings BS, Wills LP, Schnellmann RG (2004) Measurement of cell death in mammalian cells. Curr Protoc Pharmacol 25:12.8.1–12.8.22. https://doi.org/10.1002/0471141755.ph1208s25
Echeverry N, Barbone D, Weder W, Stahel RA, Broaddus VC, Felley-Bosco E (2015) Inhibition of autophagy sensitizes malignant pleural mesothelioma cells to dual PI3K/mTOR inhibitors. Cell Death Dis 6:1757. https://doi.org/10.1038/cddis.2015.124
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16:966–975. https://doi.org/10.1038/cdd.2009.33
Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui W, Chuangqi C et al (2016) Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric cancer. Tumour Biol 37:13679–13685. https://doi.org/10.1007/s13277-016-5229-1
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319. https://doi.org/10.1038/nprot.2006.339
Haggar FA, Boushey RP (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22:191–197. https://doi.org/10.1055/s-0029-1242458
Han W, Xie J, Fang Y, Wang Z, Pan H (2012) Nec-1 enhances Shikonin-induced apoptosis in leukemia cells by inhibition of RIP-1 and ERK1/2. Int J Mol Sci 13:7212–7225. https://doi.org/10.3390/ijms13067212
Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y et al (2018) Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J Transl Med 16:201. https://doi.org/10.1186/s12967-018-1580-x
Hata AN, Engelman JA, Faber AC (2015) The BCL-2 family: key mediators of the apoptotic response to targeted anti-cancer therapeutics. Cancer Discov 5:475–487. https://doi.org/10.1158/2159-8290.CD-15-0011
Hayat MJ, Howlader N, Reichman ME, Edwards BK (2007) Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist 12:20–37. https://doi.org/10.1634/theoncologist.12-1-20
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:–93. https://doi.org/10.1146/annurev-genet-102808-114910
He L, Peng K, Liu Y, Xiong J, Zhu FF (2013) Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther 6:1539–1543. https://doi.org/10.1186/s12885-018-4655-4
Huang YH, Yang PM, Chuah QY, Lee YJ, Hsieh YF, Peng CW, Chiu SJ (2014) Autophagy promotes radiation-induced senescence but inhibits bystander effects in human breast cancer cells. Autophagy 10:1212–1228. https://doi.org/10.4161/auto.28772
Iswarya A, Vaseeharan B, Anjugam M, Ashokkumar B, Govindarajan M, Alharbi NS et al (2017) Multipurpose efficacy of ZnO nanoparticles coated by the crustacean immune molecule β-1, 3-glucan binding protein: toxicity on HepG2 liver cancer cells and bacterial pathogens. Colloids Surf B Biointerfaces 158:257–269. https://doi.org/10.1016/j.colsurfb.2017.06.035
Jiang J, Pi J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl 2018:1062562. https://doi.org/10.1155/2018/1062562
Kadhem HA, Ibraheem SA, Jabir MS, Kadhim AK, Taqi ZJ, Florin MD (2019) Zinc oxide nanoparticles induces apoptosis in human breast Cancer cells via Caspase-8 and P53 pathway. Nano Biomed Eng 11:35–43. https://doi.org/10.5101/nbe.v11i1
Kalla PK, Chitti S, Aghamirzaei ST, Senthilkumar R, Arjunan S (2014) Anti-cancer activity of silymarin on MCF-7 and NCIH-23 cell lines. Adv Biol Res 8:57–61. https://doi.org/10.5829/idosi.abr.2014.8.2.82286
Kanematsu S, Uehara N, Miki H, Yoshizawa K, Kawanaka A, Yur T, Tsubura A (2010) Autophagy inhibition enhances Sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res 30:3381–3390
Katkoori VR et al (2010) Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J Gastrointest Oncol 1:76–89. https://doi.org/10.3978/j.issn.2078-6891.2010.019.V.R
Kavithaa K, Paulpandi M, Ponraj P, Murugan K, Sumathi S (2016) Induction of intrinsic apoptotic pathway in human breast cancer (MCF-7) cells through facile biosynthesized zinc oxide nanorods. Karbala International Journal of Modern Science 2:46–55. https://doi.org/10.1016/j.kijoms.2016.01.002
Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33:1477–1488. https://doi.org/10.1016/j.biomaterials.2011.10.080
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide-from synthesis to application: a review. Materials 7:2833–2881. https://doi.org/10.3390/ma7042833
Koo GB, Morgan MJ, Lee DJ, Kim WJ, Yoon JH, Koo JS et al (2015) Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 25:707–725
Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS et al (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350. https://doi.org/10.1016/j.cell.2012.06.019
Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ (2005) Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations. Environ Sci Technol 39:9370–9376
Lin YC, Chang TW, Hsieh WH, Hung MC, Lin IH, Lai SC, Tzeng YJ (2016) Simultaneous induction of apoptosis and necroptosis by Tanshinone IIA in human hepatocellular carcinoma HepG2 cells. Cell Death Discov 2:16065. https://doi.org/10.1038/cddiscovery.2016.65
Liu M et al (2013) Gold nanoparticles trigger apoptosis and necrosis in lung cancer cells with low intracellular glutathione. J Nanopart Res 15:1745. https://doi.org/10.1007/s11051-013-1745-8
Malaikozhundan B, Vaseeharan B, Vijayakumar S, Pandiselvi K, Kalanjiam MA, Murugan K et al (2017) Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathog 104:268–277. https://doi.org/10.1016/j.micpath.2017.01.029
Marquez RT, Xu L (2012) Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2:214–221
Mikes J, Koval’ J, Jendzelovský R, Sacková V, Uhrinová I, Kello M, Kuliková L, Fedorocko P (2009) The role of p53 in the efficiency of photodynamic therapy with hypericin and subsequent long-term survival of colon cancer cells. Photochem Photobiol Sci 8:1558–1567. https://doi.org/10.1039/b9pp00021f
Moghaddam AB, Moniri M, Azizi S, Abdul Rahim R, Ariff AB, Navaderi M, Mohamad R (2017) Eco-friendly formulated zinc oxide nanoparticles: induction of cell cycle arrest and apoptosis in the MCF-7 cancer cell line. Genes (Basel) 8:10. https://doi.org/10.3390/genes8100281
Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FKM (2015) Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis 6:16. https://doi.org/10.1038/cddis.2015.16
Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM et al (2009) The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer Res 69:2809–2816. https://doi.org/10.1158/0008-5472.CAN-08-4079
Park UH, Jeong JC, Jang JS, Sung MR, Youn H, Lee SJ, Kim EJ, Um SJ (2012) Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol Pharm Bull 35:1525–1533. https://doi.org/10.1248/bpb.b12-00254
Polito L, Bortolotti M, Farini V, Marzano C, Björnstedt M, Gandin V et al (2013) Saporin induces multiple death pathways in lymphoma cells with different intensity and timing as compared to ricin. Biochim Biophys Acta 1833:3448–3459. https://doi.org/10.1016/j.biocel.2008.09.021
Qi Y, Ding Z, Yao Y, Ma D, Ren F, Yang H, Chen A (2019) Novel triazole analogs of apigenin-7methyl ether exhibit potent antitumor activityagainst ovarian carcinoma cells via the induction of mitochondrial-mediatedapoptosis. Exp Ther Med 17:1670–1676. https://doi.org/10.3892/etm.2018.7138
Reggiori F, Klionsky DJ (2005) Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol 17:415–422. https://doi.org/10.1016/j.ceb.2005.06.007
Ricci MS, Zong WX (2006) Chemotherapeutic approaches for targeting cell death pathways. Oncologist 11:342–357. https://doi.org/10.1634/theoncologist.11-4-342
Roya R, Singh SK, Chauhand LKS, Dasa M, Tripathia A, Dwivedi PD (2014) Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett 227:29–40. https://doi.org/10.1016/j.toxlet.2014.02.024
Ruan J, Mei L, Zhu Q, Shi G, Wang H (2015) Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol 8:15035–15038
Selvakumari D, Deepa R, Mahalakshmi V, Subhashini P, Lakshminarayan N (2015) Anticancer activity of ZnO nanoparticles on MCF7 (breast cancer cell) and A549 (lung cancer cell). ARPN J Eng Appl Sci 10:5418–5421
Shahsavari Z, Karami-Tehrani F, Salami S (2018) Targeting cell necroptosis and apoptosis induced by shikonin via receptor interacting protein kinases in estrogen receptor positive breast cancer cell line, MCF-7. Anti Cancer Agents Med Chem 18:245–254. https://doi.org/10.2174/1871520617666170919164055
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Habsah H, Dasmawati M (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242. https://doi.org/10.1007/s40820-015-0040-x
Song J, Du L, Feng Y, Wu W, Yan Z (2013) Pyroptosis induced by zinc oxide nanoparticles in A549 cells. Wei Sheng Yan Jiu 42(2):273–276 [Article in Chinese]
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 14:48. https://doi.org/10.1186/s12943-015-0321-5
Sun M, Nie S, Pan X, Zhang R, Fan Z, Wang S (2014) Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces 113:15–24. https://doi.org/10.1016/j.colsurfb.2013.08.032
Taccola L, Raffa V, Riggio C, Vittorio O, Iorio MC, Vanacore R, Pietrabissa A, Cuschieri A (2011) Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine 6:1129–1140. https://doi.org/10.2147/IJN.S16581
Wahab R, Siddiqui MA, Saquib Q, Dwivedi S, Ahmad J, Musarrat J, Al-Khedhairy AA, Shin HS (2014) ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf B Biointerfaces 117:267–276. https://doi.org/10.1016/j.colsurfb.2014.02.038
Wang Q, Chen W, Xu X, Li B, He W, Padilla MT et al (2013) RIP1 potentiates BPDE-induced transformation in human bronchial epithelial cells through catalase-mediated suppression of excessive reactive oxygen species. Carcinogenesis 34:2119–2128. https://doi.org/10.1093/carcin/bgt143
Wang B, Zhang J, Chen C, Xu G, Qin X, Hong Y, Bose DD, Qiu F, Zou Z (2017) The size of zinc oxide nanoparticles controls its toxicity through impairing autophagic flux in A549 lung epithelial cells. Toxicol Lett 28:51–59. https://doi.org/10.1016/j.toxlet.2017.12.025
Whelan RS et al (2012) Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A 109:6566–6571. https://doi.org/10.1073/pnas.1201608109
Williams MM, Cook RS (2015) Bcl-2 family proteins in breast development and cancer: could Mcl-1 targeting overcome therapeutic resistance? Oncotarget 6:3519–3530. https://doi.org/10.18632/oncotarget.2792
Yang H, Ma Y, Chen G, Zhou H, Yamazaki T, Klein C et al (2016) Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology 5:e1149673
Yao F, Wang G, Wei W, Tu Y, Tong H, Sun S (2012) An autophagy inhibitor enhances the inhibition of cell proliferation induced by a proteasome inhibitor in MCF-7 cells. Mol Med Rep 5:84–88. https://doi.org/10.3892/mmr.2011.590
Ye LH, Li WJ, Jiang XQ, Chen YL, Tao SX, Qian WL et al (2012) Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat Rec 295:417–422. https://doi.org/10.1002/ar.21528
Zhang J, Qin X, Wang B, Xu G, Qin Z, Wang J et al (2017) Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis 8:e2954. https://doi.org/10.1038/cddis.2017.337
Acknowledgments
This article was supported by a grant (CMRC-9414) from the research council of Ahvaz Jundishapur University of Medical sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farasat, M., Niazvand, F. & Khorsandi, L. Zinc oxide nanoparticles induce necroptosis and inhibit autophagy in MCF-7 human breast cancer cells. Biologia 75, 161–174 (2020). https://doi.org/10.2478/s11756-019-00325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00325-9