Abstract
Polylactic acid (PLA) microspheres are primarily used in drug delivery applications and rarely manufactured commercially. In encapsulation, the drug encapsulation and microsphere synthesis process happen simultaneously. Simultaneous synthesis necessitates conditions that ensure the drugs to be encapsulated remain viable in the synthesis process. In the present work, solid pure PLA microspheres are prepared using a repeatable centrifugal mixing method to create a single emulsion system. A planetary thinky mixer is used to achieve centrifugal mixing. PLA acts as the dispersed phase of the emulsion, whereas Polyvinyl alcohol (emulsifier) dissolved in deionized water serves as the bulk phase. The emulsions are stirred for about 4 min at 2000 rpm. Microspheres prepared by mechanical mixing are compared with microspheres prepared by centrifugal mixing. The microspheres prepared by centrifugal mixing proved to be narrower and closer to a normal distribution with a mean size (Dx 50) of 30 microns. The size distribution of planetary mixed microspheres did not change from one batch to another.
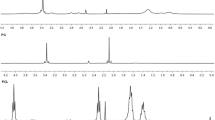
Graphical Abstract
SEM image of PLA Microspheres from emulsion-based processing and size distribution

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polylactic acid (PLA), a material of interest in this research, is a biodegradable and biocompatible polyester [1, 2]. PLA has garnered significant attention in recent decades, particularly in biomedical applications [3], pharmaceutical applications, and drug delivery applications [4,5,6], owing to its biodegradability and biocompatibility [1]. PLA, being a thermoplastic, is also used in packaging for bottling, plastic films, shrink wrapping, etc. [7]. PLA can be mass-produced on a large scale and is commercially available in a wide range of molecular weights [1]. This aliphatic polyester can be synthesized using different precursors, but the ring-opening polymerization of lactide is prominent among these.
PLA has two enantiomers, namely poly (1-lactic) acid (PLLA) and poly (d-lactic) acid (PDLA), derived from the optical monomers l-lactide and d-lactide, respectively. Another version of the polymer, poly (d, l-lactic) acid (PDLLA), also exists and is entirely amorphous, unlike PDLA and PLLA, which are semi-crystalline and have similar physical and chemical properties [8, 9]. The polymer usually consists of a mixture of poly-d-lactide/poly-l-lactide and poly-meso-lactide isomers in most cases [10]. The isomeric composition of the polymer provides variability in the crystallinity of the polymer [1]. Depending on the intended application, PLA can be manufactured as soft, elastic polymers or high-strength polymers. PLA remains thermally crystallizable in an amorphous state, exhibiting slow crystallization kinetics [1].
Table S-1(Supplementary Material) shows PLA’s typical thermal transition temperatures [1]. The thermal properties shown in the table indicate that PLA remains solid at room temperature and is desired for many targeted applications. PLA has a relatively low thermal decomposition temperature, which can be explored in the generation of porosity in thermally stable materials (e.g., silicone) by calcination. PLA can also be degraded by hydrolysis or transesterification, owing to the presence of carboxylic and ester groups, respectively [1]. The presence of the carboxylic and ester groups also suggests that PLA could either surface modified or conjoined chemically with other polymer chains to either alter the surface properties of pure PLA (such as degree of hydrophobicity) [11, 12] or to enhance its thermal and chemical properties [2, 13]. PLA is hydrophobic and can be copolymerized with hydrophilic polymers to form amphiphiles, which can be further converted to polymersomes [14], and microspheres [15]. In many drug delivery applications, micro- and nanoparticles of PLA and copolymers of PLA (common amongst them being PLA-PEG (polyethylene glycol), PLA-PEG-PLA, PLA-PLGA (polylactic-co-glycolic acid), PLA-PVA (polyvinyl alcohol), among others) are used for encapsulation [2, 3, 16]. Due to its moldability, PLA can be fabricated as films, scaffolds, micelles, and micro- and nanoparticles [9].
Polylactic acid can dissolve in various solvents; these include chlorinated and fluorinated organic compounds, tetrahydrofuran (THF), acetone, dimethylformamide (DMF), benzene, pyrrolidine, benzylamine [17]. The choice of solvent for PLA may depend on the polymer’s molecular weight, stereochemistry, and percent crystallinity. Amorphous PLA is easier to dissolve than crystalline PLA [17]. The flexibility of PLA makes it an attractive candidate material for further research for several applications, including additive manufacturing and porous material formation [18].
Polymeric microspheres are prevalent in several applications. They are used as absorbents, latex diagnostics, affinity bio-separators, pore formers, and drug and enzyme applications [6, 19]. The choice of polymer for microsphere formation is dependent on its intended purpose. In biologically related applications, for instance, the polymers must have suitable biocompatibility, bioavailability, biodegradability, and non-immunogenicity [16]. In industrial packaging applications, properties such as gas permeability are desirable [7, 20]. Ideally, the polymers must be soluble and miscible in various solvents. In drug delivery applications, the drug to be encapsulated within the core of a polymer and the polymer itself must be soluble in the same precursor solvent; hence, compatibility with more than a few solvents becomes a prime factor for consideration [8, 21]. Polymer microspheres must also possess the desirable thermal, mechanical, and chemical properties for the application. Among the homopolymers studied, polylactic acid (PLA) exhibits properties that make them preferable across several applications.
PLA and PLA block copolymer microspheres are synthesized primarily for drug delivery applications. The microsphere synthesis and the drug encapsulation process happen simultaneously, so PLA microspheres are generally not produced commercially. The properties of PLA aforementioned make the polymer a great choice pore former candidate in the formation of evenly distributed micropores in any polymer matrix. As such, there is a need for readily available PLA microspheres for related applications. This article proposes a simple and effective approach for preparing PLA microspheres.
Methodology and experiments
The starting solution for synthesizing PLA microspheres is prepared by dissolving about 40 g of the PLA pellets in 800 mL of dichloromethane (DCM) to form an oil-in-water (O/W) single emulsion. The oil-in-water emulsion was prepared by dispersing 5 mL of the starting solution for every 200 mL DCM. The dispersion of the oil phase in the water phase was conducted by (1) using a magnetic stir plate, (2) using an overhead mixer, as well as (3) stirring with a planetary (Thinky) mixer. In each case, the microspheres were formed at room temperature. The microspheres prepared by mixing with the magnetic plate were stirred at 1200 revolutions per minute (rpm) for 60 min.
The microspheres prepared by mixing with the overhead mixer were stirred at the highest setting on the Hamilton Beach overhead mixer for 4 min per every 200 mL emulsion system. The microspheres prepared using the planetary vacuum mixer were prepared by mixing at 2000 rpm for 4 min under no vacuum. After dispersion, the microspheres prepared are obtained by eliminating the organic phase. The organic phase elimination is done by pouring the 200 mL emulsion into a 1 L beaker filled with distilled water (shown in Figure S-1: Supplementary Material) and chilling for 2 h. (In the absence of distilled water, deionized water can be used). The cold jar of water extracts the solvent from the emulsion whiles solidifying the liquid microsphere droplets formed. The microspheres are then obtained by vacuum filtration. After washing with deionized water, the microspheres are dried in a fume hood.
Results and discussions
Figure 1 shows the Scanning Electron Microscope (SEM) images of the emulsion microspheres prepared with the planetary mixer (a), a stir plate (b), and an overhead mixer (c). SEM characterization confirms that microspheres were formed in the various emulsion processes. Microspheres are formed in each single emulsion process with the morphology and size of the particles shown in Fig. 1.
Figure 2 shows the particle size distribution data for the microspheres prepared in the planetary (Thinky) mixer (a), particles prepared on the stir plate (b), and with an overhead mixer (c). Among the three mixing conditions, the planetary mixer produced the smallest spheres, recording sizes as small as 15 microns. Planetary mixers operate at relatively high speeds (up to 2000 rpm) without added shear from a mixing blade that may deform the formed particles, which is the case for overhead mixers (see SEM images in Figure 1). Higher mix speeds generally correlate to smaller particle sizes and narrow size distributions. Emulsions by magnetic stirring can generate relatively small particles but may not be as efficient in generating a narrow distribution and do not constitute a high amount of shear mixing. However, when the phases are uniform and have a low viscosity, uniform particles with a narrow size distribution can be generated with the stir plate method. 87% of the microspheres formed by the planetary (thinky) mixer fall between 15 and 50 microns, compared with 70% for the overhead mixer and 50% for the stir plate within the same size range. Planetary mixing proved to be the most efficient of the three emulsion methods discussed for emulsion preparation, given that it can be carried out quickly, mixed with little to no filming/crystallization of the PLA, while producing microspheres with a relatively narrow size distribution. The collected PLA microspheres represent up to a 95% conversion rate of our starting PLA material. While the conversion rate is reasonable, lab-scale synthesis has quantity limitations, given the amount of water needed to disperse even a small amount of PLA/DCM from the starting solution.
Figure 3 compares microspheres prepared on a stir plate, mixed for 10, 30, and 60 minutes, and stirred at 1200 rpm. The particle size distribution indicates that the mixing speed and size distribution are not strongly correlated for the stir plate. Each mixing speed shows similar distributions across the particle size range shown. However, more particles are seen in the lower micron size range at longer mix times.
The Hamilton Beach mixer used had three-speed settings. Microspheres were prepared with the highest mixing speeds, introducing the most shear into the emulsion. When the emulsion is stirred for 4 min, the formed microspheres deform under the continuous shear applied. Mixing at the highest speed setting on the overhead mixer (3600 rpm) for 4 min was the optimum mixing criteria for the overhead mixer.
Figure S-2 (Supplementary Material) compares three particle size measurements of a sample of solid PLA microspheres prepared using a Planetary Mixer obtained using a Malvern Mastersizer. The data shows that the microspheres have a mean size of 30.8 ± 0.205 microns. The processing parameters chosen for this mixing method (4-minute mix speed at 2000 rpm under vacuum in the planetary mixer) produced microspheres closest to a normal distribution in the desired size range. These settings were found to be optimal for the planetary mixer.
Figure 4 compares the particle size distribution of the microspheres from three methods of preparing single emulsions. The stir plate requires considerable mixing time, while the overhead and Thinky mixer had shorter mixing times. The planetary mixer appears to be the best method for creating micron-sized spherical particles in a repeatable, efficient way with a high conversion rate.
Table S-2 (Supplementary Material) compares all three mixing methods, their process times, particle size range, and particle size distributions. We deduce from the above results that planetary mixing is the most efficient way to prepare smaller-sized, monodisperse PLA microspheres. Table S-2 and the particle size distribution graphs in Figure 4 further reveal that tunable parameters directly impact the sizes and size distribution of the microspheres for each of these processes. Mixing speed, time duration of mixing, the concentration of the starting solution, and even the concentration of the precursor are all parameters that can be tuned to obtain a desired and narrow size distribution of microspheres. The narrow size distribution might take longer for mixing methods such as the stir plate, but it is feasible.
Conclusions
The paper presented the emulsion-based synthesis and characterization of PLA microspheres. The chosen mixing mechanism during synthesis depends mainly on the mixing time, desired particle size, and size distribution. Higher mix speeds generally correlate to smaller particle sizes and narrow size distributions. High shear mixing leads to the formation of microspheres quickly within a few min. Magnetic stirrers can generate relatively small particles but may not be as efficient in generating a narrow size distribution and do not constitute a high amount of shear mixing. Overhead mixers provide high shear and mix speeds, but too high speeds may destroy the morphology of the formed particles in the emulsion.
In comparison, planetary mixers can form PLA microspheres within a short time with a narrow size distribution. They can operate at high speeds without added shear that may deform the formed particles. The planetary mixer is the ideal choice of the three methods used for PLA synthesis through emulsion preparation. It can be carried out quickly, mixed with little to no filming/crystallization of the PLA, and can produce microspheres with a narrow size distribution. The only downside to this method, along with the other methods described in this chapter, is that each method is a batch process. A straightforward, scalable, continuous approach for narrowly distributed microsphere production is required and will be the focus of future work.
Data availability
Data will be made available on reasonable request and upon clearance for availability by KCNSC.
References
L. Avérous, Chapter 21 - Polylactic Acid: Synthesis, Properties and Applications, in Monomers, Polymers and Composites from Renewable Resources. ed. by M.N. Belgacem, A. Gandini (Elsevier, Amsterdam, 2008), pp.433–450
J.-M. Raquez et al., Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 38(10), 1504–1542 (2013)
M. Tobío et al., Stealth PLA-PEG Nanoparticles as protein carriers for nasal administration. Pharm. Res. 15(2), 270–275 (1998)
H. Wang et al., Preparation and degradability of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid)/SiO2 hybrid material. J. Appl. Polym. Sci. 110(6), 3985–3989 (2008)
G. Gorrasi, R. Pantani, Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 98(5), 1006–1014 (2013)
S.M. Davachi, B. Kaffashi, Polylactic acid in medicine. Polym.-Plast. Technol. Eng. 54(9), 944–967 (2015)
L. Bao et al., Gas permeation properties of poly(lactic acid) revisited. J. Membr. Sci. 285(1–2), 166–172 (2006)
M. Brzeziński et al., Stereocomplexed PLA microspheres: Control over morphology, drug encapsulation and anticancer activity. Colloids Surf. B 184, 110544 (2019)
B. Tyler et al., Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 107, 163–175 (2016)
M. Brzeziński, T. Biela, Stereocomplexed Polylactides, in Encyclopedia of Polymeric Nanomaterials. ed. by S. Kobayashi, K. Müllen (Springer, Heidelberg, 2015), pp.2274–2281
C.Y. Tham, Z.A.A. Hamid, Z.A. Ahmad, H. Ismail, Surface engineered poly(lactic acid) (PLA) microspheres by chemical treatment for drug delivery system. Key Eng. Mater. (2013). https://doi.org/10.4028/www.scientific.net/KEM.594-595.214
J.-P. Chen, C.-H. Su, Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 7(1), 234–243 (2011)
L.T. Sin, A.R. Rahmat, W.A.W.A. Rahman, Polylactic acid: PLA biopolymer technology and applications. Plastics design library, 1st edn. (Elsevier, Boston, 2013), p.341
J.P. Jain, N. Kumar, Self assembly of amphiphilic (PEG)3-PLA copolymer as polymersomes: preparation, characterization, and their evaluation as drug carrier. Biomacromol 11(4), 1027–1035 (2010)
Y. Wei et al., Microcosmic Mechanisms for protein incomplete release and stability of various amphiphilic mPEG-PLA microspheres. Langmuir 28(39), 13984–13992 (2012)
G. Ruan, S.-S. Feng, preparation and characterization of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials 24(27), 5037–5044 (2003)
Persson, A., Identification of Alternative Solvents to DCM for Dissolving PLA. 2018.
Y. Wang, F. Li, An emerging pore-making strategy: confined swelling-induced pore generation in block copolymer materials. Adv. Mater. 23(19), 2134–2148 (2011)
H. Kawaguchi, Functional polymer microspheres. Prog. Polym. Sci. 25(8), 1171–1210 (2000)
H. Ebadi-Dehaghani et al., On O2 gas permeability of PP/PLA/clay nanocomposites: a molecular dynamics simulation approach. Polym. Testing 45, 139–151 (2015)
H. Deng, Z. Lei, Preparation and characterization of hollow Fe3O4/SiO2@PEG–PLA nanoparticles for drug delivery. Compos. B Eng. 54, 194–199 (2013)
Acknowledgments
The Department of Energy’s Kansas City National Security Campus is operated and managed by Honeywell Federal Manufacturing & Technologies, LLC, under contract number DE-NA0002839. The authors from North Carolina A&T State University acknowledge the support and funding from the Pipeline Development of Skilled Workforce in STEM through Advanced Manufacturing (STEAM) under a program from the National Nuclear Security Administration (NNSA)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Damptey, R., Torres, S., Cummings, L. et al. Synthesis and characterization of polylactic acid microspheres via emulsion-based processing. MRS Advances 8, 982–987 (2023). https://doi.org/10.1557/s43580-023-00634-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00634-x