Abstract
We investigated growth of carbon nanotubes (CNTs) by catalytic chemical vapor deposition (CVD), using catalysts of iron combined with heavy refractory metals including hafnium, tantalum, and ruthenium to increase the ultimate length to which the CNTs grow. The refractories act as diffusion inhibitors, slowing erosion, and deactivation of catalyst nanoparticles, resulting in the growth of longer CNTs than grow from pure iron catalyst. Inclusion of hafnium or tantalum prolongs the CNT growth time; however, both metals decrease the growth rate of CNTs vs pure iron. CNTs grown from Fe/Hf catalysts reach greater ultimate length than CNTs grown from pure Fe despite the slower growth rate, while ultimate CNT length from Fe/Ta catalysts is substantially less than given by pure Fe. Fe/Ru catalyst behaves interestingly, showing faster growth rate, shorter growth time, and greater ultimate length than CNTs grown from Fe. For all catalysts, the CNTs produced were primarily double-walled (DWCNTs).
Graphical Abstract

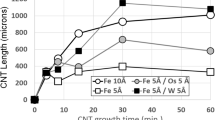
CNT growth curves for pure iron catalyst and for iron combined with diffusion inhibitors
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the discovery of carbon nanotubes (CNTs) [1], there has been growing interest in their use in many applications [2]. Taking full advantage of CNTs’ properties will require large-scale production of CNTs with lengths of tens of centimeters to meters or more. CNTs are typically grown using catalytic chemical vapor deposition (CVD): carbonaceous gases like methane are flowed at elevated temperature over nanometer-sized particles of catalytic metals, such as iron or cobalt: the gases decompose upon the particles to give carbon atoms, which can dissolve into the metal particles and precipitate out on the particle surface as a graphitic carbon cap. This cap will, if the particle’s diameter is appropriate, lift off, and grow a CNT, with new carbon continuously added at the CNT/particle interface [2]. Interest in maximizing CNT length has resulted in much research on methods to increase this length [3,4,5]. Causes of CNT growth cessation often involve the instability of the catalyst particles, which can change and deactivate over time by mechanisms including Ostwald ripening, coalescence, and diffusion of catalyst into the supporting substrate [6,7,8,9,10,11,12,13,14]. Thus, much recent work has focused on increasing the lifetime and stability of these particles [4, 5, 15,16,17,18,19,20,21,22,23,24].
One method to stabilize catalyst particles involves combining the catalysts with heavy refractory metals with higher melting points. Li and co-workers used this method to produce single-walled CNTs (SWCNTs) with narrow, reproducible distributions of chiralities by combining cobalt with tungsten [20, 21]. Amama and co-workers achieved similar results using cobalt combined with ruthenium [22]. Our group has used combination of catalyst with heavy refractories to produce stabilized catalyst particles for growth of longer CNTs [25,26,27]. These particles can give both increased CNT growth lifetime and greater CNT length, compared to pure catalyst metals. Inclusion of rhenium stabilizer with molybdenum [25] or iron [26] catalyst increased CNT growth time and ultimate CNT length, using CO as carbon source gas. Both tungsten (W, MP: 3422 °C) and osmium (Os, MP: 3033 °C) inclusion with iron were found to increase CNT growth time, with the Fe/W combination giving greater ultimate CNT length vs pure Fe, when ethylene (C2H4) was used as carbon source. The interpretation was that the refractories act as “diffusion inhibitors” (or “Ostwald ripening inhibitors”), binding catalyst atoms strongly enough that the latter cannot detach from the nanoparticle and diffuse across the substrate and thus slowing the Ostwald ripening that would eventually deactivate the particles. This results in longer particle lifetime, longer CNT growth time, and greater ultimate CNT length. Note that, in this context, catalyst particle lifetime is the same as CNT growth time, since the particle is still considered “live” for as long as the CNT is growing.
Here, we investigate Hafnium (Hf, MP: 2233 °C), Tantalum (Ta, MP: 3017 °C), and Ruthenium (Ru MP: 2334 °C) combined with iron catalyst (Fe, MP: 1538 °C) to grow CNTs using C2H4 as carbon source. We find that both Fe/Hf and Fe/Ta catalysts increase CNT growth time compared to pure Fe, but decrease growth rate. For Fe/Hf catalysts, the ultimate CNT length is greater than for pure Fe, while for Fe/Ta, the growth rate reduction is such that ultimate CNT length is less. Fe/Ru catalyst behaves interestingly: growth rate is increased over pure Fe, while the growth time is approximately the same as for Fe alone.
Experimental
The processes of supported metal catalyst preparation and CNT growth for these studies have been described previously [25,26,27]. Substrates were silicon wafers topped with 1000-nm thermal oxide (SiO2) and 15 nm evaporatively deposited Al2O3. Onto these substrates were deposited metal catalysts of controlled composition and thickness using spin-on toluene solutions with controlled concentrations of organometallic compounds containing the metal atoms. The substrates were then plasma ashed, leaving thin films of oxides of the metals, the films’ thickness being determined by the concentration of organometallics in the spin-on solution.
CNT’s were grown on catalyst-coated substrates in a tube furnace CVD reactor containing a 1-inch-diameter quartz tube through which reactive gas mixtures were flowed at elevated temperatures. Our CNT growth process used a mixture of Ar, C2H4, and H2, with flows of 100, 25, and 25 sccm, respectively. This mixture was flowed through the reactor tube at 1 atmosphere pressure at 750 °C, which resulted in growth of dense mats of CNTs growing upward from the substrate surface. CNT length was determined by measuring the thickness of these CNT mats using Scanning Electron Microscopy (SEM). We grew CNT mats from pure Fe catalyst and from mixed-metal catalyst films consisting of equal parts (same equivalent thicknesses) of Fe and refractory metal, with total thickness of 1.0 nm. These mixed-metal films contained metal amounts equivalent to 0.5-nm Fe and 0.5-nm diffusion inhibitor, although our preparation procedure insured that the metal atoms were intermixed at the atomic level.
Results
We have found previously [27] that, for our CVD system, the global maximum of CNT length achieved with Fe catalyst is given using Fe film thickness of 1.0 nm and temperature of 750 °C and so these conditions were used here. Under these conditions, CNTs grew from both pure Fe catalyst and from combinations of iron with heavy diffusion inhibitors. We tested a variety of high-melting point metals as diffusion inhibitors, including Hf, Ta, and Ru, which gave robust, consistent growth when combined with Fe, and were investigated further. Additionally, we tested as potential diffusion inhibitors iridium, niobium, molybdenum, and rhenium. These metals, combined with Fe, gave either no CNT growth (Mo), extremely non-reproducible and inconsistent growth (Re), or consistent growth of very short CNTs (ultimate length ≤ 3 μm, Ir and Nb). Note that combining Re with Fe gave good, consistent CNT growth previously with CO as carbon source gas [26], but not so here with C2H4.
CNT lengths were measured versus growth time for Fe/Hf, Fe/Ta, and Fe/Ru catalyst mixtures, with catalyst and diffusion inhibitor each present at equivalent thickness of 0.5 nm. These combinations are denoted below as Fe5Hf5, Fe5Ta5, and Fe5Ru5. We compared these results to CNT growth from pure Fe films with thickness of 1.0 nm (same total metal thickness) and 0.5 nm (same amount of catalytic Fe metal), which have also been investigated previously [27]. These films are denoted below as Fe10 and Fe5.
CNT growth gave dense CNT mats for the catalysts Fe5Hf5, Fe5Ta5, Fe5Ru5, Fe10, and Fe5, as described above. The time dependence of the CNT growth was determined using a series of CNT growth experiments, with time allowed for growth varied between 4 and 60 min. The observed CNT length-versus-time curves were qualitatively similar to previously observed curves: the CNTs grow initially at a constant rate, with growth slowing and eventually stopping over time. No growth was observed when using metal films of pure Hf, Ta, or Ru, indicating that, under the conditions we used, all catalytic activity for CNT growth from these mixed metals arises from iron. Note that it is possible that the pure refractory metals could yield CNT under different growth conditions.
Typical results are displayed in Fig. 1, which shows SEM images of CNT mats grown for increasing times from films of 0.5-nm Fe mixed with 0.5-nm Hf. Figure 2 shows CNT length vs growth time for all catalysts studied. Error bars in Fig. 2 show the standard error in measured CNT length from multiple growth runs (typically 2–4 for each point). For both pure Fe and Fe/inhibitor catalysts, TEM analysis showed that the CNTs were primarily double-walled carbon nanotubes (DWCNTs), with occasional nanotubes having three or more walls. Typical tube diameters were 5–10 nm. No significant differences were noted in wall number or diameter distribution between CNT grown from Fe vs Fe/inhibitor for any of the inhibitors studied. Figure 3 shows TEM images of representative nanotubes from each catalyst investigated.
Discussion
As previously reported [27], Fe10 and Fe5 catalyst films show qualitatively similar time-dependent CNT growth behavior. CNTs from Fe5 grow rapidly for a short time, reaching approximately 350–400 µm length in 4–5-min time, after which growth ceases. Fe10 catalysts CNT grow for a longer time, reaching approximately 800 µm after 15 min of growth. Both catalysts show some run-to-run variation in final CNT length for growth times exceeding the CNT growth lifetime; however, no systematic lengthening occurs after the above growth times.
This behavior changes if the catalyst includes refractory metal stabilizer. Consider first Fe5Hf5 catalyst. The CNT growth rate is less than that for either Fe5 or Fe10, with CNT length less than those given by the pure Fe catalysts for any time during which the latter are still growing. However, CNTs from Fe5Hf5 continue to grow for times substantially past the growth cessation times of Fe10 and Fe5, so that CNTs from Fe5Hf5 eventually grow longer than the ultimate lengths from pure Fe. Indeed, it appears that CNTs from Fe5Hf5 may still be lengthening even after growth time of 1 h, the maximum growth time investigated in this study, by which time these CNTS have reached approximately 1000 µm length. Addition of Hf thus produces two effects: it decreases the catalytic activity of the catalyst, hence the slower growth rate and increases the stability of the catalyst particles, hence the greater growth time. An analogous effect is seen if Ta is used as refractory stabilizer. Figure 2 shows that CNTs from Fe5Ta5 lengthen uniformly for approximately 30 min, after which growth ceases. However, the growth rate is substantially slower Fe5Ta5 for pure iron, so that the ultimate CNT length from Fe5Ta5 is only approximately 200 µm despite the increased growth time.
The CNT growth behavior of the Fe/Hf and Fe/Ta catalysts mirrors the behavior seen in our previous studies of W and Os diffusion inhibitors [27]. Combination of diffusion inhibitor with catalytic Fe decreases the catalytic activity of the catalyst, resulting in slower growth rate than given by pure Fe. However, all these stabilizers result in longer growth times, indicating that the catalyst particles are stabilized and retain their CNT growth-generating capability for increased times. Whether greater ultimate CNT length results depends on the balance between these two effects. For stabilizers like Hf and W [27], the decrease in CNT growth rate is more than compensated for by the increase in growth time, thus the CNTs are longer when growth ceases. On the other hand, inhibitors like Ta degrade the CNT growth rate to an extent that, even with the enhanced growth time, the final CNT length is still less than given by pure Fe catalyst. In our previous work, we saw that for Os diffusion inhibitor these two effects approximately cancel, so that the ultimate CNT length for Fe/Os was approximately the same as that given by pure Fe [27]. This combination of factors appears to be a general effect for Fe catalyst: the stabilizer results in longer-lived catalyst particles and longer growth times, at the price of decreased catalytic activity and slower growth rate. Whether ultimately longer CNTs are achieved depends on the trade-off between these two factors.
The case of Fe combined with Ru shows qualitatively different behaviors. Figure 2 shows that Fe5Ru5 catalyst gives increased initial CNT growth rate over pure Fe of either tested thickness. On the other hand, growth time is certainly no greater than and possibly less than that given by Fe10, although this time is still greater than for Fe5. This behavior is reversed from that seen for the other diffusion inhibitors. The ultimate length given by CNTs from Fe5Ru5 is approximately the same as that given by Fe10, approximately 800 µm. This pattern of behavior is difficult to explain with our diffusion inhibitor ideas, and it appears that a different type of chemical reactivity change is induced in the iron by ruthenium. Ruthenium is known as a strongly catalytic metal for many reactions, either on its own or in combination with metals like iron [28,29,30]. Instances are known for which the combination of Fe plus Ru will catalytically accelerate a chemical reaction to a greater extent than either metal does separately [28, 30]. Thus, it may simply be that the combination of Fe and Ru results in a higher catalytic activity than Fe alone for the conversion of C2H4 into solid carbon (as CNTs), giving faster CNT growth rate. Future studies will continue to explore the case of CNT growth using Fe/Ru catalysts.
Conclusion
These results provide additional evidence for and examples of the increase afforded to both CNT growth time and ultimate CNT length from inclusion of heavy refractory diffusion inhibitor metals with catalytic metals. We observe that addition of Hf to Fe catalyst gives both longer growth times and greater ultimate CNT length than given by Fe alone. Fe alone gives growth time of approximately 15 min, whereas Fe/Hf appears to give CNT growth time of at least 60 min, implying at least a factor of four increase in catalytic particle lifetime. The slower growth rate from Fe/Hf vs Fe dictates that the increase in CNT length is not this great, but comparison of the ultimate lengths observed (approximately 800 μm for Fe vs 1000 μm for Fe/Hf) implies an increase in CNT length by at least a factor of 1.25. Fe/Ta behaves qualitatively similarly, with growth time of approximately 30 min, a factor of 2 greater than pure Fe. However, the lower growth rate for Fe/Ta dictates that ultimate CNT length from Fe/Ta catalyst is still less than from Fe catalyst. Fe/Ru combined catalyst gives qualitatively different behaviors, with faster growth rate and no clear difference in growth time. Ultimate CNT length from Fe/Ru is approximately equal to that from pure Fe catalyst.
Future research will include varying catalyst/inhibitor ratio and total metal thickness of our metal catalysts, as well as CVD temperature and reactive gas mixture composition. Ultimately, it is hoped that this method will enable production of large-scale quantities of ultra-long CNTs for materials applications.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
S. Iijima, Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991)
R. Rao, C. L. Pint, A. E. Islam, R. S. Weatherup, S. Hofmann, E. R. Meshot, et al., Carbon nanotubes and related nanomaterials: critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano. 12, 11756–11784 (2018)
K. Hata, D.N. Futaba, K. Mizuno, T. Namai, M. Yumura, S. Iijima, Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science 306, 1362–1364 (2004)
W. Cho, M. Schulz, V. Shanov, Growth and characterization of vertically aligned centimeter long CNT arrays. Carbon 72, 264–273 (2014)
H. Sugime, T. Sato, R. Nakagawa, T. Hayashi, Y. Inoue, S. Noda, Ultra-long carbon nanotube forest via in situ supplements of iron and aluminum vapor sources. Carbon 172, 772–780 (2021)
P.B. Amama, C.L. Pint, L. McJilton, S.M. Kim, E.A. Stach, P.T. Murray, R.H. Hauge, B. Maruyama, Role of water in super growth of single-walled carbon nanotube carpets. Nano Lett. 9, 44–49 (2009)
S.M. Kim, C.L. Pint, P.B. Amama, D.N. Zakharov, R.H. Hauge, B. Maruyama et al., Evolution in catalyst morphology leads to carbon nanotube growth termination. J. Phys. Chem. Lett. 1, 918–922 (2010)
P.B. Amama, C.L. Pint, S.M. Kim, L. McJilton, K.G. Eyink, E.A. Stach, R.H. Hauge, B. Maruyama, Influence of alumina type on the evolution and activity of alumina-supported Fe catalysts in single-walled carbon nanotube carpet growth. ACS Nano 4, 895–904 (2010)
S. Sakurai, H. Nishino, D.N. Futaba, S. Yasuda, T. Yamada, A. Maigne, Y. Matsuo, E. Nakamura, M. Yumura, K. Hata, Role of subsurface diffusion and ostwald ripening in catalyst formation for single-walled carbon nanotube forest growth. J. Am. Chem. Soc. 134, 2148–2153 (2011)
F. Yang, X. Wang, D. Zhang, J. Yang, D. Luo, Z. Xu et al., Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 510, 522–524 (2014)
S. Jeong, J. Lee, H.-C. Kim, J.Y. Hwang, D.N. Zakharov, B. Maruyama, E.A. Stach, S.M. Kim, Direct observation of morphological evolution of a catalyst during carbon nanotube forest growth: new insights into growth and growth termination. Nanoscale 8, 2055–2062 (2016)
W. Shi, J. Li, E.S. Polsen, C.R. Oliver, Y. Zhao, E.R. Meshot, M. Barclay, D.H. Fairbrother, A.J. Hart, D.L. Plata, Oxygen-promoted catalyst sintering influences number density, alignment, and wall number of vertically aligned carbon nanotubes. Nanoscale 9, 5222–5233 (2017)
H.-H. Li, G.-J. Yuan, B. Shan, X.-X. Zhang, H.-P. Ma, Y.-Z. Tian, H.-L. Lu, J. Liu, Atomic layer deposition of buffer layers for the growth of vertically aligned carbon nanotube arrays. Nanoscale Res. Lett. 14, 119 (2019)
M. Liu, H. An, A. Kumamoto, T. Inoue, S. Chiashi, R. Xiang, S. Maruyama, Efficient growth of vertically-aligned single-walled carbon nanotubes combining two unfavorable synthesis conditions. Carbon 146, 413–419 (2019)
K. Hasegawa, S. Noda, Moderating carbon supply and suppressing Ostwald ripening of catalyst particles to produce 4.5-mm-tall single-walled carbon nanotube forests. Carbon 49, 4497–4504 (2011)
W. Cho, M. Schulz, V. Shanov, Growth termination mechanism of vertically aligned centimeter long carbon nanotube arrays. Carbon 69, 609–620 (2014)
E. Shawat, V. Mor, L. Oakes, Y. Fleger, C.L. Pint, G.D. Nessim, What is below the support layer affects carbon nanotube growth: an iron catalyst reservoir yields taller nanotube carpets. Nanoscale 6, 1545–1551 (2014)
N. Yang, M. Li, J. Patscheider, S. K. Youn, H. G. Park, A forest of Sub-1.5-nm-wide single-walled carbon nanotubes over an engineered alumina support. Nature Sci. Rep. 7, 7:46725 (2017). https://doi.org/10.1038/srep46725
O.T. Gul, Decoupling the catalyst reduction and annealing for suppressing Ostwald ripening in carbon nanotube growth. Appl. Phys. A 127, 762 (2021)
F. Yang, H. Zhao, X. Wang, X. Liu, Q. Liu, X. Liu, C. Jin, R. Wang, Y. Li, Atomic scale stability of tungsten–cobalt intermetallic nanocrystals in reactive environment at high temperature. J. Am. Chem. Soc. 141, 5871–5879 (2019)
F. Yang, X. Wang, D. Zhang, J. Yang, D. Luo, Z. Xu, J. Wei, J.-Q. Wang, Z. Xu, F. Peng, X. Li, R. Li, Y. Li, M. Li, X. Bai, F. Ding, Y. Li, Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 510, 522–524 (2014)
B.M. Everhart, R. Rao, P. Nikolaev, T.-W. Liu, D.A. Gómez-Gualdrón, B. Maruyama, P.B. Amama, High-throughput experimentation for selective growth of small-diameter single-wall carbon nanotubes using ru-promoted co catalysts. Chem. Mater. 34, 4548–4559 (2022)
M. Bedewy, E.R. Meshot, H. Guo, E.A. Verploegen, W. Lu, A.J. Hart, Collective mechanism for the evolution and self-termination of vertically aligned carbon nanotube growth. J. Phys. Chem. C 113, 20576–20582 (2009)
D.B. Geohegan, A.A. Puretzky, J.J. Jackson, C.M. Rouleau, G. Eres, K.L. More, Flux-dependent growth kinetics and diameter selectivity in single-wall carbon nanotube arrays. ACSNano 5(10), 8311–8321 (2011)
M.J. Bronikowski, Use of refractory-metal diffusion inhibitors to slow Ostwald ripening of catalytic metal particles: a route to ultra-long Carbon Nanotubes (CNTs). Carbon 107, 297–303 (2016)
M.J. Bronikowski, M. King, Rhenium and molybdenum as diffusion inhibitors in catalytic metal particles for growth of ultra-long carbon nanotubes (CNTs). MRS Adv. 5, 1697–1704 (2020)
M.J. Bronikowski, Growth of carbon nanotubes from ethylene using catalyst particles that incorporate diffusion inhibitor metals. MRS Adv. 7, 180–184 (2022)
S. Li, S. Krishnamoorthy, A. Li, G.D. Meitzner, E. Iglesia, Promoted iron-based catalysts for the Fischer-Tropsch synthesis: design, synthesis, site densities, and catalytic properties. J. Catalysis 206, 202–217 (2002)
N. Popovska, K. Danova, I. Jipa, U. Zenneck, Catalytic growth of carbon nanotubes on zeolite supported iron, ruthenium and iron/ruthenium nanoparticles by chemical vapor deposition in a fluidized bed reactor. Powder Technol. 207, 17–25 (2011)
V. Kelsen, A. Meffre, P.-F. Fazzini, P. Lecante, B. Chaudret, How to modulate catalytic properties in nanosystems: the case of iron–ruthenium nanoparticles. ChemCatChem 6, 1714–1720 (2014)
Acknowledgments
This research was funded by the University of Tampa under the Research Innovation and Scholarly Excellence (RISE) Grant Program. We thank N. G. Rudawski (College of Engineering, University of Florida) and M. G. Boebinger (Center for Nanophase Materials Science, Oak Ridge National Laboratory) for TEM imaging.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
WP contributed to Conceptualization, Visualization, Investigation, Methodology, Research, Validation, Formal analysis, and Writing-review. MJB contributed to Conceptualization, Visualization, Investigation, Methodology, Research, Validation, Formal analysis, Writing-original draft, Writing-review, Project Administration, and Supervision.
Corresponding author
Ethics declarations
Conflict of interests
The authors state that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, W., Bronikowski, M.J. Investigation of hafnium, tantalum, and ruthenium as catalyst stabilizers in the iron-catalyzed growth of carbon nanotubes from ethylene. MRS Advances (2024). https://doi.org/10.1557/s43580-024-00926-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43580-024-00926-w