Abstract
Functional biomaterials of biphasic tissue scaffolds were fabricated with polyvinyl alcohol (PVA) and different percentage of gelatin 0%, 0.1%, 0.5%, 1%, and 5% (PVAG0, PVAG0.1, PVAG0.5, PVAG1, and PVAG5) via bubbling freeze-thawing, and freeze-drying. Morphology of the scaffolds was observed by scanning electron microscopy. Swelling behavior, and degradation of the scaffolds were also evaluated. Biological performance of the scaffolds was studied. The result exhibited that PVAG0.5 showed suitable performance promising to model of resorbable framework as tissue expanding to recover oral contour shape for surgical application fitting to cleft lip and palate.
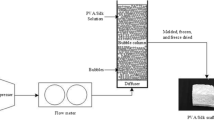
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Damaged tissue leading to the dysfunction of organ is critical problem of some patients. To treat these patients, the effective surgery to repair damaged tissue and recover contour shape is the choice to solve this problem.[1,2] To surgery with functional biomaterials of scaffolds acting as structure for cell adhesion and proliferation is the successful method to promote new tissue formation at damaged area.[3] Especially, biphasic scaffolds is selected as alternative materials to enhance new tissue formation and recover contour shape of organ.[4]
Interestingly, for some cases volume expanding with functional biomaterials has been applied as framework to promote tissue formation and recover contour shape.[3,5,6] These materials are inserted into sutured tissue at damage area. They show function to enlarge the volume of sutured tissue area. This leads to the sufficient space for complete cell growth to recover contour shape without shrinkage after surgery. Critically, they often act as non-resorbable frameworks which have to remove after the complete cell growth.[3,7]
In this research, functional biomaterial of biphasic scaffolds with function of tissue expanding was created as a resorbable framework to recover contour shape. Material selection, design, performance of biphasic scaffolds was emphasized. To evaluation their structure, function, and identification fitting to surgical application was discussed in this research.
Results
Morphological analysis of scaffolds
Figure 1 shows cross-sectional photographs and SEM images of the fabricated scaffolds. Obviously, the scaffolds possessed two-phase structure of sponge-like layer and dense layer. The two layers were separated clearly from each other at the interface. The sponge layer was highly porous and connected to dense layer. In addition, at the sponge region, the pore walls show the fibrous feature with a high volume of tiny pores in them contributing rough surfaces of the walls and fibrous networks. The PVAG5 scaffold had the biggest mean pore size compared with the other scaffolds (326.70 ± 21.80 µm) see Fig. 1. The PVAG1 scaffolds showed a mean pore size of 297.60 ± 29.75 µm which was bigger than the PVAG0.1 and PVAG0.5 scaffolds. The PVA scaffolds had the smallest mean pore size (264.86 ± 16.95 µm) compared with the other scaffolds.
Photographs of the fabricated scaffolds: (a) PVAG0, (c) PVAG0.1, (e) PVAG0.5, (g) PVAG1, and (i) PVAG5. Cross-sectional morphologies of the scaffold under SEM observation: (b) PVAG0, (d) PVAG0.1, (f) PVAG0.5, (h) PVAG1, and (j) PVAG5. Size distribution of the main pores gained from the cross-sectional views of the sponge layers: (k) PVAG0, (l) PVAG0.1, (m) PVAG0.5, (n) PVAG1, and (o) PVAG5.
Mechanical properties of scaffolds
Compressive strengths of the scaffolds both in dry and wet conditions were evaluated [Fig. 2(a–d)] the PVAG5, PVAG1, and PPVA had higher stress values than the PVAG0.1 and PVAG0.5. The PVAG5 scaffold provided the highest stress values of 1.13 MPa, whereas the lowest stress values an approximately 0.27 MPa were obtained from the PVAG0.5. The compressive strength data of the wet scaffolds is presented in Fig. 2(c, d). In the wet condition, the highest stress values of 0.31 MPa were found for the wet PVAG0. The lowest stress values of 0.07 MPa were observed on the wet PVAG5.
Swelling behavior of scaffolds
PVAG0 showed the highest swelling volume of 138.39% while PVAG0.1 demonstrated the lowest swelling percentage of 17.34%, and the PVAG0.5, PVAG1, and PVAG5 showed swelling volumes of 24.76%, 54.15%, and 60.08% respectively [Fig. 2(e)]. The swelling volume for samples increased continued to increase with time, especially during the first soaking hour. After that, the trends for samples were slightly increased except for the PVAG0.
Degradation of scaffolds
Figure 2(f) illustrates a bar graph of the degradation percentage of the scaffold samples. At the first 3 days of the soaking time, PVAG0 showed the highest degradation percentage of 45.02% while PVAG5 demonstrated the lowest degradation percentage of 18.61%, and the PVAG0.1, PVAG0.5, and PVAG1 showed degradation percentages of 24.51%, 23.55%, and 18.61% respectively. The degradation percentage for samples increased continued to increase with time, especially PVAG0 the degradation of scaffold is higher than others was noticed from 45.02% at the first 3 days to 54.67% at 21 days.
Cell proliferation
Figure 2(g) shows that cell proliferation of all the scaffolds increased and decreased with time. Cell proliferation of the PVAG0 scaffold was not much higher than that of other structures. Except on day 1, the cell proliferation of PVAG0 scaffold was highest while PVAG0.1 was the lowest the cell proliferation of each scaffold was different than on other days. On day 3, the cell proliferation of all scaffolds is the highest when compare with other days and each scaffold nearly same. On day 5, the cell proliferation of all scaffolds was reduced and PVAG0 was the highest while PVAG0.1 was lowest on Day 7, similar to Day 5. Cell proliferation on PVAG0 was still higher than the others. But the cell proliferation of other cytoskeletons was not very different.
ALP activity
In this research, ALP activity was used to evaluate the early stage of bone formation. The results were presented in Fig. 2(h). On days 7, 14, and 21. On day 7, the ALP activity of the PVAG1 was higher than the other scaffolds. On the other hand, the PVAG0 was lower than the other scaffolds and the PVAG0.5 and the PVAG5 scaffolds were similar. On day 14, the ALP activity of the PVAG5 increased and became significantly higher than the PVAG0.5 scaffold. On day 21, the ALP activity of all scaffolds was similar, especially for the PVAG0, PVAG0.5, PVAG1 and PVAG5 scaffolds.
Protein analysis
Protein analysis is shown in Fig. 2(i). Clearly, for PVAG5, the amount of protein synthesis was quite stable. For days 7, the PVAG0, PVAG0.1 scaffolds revealed significantly higher the amount of protein synthesis than the PVAG0.5, PVAG1, and PVAG5 scaffolds. In addition, The PVAG0.5, PVAG1, and PVAG5 scaffolds shown increase amount of protein synthesis follow percentage of gelatin was added. On day14, the PVAG0.1, and PVAG1 scaffolds revealed significantly highest amount of protein synthesis than the other scaffolds. The PVAG0, PVAG0.5, and PVAG5 shown amount of protein synthesis was similar. On day 21, the PVAG0 scaffolds were shown the amount of protein synthesis significantly higher than the PVAG5 scaffolds.
Calcium deposition analysis
In later stage of bone formation, calcium deposition was used as biomarker to evaluate biological performance of scaffolds. The higher calcium deposition was due to the last stage of maturation.[8] This behavior matched the lowered ALP activity related to early stage of bone formation obtained up to 14 days, while the calcium deposition referred to later stage of bone formation increased at the same time point [Fig. 2(j)]. Moreover, the lower value measured for the PVAG0 scaffold on day 21 further supports the hypothesis that our scaffold promotes calcium deposition. After days 7, 14, and 21, all the scaffolds had gelatin induced high levels of calcium deposition.
Discussion
Evaluation of materials selection and formation for biphasic scaffolds fitting to tissue expanding
In our research, for materials selection, PVA was used as based materials because of its performance of water uptake and high swelling.[9] Furthermore, PVA show good performance as resorbable and degradable function which have been selected as biomaterials.[10] According to its performance, PVA was selected as based component of our scaffolds expected to main physical performance of tissue expanding. Gelatin is another selected materials for our scaffolds because of physical and biological performance to enhance swelling behavior and promote cell adhesion and proliferation leading to enhancement of tissue formation.[11] Therefore, gelatin was incorporated with PVA to create scaffold which has performance supporting tissue expanding.
To form biphasic scaffolds, they were fabricated via micro-bubble technique following the previous works.[12] In this research, biphasic scaffolds were form via integrated techniques of bubbling with freeze-thawing before freeze drying. The bubbling was aimed to create pores in the solution of PVA and gelatin. This is the cause of the main porous formation of scaffold. For freeze-thawing, it was used to promote physical crossing of PVA and this leads to physical stability of scaffolds.[13] The freeze drying is another technique to form porous structure of scaffolds.[14] The sub-structure was formed during freeze drying. Biphasic structure was formed due to segregation of fused air bubbles at the bottom and the non-fused air bubbles at the top of the mold during the fabrication. Based their formation into biphasic scaffolds, they are suitable for using to expand area of tissue included two parts.
Physical and mechanical properties of biphasic scaffolds evaluated to support tissue expanding
For our results, PVAG0 showed higher swelling than the others. Its swelling demonstrated a sharp increase in the water uptake with time was noticed from 138.39% at the first 15 min to 234.93% at 480 min. First, this is because the pore size distribution is relatively uniform in the sponge layer. In the case PVAG0.1, PVAG0.5, PVAG1, PVAG5, the swelling percentage increases steadily as the percentage of gelatin increases. This is related to the non-uniform of their porous structure which is the cause of lower swelling than uniform porous structure of PVAG0. Importantly, the literature exhibits that the suitable swelling of scaffolds has to maintain the certain hydration without dramatic damage of their structure. Otherwise, the scaffolds were malfunction to support cell attachment and growth.[15]
Our results showed that the distinct ability in the degradation is attributed to the high-water solubility of the only constituent PVA in PVAG0 without other constituents. In the case PVAG0.1, PVAG0.5, PVAG1, PVAG5, it has been seen the degradation percentage increases steadily and be less degradable as the percentage of gelatin increases. The distinct ability in the degradation of the scaffold comes from the water solubility of the substances that form the scaffold and the structure of the created scaffolds. Furthermore, the literature demonstrated that the non-uniform morphology of scaffold has effect on enhancement of degradability.[16,17] Our result of degradability follows that literature particularly in scaffolds of PVA with gelatin.
For mechanical properties, PVA with gelatin had lower stress at maximum load and Young’s modulus than without gelatin. The results indicated that the pore size and the distribution of the internal pore size affected the mechanical properties of the scaffolds. In dry conditions, it can be seen that PVAG0, PVAG5 has a narrower pore size distribution than PVAG0.1, PVAG0.5, PVAG1 resulting in stress maximums higher than PVAG0.1, PVAG0.5, PVAG1, and vice versa. PVAG0.1, PVAG0.5, PVAG1 have a wider pore size distribution resulting in a lower stress value. In a wet state, it is also important to evaluate the mechanical strength of the scaffolds to determine their potential use during implanting when exposed to a physiological fluid inside the body. In wet conditions, the results showed that in pore size each pore was clearly separated and the pore size was relatively close more than other scaffolds. It will keep the water inside high. The water in the porous scaffolds could resist the compressive force while testing in the wet state. Therefore, the scaffolds that could hold a higher amount of water in the porous structure showed higher stress values and Young’s modulus. This was suitable performance to support tissue expanding.
Biological performance of scaffolds evaluated to identify tissue formation
In our research, after morphological observation of biphasic scaffold, it showed porous structure with spherical form and size which fit to promote bone tissue formation.[5] Hence, biphasic scaffolds were proposed to promote bone tissue formation related and supported to performance of tissue expanding. Biological performance referring to bone tissue formation was used to test on our scaffolds. Based on literatures, cell proliferation, ALP activity, protein absorption, and calcium deposition were biomarkers selected for in vitro testing. First, the results demonstrated that PVA without gelatin enhanced cell proliferation due to higher degradation than other scaffolds. This leads to occurrence of large area supporting cell adhesion, migration, and proliferation which promote tissue formation.[18,19,20]
For ALP activity, PVA with gelatin was higher than without gelatin. This is because gelatin which has amino motif acting as biological signal to activate cell for promotion of ALP activity particularly in the early stage of bone formation.[13,21] Notably, PVA with high loading of gelatin showed ALP activity than low loading of gelatin at early stage of testing. Then, the ALP activity of all sample showed non-difference of ALP activity. This demonstrated that PVA with gelatin showed prominent function at the early stage of bone formation that is the similar trend as literature.[13]
For protein absorption, PVA without gelatin is higher than with gelatin at early stage of testing of day 7. This is related to the literature that polymers showed high swelling which support protein absorption.[22] Then, after day 7 the protein absorption on polymer scaffolds showed fluctuation particularly in day 14. This is because the effect of degradability which had influence on protein absorption.[18,23] Finally, after degradability approaches to the steady state, the protein absorption shows non-fluctuation at later stage of testing of day 21.[18] The result illustrated that the unique pore size, inter-connective network structure directly affecting the deterioration behavior of the scaffold enhanced media diffusion into the whole texture of the scaffolds.[18] High diffusion of the media enhanced the deposition of the protein in the pores.[24]
In our research, calcium deposition is considered as biomarker of the final stage of osteoblast maturation to form bone. Low calcium deposition was found on PVA without gelatin. In contrast, PVA with gelatin showed the high calcium deposition. This is because PVA without gelatin showed higher degradability than with gelatin. The high degradability related to the low physical stability which shows poor performance to support calcium deposition.[8,13] Interestingly, PVA with gelatin at early stage of testing at day 7 had higher calcium deposition than later stage of testing at day 21. This was related to the degradability of scaffolds. The literature demonstrated that degradability of scaffolds at early stage of testing is low and they had sufficient physical stability to support calcium deposition.[13] For the intermediate for testing of day 14, the scaffolds exhibited fluctuation of degradability of PVA with gelatin. This had effect on fluctuation of calcium deposition on scaffolds. The literature described that the polymer with many component has fluctuation of degradability particularly in the intermediate time of testing.[13,25] After degradability reach to the steady stage, it has effect on non-fluctuation of calcium deposition.[26,27] The results demonstrated that our biphasic scaffolds is suitable materials to support bone tissue formation particularly in sponge part.
To evaluate identification of biphasic scaffolds fitting to surgical application
According to the physical, mechanical, and biological performance, our biphasic scaffold was proposed to recover contour shape for cleft lip and palate. This is because our biphasic scaffolds has the suitable physical and mechanical performance fitting to expand tissue volume. Furthermore, they showed biological performance to promote bone tissue formation. As our design into biphasic structure, the dense part was proposed to contact soft tissue. The dense part has the sufficient physical and mechanical stability to expand volume without collapse during tissue formation. This is the method to solve problem of tissue shrinkage which often form in surgery of cleft lip and palate. On the other hand, the part of porous structure has the good biological performance to promote bone tissue formation. Our research demonstrated that biphasic scaffolds showed the suitable model as resorbable framework to expand tissue volume supporting tissue formation and recover contour shape following Figure 3.
In this research, we emphasized on in vitro testing of tissue formation at bone defect fitting to cleft lip and palate. This is because the regular tissue formation in bone part acts stable structure to support the function of soft tissue formation.[28,29] Therefore, in this research we focused bone tissue formation. For the soft tissue part, the main expected function is the physical and mechanical performances which are sufficient stability to hold the contour shape without collapse during application. Hence, testing of physical and mechanical properties was performed as the related function of volume expanding in soft tissue. Nevertheless, to improve performance of tissue expanding by materials with sufficient mechanical strength and high swelling behavior is the attractive topic and in vivo testing needs for the future works and to develop bioactive function of biphasic scaffolds as osteo-induction in the part of porous structure in the future works.
Conclusion
In this research, functional biomaterials of resorbable framework for tissue expanding were created. They were evaluated performance to identify fitted surgical application. Functional biomaterials were fabricated into biphasic scaffolds based on materials section of PVA and gelatin. Our biphasic scaffold showed two part: (1) dense and (2) porous structure. Our scaffolds had the suitable swelling behavior and mechanical stability supporting tissue expanding. Because of their pore size and structure fitting to osteoblast growth, they were proposed to apply in two parts of defect area included bone tissue. They showed the suitable biological performance; cell proliferation, protein synthesis, ALP activity, and calcium deposition to supporting bone formation. Especially, PVA with 0.5% of gelatin was evaluated as functional biomaterials of resorbable framework exhibited the performance promising to support tissue expanding and recover oral contour shape for surgical application fitting to cleft lip and palate which has the defect included two part of bone and soft tissue.
Materials and methods
Raw materials
Polyvinyl Alcohol (PVA, Mw = 47,000 g/mol, 98% hydrolyzed) and Gelatin from porcine skin, purchased from Fluka chemika, were used to produce scaffolds. Glacial acetic acid (CH3COOH, 80% purity), supplied from J. T. Baker Chemical, was used as a solvent. Phosphate Buffered Saline solution (PBS) was prepared using Sodium chloride, Potassium chloride, Sodium hydrogen phosphate, and Potassium dihydrogen phosphate (NaCl, KCl, Na2HPO4, KH2PO4) purchased from Fluka chemika.
Preparation of biphasic scaffolds
Gelatin-mixed PVA solution was prepared by dissolving PVA powder (12.5 g) in 0.01 molar acetic acid (50 ml) at 90°C for 1 h, after which gelatin powder was added, and stirred for another 30 min. Then pour the solution into the bubble column while flowing air through a porous diffuser (pore range 10–16 µm), a constant air flow rate of 100 mL/min was applied for 15 min. After that, pour the solution bubbled into the mold. The samples were frozen at − 4°C for 20 h and the frozen samples were then thawed at room temperature for 6 h. The freeze–thaw process was repeated 3 cycles before samples were immersed in PBS solution to pH 7 and then freeze-dried before further characterization. The production process is based on the research of Parivatphun et al.[30] which there are different steps and parameters. A list of the samples fabricated for scaffold fabrication is shown in Table I.
Morphology, and thermal behavior
Morphological structure of the scaffold was observed by Scanning Electron Microscopy (SEM, JEOL, JSM5800LV) at an accelerating voltage of 15 kV. The scaffold was cut into a cubic shape and sticked on a SEM stub prior to gold sputtering for conductive coating. ImageJ software was used to measure pore size of the scaffold via SEM images.
Compressive strength
The compressive strength of the scaffolds was estimated by compression test using a universal testing machine (Lloyd model LRX-Plus, Lloyd Instrument Ltd., London, UK). All scaffolds were cut into a shape of 10 × 10 × 6 mm3 and tested at room temperature under both wet and dry conditions with a 250 N load cell at a displacement speed of 1 mm·min−1 that stopped at a strain of 40%. For the wet condition, the samples were immersed in PBS for 24 h at room temperature.
Degradation and swelling behaviors
The degradation behavior of scaffolds was estimated using lysozyme enzyme. The scaffolds were soaked in a lysozyme solution at 2 mg/ml of PBS at 37°C. The scaffolds were weighed before and after soaking at day 3, 5, 7, 14, and 21. The degradation percentage was calculated using Eq. (1)
where D is the degree of degradation \({W}_{a}\) and \({W}_{b}\) represent the weight of scaffolds after and before soaking, respectively.
The swelling behavior of scaffolds was studied by incubation in PBS at 37°C. The PBS was prepared by mixing 8 g NaCl, 200 mg KCl, 1.44 g Na2HPO4, 245 mg KH2PO4 into 800 ml of distilled water, while stirring and adjusting the pH of solution to pH 7.4. Then distilled water was added until the total volume was 1 L. The scaffolds were soaked in the PBS for 8 h. Samples were weighed before and after soaking at an interval time. The swelling percentage was calculated from Eq. (2)
where S is the degree of swelling \({W}_{w}\) and \({W}_{d}\) are the wet weight and the dry weight of scaffolds.
Cell culture
MC3T3E1 cells were cultured in the alpha MEM composed of 10% fetal bovine serum (FBS, Gibco, Invitrogen, USA), 1% penicillin and streptomycin (Gibco, Invitrogen, USA), and 0.1% Fungizone® (Gibco, Invitrogen, USA). The cells were then seeded at a density of 1 × 106 onto the scaffold, and the culture was incubated at 37°C in a humidified incubator at 5% CO2 and 95% air.
Cell proliferation
Cell proliferation of the scaffolds were tested with PrestoBlue cell reagent (Thermo Fisher Scientific), after cell seeding at a density of 2 × 106 cells per scaffold on day 1, 3, 5, and 7. Old media was removed and new alpha MEM media was added with 10% PrestoBlue cell reagent (400 µl) and incubated for 1 h. The solution was then added into 96 well plates for optical density (OD) measurement at 600 nm.
Alkaline phosphatase (ALP) activity, calcium analysis
The osteoblast cells were cultured in OS media for 7, 14, and 21 days to extract substances from cells by the lysis method. Triton (1%) in PBS was used for cell extraction. The old media was removed, the scaffolds were washed twice with PBS, and 800 µl lysis buffer was added into the scaffolds. Subsequently, the scaffolds were freeze-thawed up to 3 cycles at − 80°C and left at room temperature. The scaffolds were mashed and centrifuged to remove the precipitation from the solution. The solution from the lysis process was used for the ALP activity test that followed the manufacturer’s instructions of the Alkaline Phosphatase LiquiColor® Test kit (Human, Germany). For concentration of calcium measurement with that followed the manufacturer’s instructions of the Calcium Colormetric Assay® Test kit (Darmastadt, Germany).
Protein analysis
Lysis solutions from day 7, 14, and 21 were used for protein measurement with Bio-Rad Protein Assay Dry Reagent Concentrate (Bio-Rad Laboratories, Inc. USA). The ratio of the reagent to deionized water was 1:4 which was added to 20 µl of the sample solution followed by OD measurement at 595 µm.
Statistical analysis
In this research, five samples were tested in experiments. All data were shown as mean ± standard deviation. The samples were measured and statistically compared by one-way ANOVA and Tukey’s HSD test (SPSS 17.0 software) The acceptance for statistical significance was set at *P < 0.05.
References
M.A. Farfán, C.M. Olarte, R.F. Pesantez, S. Suárez, L. Vallejo, Recommendations for fracture management in patients with osteopetrosis: case report. Arch. Orthop. Trauma Surg. 135, 351 (2015)
A.B. Mink van der Molen, J.M. van Breugel, N.G. Janssen, R.J. Admiraal, L.N. van Adrichem, F. Bierenbroodspot, D. Bittermann, M.-J.H. van den Boogaard, P.H. Broos, J.J. Dijkstra-Putkamer, Clinical practice guidelines on the treatment of patients with cleft lip, alveolus, and palate: an executive summary. J. Clin. Med. 10, 4813 (2021)
F.-M. Chen, X. Liu, Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 53, 86 (2016)
X. Li, J. Ding, J. Wang, X. Zhuang, X. Chen, Biomimetic biphasic scaffolds for osteochondral defect repair. Regen. Biomater. 2, 221 (2015)
G. Jia, C. Chen, J. Zhang, Y. Wang, R. Yue, B.J. Luthringer-Feyerabend, R. Willumeit-Roemer, H. Zhang, M. Xiong, H. Huang, In vitro degradation behavior of Mg scaffolds with three-dimensional interconnected porous structures for bone tissue engineering. Corros. Sci. 144, 301 (2018)
R. Xie, J. Hu, O. Hoffmann, Y. Zhang, F. Ng, T. Qin, X. Guo, Self-fitting shape memory polymer foam inducing bone regeneration: a rabbit femoral defect study. Biochim. Biophys. Acta Gen. Subj. 1862, 936 (2018)
J.W. Von den Hoff, J.C. Maltha, A.M. Kuijpers-Jagtman, Palatal Wound Healing: The Effects of Scarring on Growth, in Cleft Lip Palate: Diagnosis Management. ed. by S. Berkowitz (Springer, Berlin, 2013), p.309
A. Koroleva, A. Deiwick, A. Nguyen, S. Schlie-Wolter, R. Narayan, P. Timashev, V. Popov, V. Bagratashvili, B. Chichkov, Osteogenic differentiation of human mesenchymal stem cells in 3-D Zr-Si organic-inorganic scaffolds produced by two-photon polymerization technique. PLoS ONE 10, e0118164 (2015)
M. Sun, Y. Wang, L. Yao, Y. Li, Y. Weng, D. Qiu, Fabrication and characterization of gelatin/polyvinyl alcohol composite scaffold. Polymers 14, 1400 (2022)
M.A. Teixeira, M.T.P. Amorim, H.P. Felgueiras, Poly (vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers 12, 7 (2019)
S.J. You, W.S. Ahn, H.S. Jang, M.I. Kang, H.J. Chun, Y.M. Lim, Y.C. Nho, Preparation and characterization of gelatin-poly (vinyl alcohol) hydrogels for three-dimensional cell culture. J. Ind. Eng. Chem. 13, 116 (2007)
T. Parivatphun, S. Sangkert, J. Meesane, R. Kokoo, M. Khangkhamano, Constructed microbubble porous scaffolds of polyvinyl alcohol for subchondral bone formation for osteoarthritis surgery. Biomed. Mater. 15, 055029 (2020)
A. Thangprasert, C. Tansakul, N. Thuaksubun, J. Meesane, Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 183, 108113 (2019)
H.-W. Kang, Y. Tabata, Y. Ikada, Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 20, 1339 (1999)
K.R. Aadil, A. Nathani, C.S. Sharma, N. Lenka, P. Gupta, Fabrication of biocompatible alginate-poly (vinyl alcohol) nanofibers scaffolds for tissue engineering applications. Mater. Technol. 33, 507 (2018)
K. Zhang, Y. Fan, N. Dunne, X. Li, Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 5, 115 (2018)
S. Wu, X. Liu, K.W. Yeung, C. Liu, X. Yang, Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 80, 1 (2014)
S. Sangkert, S. Kamolmatyakul, M. Gelinsky, J. Meesane, 3D printed scaffolds of alginate/polyvinylalcohol with silk fibroin based on mimicked extracellular matrix for bone tissue engineering in maxillofacial surgery. Mater. Today Commun. 26, 102140 (2021)
S.B.A. Boraei, J. Nourmohammadi, F.S. Mahdavi, J. Yus, A. Ferrandez-Montero, A.J. Sanchez-Herencia, Z. Gonzalez, B. Ferrari, Effect of SrR delivery in the biomarkers of bone regeneration during the in vitro degradation of HNT/GN coatings prepared by EPD. Colloids Surf. B 190, 110944 (2020)
N. Abbasi, S. Hamlet, R.M. Love, N.-T. Nguyen, Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Dev. 5, 1 (2020)
M.A. Alvarez Perez, V. Guarino, V. Cirillo, L. Ambrosio, In vitro mineralization and bone osteogenesis in poly (ε-caprolactone)/gelatin nanofibers. J. Biomed. Mater. Res. A 100, 3008 (2012)
J.H. Jeong, D.W. Lim, D.K. Han, T.G. Park, Synthesis, characterization and protein adsorption behaviors of PLGA/PEG di-block co-polymer blend films. Colloids Surf. B 18, 371 (2000)
M. Lee, T.T. Chen, M.L. Iruela-Arispe, B.M. Wu, J.C. Dunn, Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28, 1862 (2007)
Z. Chen, P. Wang, B. Wei, X. Mo, F. Cui, Electrospun collagen–chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta. Biomater. 6, 372 (2010)
T. Inoue, Reaction-induced phase decomposition in polymer blends. Prog. Polym. Sci. 20, 119 (1995)
H. Jeon, H. Lee, G. Kim, A surface-modified poly (ɛ-caprolactone) scaffold comprising variable nanosized surface-roughness using a plasma treatment. Tissue Eng. Part C Methods 20, 951 (2014)
K. Panjapheree, S. Kamonmattayakul, J. Meesane, Biphasic scaffolds of silk fibroin film affixed to silk fibroin/chitosan sponge based on surgical design for cartilage defect in osteoarthritis. Mater. Des. 141, 323 (2018)
D.W. Hutmacher, J.T. Schantz, C.X.F. Lam, K.C. Tan, T.C. Lim, State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 1, 245 (2007)
M. Tarchala, E.J. Harvey, J. Barralet, Biomaterial-stabilized soft tissue healing for healing of critical-sized bone defects: the Masquelet technique. Adv. Healthc. Mater. 5, 630 (2016)
T. Parivatphun, S. Sangkert, R. Kokoo, M. Khangkhamano, J. Meesane, Biphasic scaffolds of polyvinyl alcohol with silk fibroin for oral and maxillofacial surgery based on mimicking materials design: fabrication, characterization, properties. J. Mater. Sci. 1 (2022)
Acknowledgments
This research was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No ENG6601237S) and the project REC.63-305-25-2. The authors would like to thank Division of Biomedical Science and Biomedical Engineering, Faculty of Medicine and the Department of Mining and Material Engineering, Faculty of Engineering, Prince of Songkla University for their provision of both facilities and equipment utilized in this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chukaew, S., Parivatphun, T., Thonglam, J. et al. Functional biomaterials as model of resorbable framework for tissue expanding to recover contour shape in surgical application: Performance evaluation. MRS Communications 13, 1218–1225 (2023). https://doi.org/10.1557/s43579-023-00428-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-023-00428-0