Abstract
The oral and maxillofacial region contains oral organs and facial soft tissues. Due to the complexity of the structures and functions of this region, the repair of related defects is complicated. Different degrees of defects require different repair methods, which involve a great combination of medicine and art, and the material requirements are extremely high. Hence, clinicians are plagued by contemporary oral repair materials due to the limitations of bone harvesting, immune rejection, low osteogenic activity and other problems. Decellularized extracellular matrix has attracted much attention as a bioactive scaffold material because of its nonimmunogenic properties, good osteogenic properties, slow release of growth factors, promotion of seed cell adhesion and maintenance of stem cell characteristics. This article reviews the sources, preparation methods, application and research progress of extracellular matrix materials in the repair of oral and maxillofacial defects to provide an overview for fundamental research and clinical development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The oral cavity and maxillofacial region is an important area of the human anatomy. This region has a variety of physiological functions, such as mastication, swallowing, language, expression and respiration. Tissue defects not only critically affect physiological function but also lead to facial deformities and serious damage to beauty, deteriorating patients’ quality of life. Causes of oral and maxillofacial defects include developmental malformation, infection, trauma, and tumor surgery. With the development of reconstructive surgery, simultaneous or secondary repair of tissue defects that remain after tumor resection can not only ensure the safe boundary of tumor resection but also restore the original shape and function of patients to the maximum extent. It is of great importance to adopt appropriate repair methods for different defects to repair the function and appearance of patients with oral and maxillofacial defects. The traditional repair method is predominantly autologous tissue transplantation; although it has achieved obvious clinical outcomes, it likewise has obvious disadvantages: causing new injuries. With the development of science, decellularized scaffolds have attracted great attention. Due to the biodegradability of decellularized scaffolds, they provide space for cell growth in the surrounding tissue of the defect. More importantly, they can guide tissue regeneration induction by inducing angiogenesis, cell proliferation and differentiation [1, 2].
Scaffold materials have been developed along with tissue engineering and regenerative medicine and include natural, synthetic, and composite materials. However, most materials cannot regenerate the microenvironment in vivo and provide an environment to rebuild the connection between cells. Compared with other engineering materials, natural extracellular matrix (ECM) scaffolds seem to be the optimal choice. After the use of the decellularization method, the biological scaffold, which is called decellularized extracellular matrix (dECM), retains the composition and properties of the ECM to the greatest extent, shows no cytotoxicity, and does not result in rejection or inflammatory reactions [1]. Decellularization mainly aims to remove cell components and DNA fragments. If these components remain, the residuum may result in incompatibility in vitro and immunological responses in vivo [3]. The dECM is believed to reconstitute a microenvironment that is similar to an internal environment, serving as a cellular connection and three-dimensional culture system and producing no adverse reactions [4]. Thus, these scaffolds have been suggested as a physiologically relevant, low cost, scalable, and reproducible in vitro platform [5]. Recent reports validate the use of decellularized ECM scaffolds for a variety of stomatology fields, including periodontium, face, salivary gland and bone. However, the decellularization method can affect the biological characteristics and mechanical properties of the treated tissues and the host response after transplantation. Therefore, the most suitable decellularization method needs to be explored in different tissues to achieve the best decellularization effect and properly retain the original tissue structure. Hence, we focus on the sources, optimization of methods, products and applications of decellularization in stomatology to provide an overview for fundamental research and clinical development.
2 Decellularized sources
Decellularized scaffolds mostly originate from both tissues and cells. Generally, scientists choose whole organs or partial tissues, such as the heart, kidney, liver, etc., to achieve decellularization. In terms of stomatology, researchers have assessed salivary gland [6], tongue [3, 7, 8] and gingival [9, 10] scaffolds with integral structures. For example, the whole-organ decellularized scaffold of rat submandibular glands (SMG) preserved the ECM composition and expression pattern of native SMGs. Moreover, this process effectively removed cellular material and retained proteins and natural morphology. Thus, decellularized SMGs have the potential to regenerate the salivary gland [6]. Likewise, decellularized tongue has also been realized. Decellularization of the tongue is a complex process. This process requires moderate-strength treatment and an appropriate DNase digestion time to minimize damage. After adjustment, researchers developed tongue scaffolds and used decellularized tongue extracellular matrix to recapitulate a tongue squamous cell carcinoma model in vitro [8]. Furthermore, scientists plated blast cells in a decellularized gingiva scaffold by placing the scaffold within the rings of rabbit blastemal tissues; cell migration was found across the scaffold. As a result of protein glycosylation, the number of glycosaminoglycans increased in the scaffold while decreasing in cells during migration [10].
In addition to tissues, cells are also being gradually used as decellularized material. After two-dimensional culture, human dental pulp stem cells are decellularized and show preserved extracellular matrix, which is called DPM. DPM provides a microenvironment for replication and mineralization and can support periodontal ligament cell proliferation [11]. With the development of cell sheet engineering, decellularized cell sheets, such as human periodontal ligament cell (hPDLC) sheets, are also preferred [12]. These cells secrete high levels of ECM and form cell sheets with the assistance of ascorbic acid. After decellularization, allogenic hPDLCs were seeded in this construct and cultured in vitro for 21 days. Farag et al. found retained growth factors in the decellularized cell sheets by ELISAs, and periodontal ligament cells could attach to the construct, as shown by confocal imaging and SEM, and increase collagen content, as shown by collagen quantification. These series of experiments showed that the matrices of scaffolds support human periodontal ligament cells in proliferation and adhesion and allow matrices to be an “off-the-shelf” tissue engineering application [13]. Fourteen days post-seeding in sheets in vivo, hPDLC cells expressed mineralized tissue markers such as ALPL, BMP2, osteocalcin, VEGF, OPN and collagen III, especially high levels of BMP2, osteocalcin and osteopontin. Primary human placental mesenchymal stem cells (hp-MSCs) have also been used to regenerate the periodontal ligament. Nevertheless, hp-MSCs express only the various collagen variant genes COL1A1, COL1A2, and COL3A1. However, collectively, decellularized constructs have no negative effects on the regeneration of periodontal defects and promote cell differentiation [14].
3 Optimization of the decellularization methods
3.1 Common classification
Standard decellularization methods include physical, chemical, and enzymatic techniques. Physical methods include freezing, force, hydrostatic pressure and vacuum-assisted decellularization. Among them, the perfusion method and mechanical agitation are the most commonly used methods of decellularization in stomatology. Perfusion is acceptable for organs with vasculature, such as the salivary gland. Mechanical agitation is a more appropriate method for decellularized tissues that lack convective transport catheters, such as the tongue [3, 8]. Physical procedures are usually combined with other methods to increase the effects. Reagents for the chemical method (containing acid or alkali base, sodium dodecyl sulfate (SDS), Triton X-100, Triton X-200, CHAPS, TBP, hypertonic and hypotonic solutions) change the chemical composition of the ECM. For example, ammonium hydroxide (NH4OH) is used to remove cellular components, Triton X-100 dissolves lipids and preserves protein interactions [15], and SDS can solubilize the basement membrane [3]. Enzymes include trypsin and pepsin with tetrasodium ethylenediamine tetraacetic acid (EDTA). Combinations of these factors usually achieve more specific decellularization than a single approach. The chemical and enzymatic methods are the main methods, while physical methods are complementary [16]. Based on dynamic classification, decellularization methods can also be divided into dynamic and static categories. Amro et al. used ten protocols to decellularize PCL membrane-supported PDL cell sheets. Two of these protocols involved NH4OH/Triton X-100 with or without DNase. The other two methods are SDS treatment with or without DNase. Both of these protocols belong to the static decellularization group. The remaining perfusion protocols include (1) perfusion + NH4OH/Triton X-100; (2) perfusion + NH4OH/Triton X-100 + DNase; (3) perfusion + SDS; (4) perfusion + SDS + DNase; (5) thermal freezing/thawing cycles (F/T); and (6) thermal freezing/thawing cycles (F/T) + DNase. Measurements showed no significant differences in collagen integrity, collagen content or growth factor retention among all the methods. However, static decellularization is generally more effective than perfusion in eliminating DNA [17].
3.2 Diverse decellularization methods for oral-related tissues
Due to different combinations of traditional decellularization methods, the same organs have diverse protocols. Hence, scientists have compared various combinations to identify the optimal decellularization method. As a biological substitute of tooth being a promising material to regenerate teeth. Scientists have compared five decellularization methods, phosphate-buffered saline (PBS), EDTA, sodium hypochlorite (SH), enzymatic detergent and hydrogen peroxide, to decellularize teeth and tested them in different combinations. Despite this, all five protocols cannot guarantee complete decellularization. In the end, the study showed that the combination of PBS, EDTA, enzymatic detergent and hydrogen peroxide is the optimal method to obtain a natural scaffold [18]. Undoubtedly, materials with diverse methods are used not only for teeth but dental pulp, PDL and other oral tissues. Because decellularized dental pulp provides an ideal scaffold for a regenerative endodontic procedure, Song et al. evaluated three protocols published previously in 2017: (1) Treatment of dental pulp with 2% Triton X-100 and 0.1% NH4OH for 72 h. (2) Treatment of dental pulp with hypertonic buffer (0.01 M Tris–HCl, 1 mm EDTA; pH 8.2) for 48 h and then three repeated cycles of incubation in 1% SDS for 24 h and 1% Triton X-100 for 24 h. (3) Treatment of dental pulp with only 1% SDS for 24 h and then 1% Triton X-100 for 24 h. The researchers concluded that the second protocol was the most effective method. Furthermore, the scaffold is conducive to 3-dimensional attachment and proliferation of stem cells of the apical papilla, which are seeded onto it [19]. However, human dental pulp is not always available. To solve the shortage of human dental pulp ECM, researchers have focused on exogenous sources and tried to develop an optimization method for bovine dental pulp decellularization. With distinct combinations and durations of trypsin/EDTA, SDS and Triton X-100, seven protocols are described in the article. The authors noted that 12 h of EDTA/trypsin and 1-h Triton X-100 treatment without SDS did not change the microstructure, and the protocols were low cost [20]. In addition to analysis of dental pulp, Son et al. also compared two methods of decellularized periodontal ligament (PDL) scaffolds. In contrast to treatment of PDL with 2% Triton X-100 and 0.1% NH4OH for 72 h, they used three cycles of 1% SDS for 24 h and 1% Triton X-100 for 24 h, and there was less DNA residuum and almost no nuclei. Moreover, the scaffold could induce the proliferation and differentiation of stem cells and even regenerate the PDL [21]. Unexpectedly, there are three protocols for decellularized muscles: a detergent‐only treatment, detergent‐enzymatic treatment protocol, and nondetergent nonenzymatic protocol. Regardless of the zygomaticus major or masseter muscle, researchers found that a detergent‐only treatment protocol is the optimal choice for decellularizing muscle tissues [22]. Overall, these findings demonstrate that decellularized protocols are specific for particular tissues.
4 The product of decellularization
The characterization of decellularized products depends on the experimenters’ aims. Scaffolds with unbroken structures are more likely to rebuild their original morphology. However, maintaining the intact morphology is difficult. Fortunately, researchers have found a feasible way to obtain a whole salivary gland when they face SMG arteries and veins that are not sufficiently thick. After recellularization of the scaffold with allogeneic primary salivary gland cells, duct-like structures are found in cells [6]. In addition to organs, decellularized tissues with an intact appearance can also be used in regeneration studies. Decellularized swine dental pulp, which was decellularized with 10% SDS combined with Triton X-100, preserved its natural shape and structure. This material is suitable to regenerate human dental pulp [23].
Another type of scaffold is called a decellularized matrix hydrogel. After lyophilization of the human dental pulp (hDDPM) from the third molars, the pulp was smashed into powder. Then, the powder was treated with 0.5 M acetic acid containing pepsin to digest undissolved particulates. The most important part of gelatinizing the powder is the pH value. If the solution pH is adjusted to 7.4, it is more feasible to gel the powder at 37 °C. Li et al. prepared two hydrogel concentrations (both of which were lower than 1%) and seeded human dental pulp cells onto hDDPM hydrogel-coated surfaces for regeneration [24]. Compared to scaffolds with intact structures, hydrogels can be injected into a root canal or cavity independent of morphology. Acellular salivary glands have a hydrogel form as well. Decellularized rat salivary gland ECM hydrogel, which is used to culture rat salivary gland stem/progenitor cells, can not only regenerate gland tissue but also promote cytodifferentiation in gene expression [25]. In addition to the oral scaffold, decellularized bovine tibia (bECM) hydrogels are used in stomatology. Human dental pulp stem cells are seeded on bECM. Compared to osteo/odontogenic medium and Col-I hydrogel scaffolds, bECM hydrogel scaffolds can also induce odontogenic differentiation without odontogenic factors and upregulate the expression levels of the odontogenic differentiation markers DSPP, DMP-1 and MEPE. Combined with osteo/odontogenic medium, bECM can promote the expression levels. Moreover, the combination of bECM and inducers, basic fibroblast growth factors, can improve the odontogenic differentiation potential of dental pulp stem cells (DPSCs) [26].
In addition to being the main structure of scaffolds, decellularized materials also have a modified role. Decellularized human dental pulp and collagen were prepared in coated solutions, and researchers modified silk fibroin scaffolds. Compared with unmodified scaffolds, scaffolds coated with collagen/decellularized pulp not only improved osteoblast cell proliferation and adhesion but also induced protein synthesis. This result shows that decellularized ingredients enhance the properties of scaffolds [27].
5 Applications in stomatology
Cell differentiation is the basis for mammals to develop from a single fertilized egg into an organism composed of a variety of tissues. Applying the material to experiments, researchers found that decellularized scaffolds provide the microenvironment of the ECM and influence the differentiation of stem cells. For the same cells, scaffolds from diverse sources function differently. For example, for DPSCs, human exfoliated deciduous tooth (SHED) dECM is suitable for the expansion and periodontal ligament of stem cell dECM for osteogenic induction in vivo. However, even stem cells originate from completely different sources, and they will differentiate in a specific direction under the influence of ECM. For example, human dental follicle cells are impacted by decellularized mouse salivary gland scaffolds but can differentiate into SG cells in the renal capsule of nude mice [28]. Thus, according to the effect of extracellular matrix on cell differentiation, we can apply decellularized scaffolds with specific ECM for repair.
Based on the differentiation-inducing properties of the scaffold, this material is used to repair the defects. Generally, scaffolds are divided into homologous and heterologous scaffolds according to their biogenetic derivations and diverse sources. Although autologous or allogenic scaffolds can more effectively regenerate tissues and treat disease, in regard to humans, the difficulty and ethics of these sources have prompted scientists to turn to xenogeneic scaffolds. With the further exploration of scaffolds, decellularized materials have also been used to repair defects both in the laboratory and clinic.
5.1 Homemade-decellularized scaffold (classified by repair site)
5.1.1 Periodontium
Periodontal disease is a common oral disease that leads to gingival atrophy, alveolar bone resorption and periodontal ligament destruction, and decellularized stents can be used as scaffolds to guide gingival tissue regeneration. Therefore, the application of acellular scaffolds in periodontitis is popular in the oral and maxillofacial regions. Heng et al. used decellularized human SHEDs and decellularized periodontal ligament stem cells (PDLSCs) to culture dental pulp stem cells. Both scaffolds enhanced the proliferation of DPSCs. However, SHED-DECM showed a stronger potential for adhesion, osteogenic differentiation and proliferation of DPSCs in vitro, while PDLSC-derived cells are more suitable for osteogenic induction of DPSCs [29]. Moreover, for human PDLSCs, dECM derived from human urine-derived stem cells (UECM) and decellularized ECM derived from hPDLSCs (PECM) can promote hPDLSC proliferation, attachment, spreading, and differentiation. However, UECM has more effects on osteogenic and vascular differentiation, while PECM promotes adipogenic differentiation of cells [30]. Tsuyoshi Kimura’s group treated mouse PDL with high hydrostatic pressure (HHP) decellularization. To preserve unbroken PDL matrix, they retained tooth and bone and then dissolved collagen fibers by SDS to extract teeth easily after HHP decellularization. Moreover, they implanted this scaffold into the subrenal capsule and observed a lack of PDL cells, and the cells surrounded by the scaffold migrated and oriented along the matrix. This decellularized PDL scaffold can regenerate PDL tissue even without PDL cells and is also suitable for tooth transplantation [31]. On the basis of the same osteogenic effect, Yi et al. investigated the induction level of two extracellular matrices on the same cells. PDLSCs were cultured on periodontal ligament cell-derived and bone marrow cell (BMC)-derived decellularized ECMs (P-dECM and B-dECM, respectively). PDLSCs displayed more vigorous proliferation, stemness, and osteogenic differentiation when cultured on B-dECM than P-dECM [32]. Scientists have decellularized the roots of dog premolars, coated the roots with fibronectin and/or calcium phosphate (CaP) and planted autologous dog PDLSCs on the surface of a scaffold. Scaffolds modified with fibronectin and CaP enhanced the attachment of dog PDLSCs. A study of scaffolds in vivo showed that periodontal ligament-like tissues formed [33]. When dog teeth with decellularized PDL were replanted into the extraction sockets, scientists observed PDL reattachment and cementogenesis. ECM from autologous PDL is a potential scaffold for periodontal tissue engineering [34]. Moreover, decellularized bovine pericardium (BP) membranes extracted and modified with sodium dodecyl sulfate (SDS) and glutaraldehyde (GA) supported the migration, attachment, and proliferation of human gingival fibroblasts in vitro. Modified BP has a much greater thickness, stronger mechanical strength and more suitable pore size than BP with SDS alone. Thus, Nguyen’s teammates optimized decellularized BP in periodontal disease treatment and regeneration [35].
5.1.2 Facial skin
Facial skin often needs cosmetic repair due to trauma, scars and other reasons. Keratinocytes are one of the most essential cells in skin repair. A decellularized gingival tissue scaffold was used for rat bone marrow mesenchymal stem cells and differentiation toward keratinocytes. Influenced by the epithelial ECM, BM-MSCs show potential for epithelial differentiation. Thus, this scaffold may be appropriate to reconstruct the skin [9]. Most encouragingly, scientists successfully decellularized segmental and total faces from fresh human cadavers, including vessels, nervous tissue, adipose tissue, cartilage and muscle. The scaffold maintains partial growth factors, allowing cell engraftment and sustaining vascular perfusion [36]. It promises to be a tissue engineering material for human facial repair in the future.
5.1.3 Salivary gland
The salivary glands also need to be repaired or treated due to a number of factors, such as trauma, tumor radiotherapy, and Schergren syndrome to restore salivary secretion. Human salivary gland epithelial cells (SGECs) cocultured with microvascular endothelial cells (mvECs) in the decellularized porcine gut matrix (SIS-muc) can develop SG-like tissue in vitro. SIS-muc with remaining vascular structures and mvECs provides adequate nutrition for SGECs so that the regenerative tissue can secrete high levels of amylase and generate biological vascularized structures [37].
5.1.4 Bone
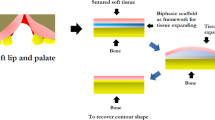
In the oral and maxillofacial regions, trauma, tumor operations and chronic inflammation often lead to bone injury. To better repair human maxillofacial bone defects, scientists have been trying to find various types of engineering materials. Acellular decalcified teeth have applied to bone graft material to repair simulated cranio-parietal bone defects in rats. Compared to decalcified teeth, this kind of scaffold had less or no antigenicity and better osteogenic potential [38]. Decellularized tooth matrix was also used to culture bone mesenchymal stem cells (BMSCs), which can spontaneously differentiate into osteoblasts after 30 days of culture. These results further confirmed the potential of the acellular dental matrix to induce osteogenesis and provide the possibility of natural allografts for bone defect repair [39]. In the maxillofacial repair process, the maxillary sinus is one reason the posterior maxilla is a problematic region for endosseous implants. Thus, doctors usually choose decellularized bovine femur compact bones to augment the sinus [40]. Decellularized bovine femur bones, likewise, are covered with freeze-dried bone marrow stem cell paracrine factors so that the decellularized human amnion/chorion membrane can reconstruct human mandibular defects and prevent fibrous tissue invasion [41]. Li’s group made use of a human decellularized amnion membrane with integrating poly(1,8-octamethylene-citrate) (DAM-POC) to repair cleft palate. Combining the advantages of DAM and POC, DAM-POC can better withstand long-term exposure to the complicated oral environment. Compared to that in the DAM group, the repaired palate in the DAM-POC scaffold became thicker and remained wider. The ability to further promote wound healing seems to cause increased bone formation and reduced vasculature. This cell-free and resorbable DAM-POC scaffold is expected to be a new approach for cleft palate repair [42] (Table 1).
5.2 Commercial-decellularized scaffold (Table 2)
5.2.1 Acellular-dermal matrix
Acellular-dermal matrix (ADM) is a kind of dermal substitute obtained by special treatment of allogeneic dermis to remove its cellular components. It was first developed by Liesey’s group of LifeCell Corporation in 1994 and named AlloDerm. In the next year, this material was used in the management of full-thickness burns by Wainwright [43]. To date, ADM has been widely used in various fields, including stomatology, and many businesses are devoted to the production of ADM. At present, ADM products on the market for stomatology can be used to repair various soft and hard tissues of the oral cavity.
5.2.1.1 Gingival recession
Periodontitis, including periodontitis and gingivitis, is one of the major diseases that seriously affects human oral health. Typically, healthy gingiva covers the entire root of the tooth. However, with the occurrence of periodontitis, the gums begin to recede toward the root, leading to root exposure. According to Millers, gingival recession can be classified into four classes: Miller’s Class I to IV. Coronally advanced flaps, combined with subepithelial connective tissue grafts (SCTGs), are considered the gold standard. However, researchers found that the marginal tissue contour at the site repaired with SCTG was greater than that in adjacent areas in 2012, and Thamil tried to look for other alternatives [44]. Similar to other researchers who utilized acellular dermal matrix in periodontal surgery [45, 46], they also investigated this matrix. Fifteen patients with Miller’s Class I gingival recession were divided into the SCTG group and the ADM group. Six months after surgery, the researchers confirmed that the results of the ADM group were equal to those of the SCTG group, such as probing pocket depth, gingival recession depth, clinical attachment level and recession coverage. The color of the ADM group was better than that of the SCTG group, indicating that decellularized dermal matrix could be a substitute for SCTG [47]. This conclusion is consistent with other studies that described the effect of ADM and SCTG in the treatment of Miller Class I and II gingival recession defects [48, 49]. For gingival recession repair, coronally advanced flaps can also result in good clinical results [50]. A meta-analysis in 2020 showed that compared to coronally advanced flaps with or without CTG, ADM had a more significant effect on recovering root coverage [51]. Although ADM seems like an optimal method, it also has disadvantages. During long-term use, although ADM can promote keratinized tissue and speed up the healing rate, a slight recession may occur [52,53,54,55].
5.2.1.2 Frey’s syndrome
Frey’s syndrome, or gustatory sweating, almost always appears after parotidectomy. The cause of the disease is neurotmesis of the auriculotemporal nerve and the parasympathetic nerve, which dominates the secretory function of the parotid gland regeneration. The nerves are dislocated and healed with the sympathetic nerve endings of the severed original innervating sweat glands and subcutaneous blood vessels. Therefore, chewing and taste stimulation cause facial flushing, sweating and parasympathetic nerve excitement. Decellularized dermal matrix was used to prevent Frey’s syndrome. Generally, judgments of the repair effect are divided into subjective and objective. The subjective evaluation is mainly that patients independently noted the phenomenon of flushing and sweating in the operation area when eating, while the objective assessment is mainly starch-iodine test. Ye et al. used ADM to treat 64 patients after parotidectomy. Compared to that of 104 patients with superficial or partial parotidectomy alone, the incidence of Frey’s syndrome in the ADM group was significantly lower [56]. In another study, twenty-four of fifty patients with benign parotid gland tumors underwent dermal matrix repair surgeries after superficial parotidectomy. The remaining patients were assigned to the non-ADM group. According to the feedback of the clinical questionnaire and starch-iodine test within one and a half years after the operation, doctors observed that ADM could reduce the incidence of Frey’s syndrome. Patients in the repair group showed no signs of immune rejection, infection, hematoma, or salivary fistula [57]. Moreover, the effectiveness of ADM in preventing infraauricular depressed deformities following parotidectomy has also been demonstrated in some cases [58, 59]. In summary, ADM can effectively control the occurrence of Frey’s syndrome.
5.2.1.3 Cleft palate repair
Cleft palate is a very common type of oral malformation. It manifests as a soft or hard palate inside the oral cavity, or the bony structure has cracked. Cleft palate repair surgery is the most important means of repair. Unfortunately, palatal or oronasal fistula is a common complication after surgery [60,61,62,63]. Thus, Aziz’s group used cellular dermal grafts (DermaMatrix, Synthes, PA, USA) to assist primary palatoplasty to control the incidence of palatal fistula [64]. Yi et al. also tried to use ADM to control this complication. These researchers found that ADM can prevent fistula formation and that even exposure will not cause fistula formation [65]. In the case where fistula has already formed, Calì and his team combined ADM with a mucoperiosteal flap to repair oronasal fistula after palatoplasty and obtained a 100% closure rate of fistula [66]. It was reported that five patients with recurrent oronasal fistulas underwent ADM grafting surgeries in 2006, and they had not recrudesced again after treatment [67]. Nonetheless, more data are needed to prove its feasibility for widespread use [68, 69].
Apart from those diseases, this material is also used to prevent alveolar osteitis [70], reconstruct the tongue [71], repair peri-implantitis [72], reconstruct periodontal tissue [73] and relieve disturbance syndrome of the temporomandibular joint [74]. Overall, acellular-dermal matrix is a promising material for repairing various oral and maxillofacial prosthetics.
5.2.2 Other products
With the development of acellular-dermal matrix, there are other decellularized products that are gradually being used in stomatology. A thoral GEN surgical patch, a porcine decellularized pericardial patch, was also used after superficial parotidectomy by Wang et al. These researchers found that the patch reduced the incidence rate of the syndrome, and it may be an excellent choice to prevent this complication as well [75].
Rabie et al. used Avance, a decellularized nerve allograft, to cure inferior alveolar nerve injury. The patient regained partial sensation by five months after surgery. Nevertheless, the postponement of referral could result in additional degeneration [76]. Although there are few commercial products used for dental restorations, with more research and attempts, there will be an increasing number of commercialized decellularized scaffolds in the future.
In brief, decellularization, a tissue engineering technique, has only three main methods, but many protocols can be developed according to different combinations of the methods. Interestingly, there are different decellularization methods for different organs and tissues, and the decellularization methods are multitudinous for organs. Although there is no way to completely remove the cellular components, scientists are actively applying decellularized materials for disease treatments to find the best scaffold for regeneration and repair. We believe that decellularized scaffolds will fully meet the requirements of repair in the future, not only in stomatology but in other medical fields as well.
References
Matoug-Elwerfelli M, Duggal MS, Nazzal H, Esteves F, Raïf E. A biocompatible decellularized pulp scaffold for regenerative endodontics. Int Endod J. 2018;51:663–73.
Zhou L, Wang Z, Wang Z, Zhu J, Feng Y, Zhang D, et al. Effect of heparinization on promoting angiogenesis of decellularized kidney scaffolds. J Biomed Mater Res A. 2021;109:1979–89.
Zhao L, Huang L, Yu S, Zheng J, Wang H, Zhang Y. Decellularized tongue tissue as an in vitro model for studying tongue cancer and tongue regeneration. Acta Biomater. 2017;58:122–35.
Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
McCrary MW, Bousalis D, Mobini S, Song YH, Schmidt CE. Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues. Acta Biomater. 2020;111:1–19.
Gao Z, Wu T, Xu J, Liu G, Xie Y, Zhang C, et al. Generation of bioartificial salivary gland using whole-organ decellularized bioscaffold. Cells Tissues Organs. 2014;200:171–80.
Lee JS, Cho AN, Jin Y, Kim J, Kim S, Cho SW. Bio-artificial tongue with tongue extracellular matrix and primary taste cells. Biomaterials. 2018;151:24–37.
Yao Y, Lin W, Zhang Y. Fabrication of tongue extracellular matrix and reconstitution of tongue squamous cell carcinoma in vitro. J Vis Exp. 2018;136:57235.
Mahdavishahri N, Moghatam Matin M, Fereidoni M, Yarjanli Z, Banihashem Rad SA, Khajeh Ahmadi S. In vitro assay of human gingival scaffold in differentiation of rat’s bone marrow mesenchymal stem cells to keratinocystes. Iran J Basic Med Sci. 2012;15:1185–90.
Naderi S, Khayat Zadeh J, Mahdavi Shahri N, Nejad Shahrokh Abady K, Cheravi M, Baharara J, et al. Three-dimensional scaffold from decellularized human gingiva for cell cultures: glycoconjugates and cell behavior. Cell J. 2013;15:166–75.
Zhang X, Li H, Sun J, Luo X, Yang H, Xie L, et al. Cell-derived micro-environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif. 2017;50:e12361.
Farag A, Vaquette C, Hutmacher DW, Bartold PM, Ivanovski S. Fabrication and characterization of decellularized periodontal ligament cell sheet constructs. Methods Mol Biol. 2017;1537:403–12.
Farag A, Vaquette C, Theodoropoulos C, Hamlet SM, Hutmacher DW, Ivanovski S. Decellularized periodontal ligament cell sheets with recellularization potential. J Dent Res. 2014;93:1313–9.
Farag A, Hashimi SM, Vaquette C, Bartold PM, Hutmacher D, Ivanovski S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J Clin Periodontol. 2018;45:586–96.
Gong T, Heng BC, Xu J, Zhu S, Yuan C, Lo EC, et al. Decellularized extracellular matrix of human umbilical vein endothelial cells promotes endothelial differentiation of stem cells from exfoliated deciduous teeth. J Biomed Mater Res A. 2017;105:1083–93.
Mendibil U, Ruiz-Hernandez R, Retegi-Carrion S, Garcia-Urquia N, Olalde-Graells B, Abarrategi A. Tissue-specific decellularization methods: rationale and strategies to achieve regenerative compounds. Int J Mol Sci. 2020;21:5447.
Farag A, Hashimi SM, Vaquette C, Volpato FZ, Hutmacher DW, Ivanovski S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch Oral Biol. 2018;88:67–76.
de Sousa Iwamoto LA, Duailibi MT, Iwamoto GY, Juliano Y, Duailibi MS, Ossamu Tanaka FA, et al. Tooth tissue engineering: tooth decellularization for natural scaffold. Future Sci OA. 2016;2:FSO121.
Song JS, Takimoto K, Jeon M, Vadakekalam J, Ruparel NB, Diogenes A. Decellularized human dental pulp as a scaffold for regenerative endodontics. J Dent Res. 2017;96:640–6.
Bakhtiar H, Rajabi S, Pezeshki-Modaress M, Ellini M, Panahinia MR, Alijani S, et al. Optimizing methods for bovine dental pulp decellularization. J Endod. 2020;47:62–8.
Son H, Jeon M, Choi HJ, Lee HS, Kim IH, Kang CM, et al. Decellularized human periodontal ligament for periodontium regeneration. PLoS One. 2019;14:e0221236.
Naik A, Griffin MF, Szarko M, Butler PE. Optimizing the decellularization process of human maxillofacial muscles for facial reconstruction using a detergent-only approach. J Tissue Eng Regen Med. 2019;13:1571–80.
Hu L, Gao Z, Xu J, Zhu Z, Fan Z, Zhang C, et al. Decellularized swine dental pulp as a bioscaffold for pulp regeneration. Biomed Res Int. 2017;2017:9342714.
Li J, Rao Z, Zhao Y, Xu Y, Chen L, Shen Z, et al. A decellularized matrix hydrogel derived from human dental pulp promotes dental pulp stem cell proliferation, migration, and induced multidirectional differentiation in vitro. J Endod. 2020;46:1438-47.e5.
Shin K, Koo KH, Jeong J, Park SJ, Choi DJ, Ko YG, et al. Three-dimensional culture of salivary gland stem cell in orthotropic decellularized extracellular matrix hydrogels. Tissue Eng Part A. 2019;25:1396–403.
Paduano F, Marrelli M, White LJ, Shakesheff KM, Tatullo M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS One. 2016;11:e0148225.
Sangkert S, Meesane J, Kamonmattayakul S, Chai WL. Modified silk fibroin scaffolds with collagen/decellularized pulp for bone tissue engineering in cleft palate: morphological structures and biofunctionalities. Mater Sci Eng C Mater Biol Appl. 2016;58:1138–49.
Xu QL, Furuhashi A, Zhang QZ, Jiang CM, Chang TH, Le AD. Induction of salivary gland-like cells from dental follicle epithelial cells. J Dent Res. 2017;96:1035–43.
Heng BC, Zhu S, Xu J, Yuan C, Gong T, Zhang C. Effects of decellularized matrices derived from periodontal ligament stem cells and SHED on the adhesion, proliferation and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue cell. 2016;48:133–43.
Xiong X, Yang X, Dai H, Feng G, Zhang Y, Zhou J, et al. Extracellular matrix derived from human urine-derived stem cells enhances the expansion, adhesion, spreading, and differentiation of human periodontal ligament stem cells. Stem Cell Res The. 2019;10:396.
Nakamura N, Ito A, Kimura T, Kishida A. Extracellular matrix induces periodontal ligament reconstruction in vivo. Int J Mol Sci. 2019;20:3277.
Wen Y, Yang H, Wu J, Wang A, Chen X, Hu S, et al. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics. 2019;9:4265–86.
Lee JS, Kim HS, Park SY, Kim TW, Jung JS, Lee JB, et al. Synergistic effects of a calcium phosphate/fibronectin coating on the adhesion of periodontal ligament stem cells onto decellularized dental root surfaces. Cell Transplant. 2015;24:1767–79.
Lee JS, Kim SK, Gruber R, Kim CS. Periodontal healing by periodontal ligament fiber with or without cells: a preclinical study of the decellularized periodontal ligament in a tooth replantation model. J Periodontol. 2020;91:110–9.
Nguyen MTN, Tran HLB. Effect of modified bovine pericardium on human gingival fibroblasts in vitro. Cells Tissues Organs. 2018;206:296–307.
Duisit J, Maistriaux L, Taddeo A, Orlando G, Joris V, Coche E, et al. Bioengineering a human face graft: the matrix of identity. Ann Surg. 2017;266:754–64.
Burghartz M, Lennartz S, Schweinlin M, Hagen R, Kleinsasser N, Hackenberg S, et al. Development of human salivary gland-like tissue in vitro. Tissue Eng Part A. 2018;24:301–9.
Guo K, Wang W, Liu Z, Xu W, Zhang S, Yang C. Reliability of acellular decalcified and decalcified teeth as bone graft material: an experimental and pathological study in rats. Int J Clin Exp Pathol. 2020;13:837–45.
Ivanov AA, Latyshev AV, Butorina NN, Domoratskaya EI, Danilova TI, Popova OP. Osteogenic potential of decellularized tooth matrix. Bull Exp Biol Med. 2020;169:512–5.
Scarano A. Maxillary sinus augmentation with decellularized bovine compact particles: a radiological, clinical, and histologic report of 4 cases. Biomed Res Int. 2017;2017:2594670.
Kakabadze A, Mardaleishvili K, Loladze G, Karalashvili L, Chutkerashvili G, Chakhunashvili D, et al. Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncol Lett. 2017;13:1811–8.
Li W, Fu Y, Jiang B, Lo AY, Ameer GA, Barnett C, et al. Polymer-integrated amnion scaffold significantly improves cleft palate repair. Acta Biomater. 2019;92:104–14.
Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8.
McGuire MK, Scheyer ET, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue: comparison of clinical parameters at 10 years. J Periodontol. 2012;83:1353–62.
Yan-mei Z, Yue-qin S. Study of acellular allogeneic dermal matrix on periodontal surgery. Chin J Practical Stomatol. 2009;2:55–7.
de Resende DRB, Greghi SLA, Siqueira AF, Benfatti CAM, Damante CA, Ragghianti Zangrando MS. Acellular dermal matrix allograft versus free gingival graft: a histological evaluation and split-mouth randomized clinical trial. Clin Oral Investig. 2019;23:539–50.
Muthuraj TS, Bagchi S, Bandyopadhyay P, Mallick S, Ghosh P, Renganath MJ. A randomized split mouth clinical study to compare the clinical outcomes of subepithelial connective graft and acellular dermal matrix in Miller’s Class I recession coverage therapy. J Indian Soc Periodontol. 2020;24:342–7.
Taiyeb Ali TB, Shapeen IM, Ahmed HB, Javed F. Efficacy of acellular dermal matrix and autogenous connective tissue grafts in the treatment of gingival recession defects among Asians. J Investig Clin Dent. 2015;6:125–32.
Suzuki KT, de Jesus Hernandez Martinez C, Suemi MI, Palioto DB, Messora MR, de Souza SLS, et al. Root coverage using coronally advanced flap with porcine-derived acellular dermal matrix or subepithelial connective tissue graft: a randomized controlled clinical trial. Clin Oral Investig. 2020;24:4077–87.
Cortellini P, Pini Prato G. Coronally advanced flap and combination therapy for root coverage Clinical strategies based on scientific evidence and clinical experience. Periodontol 2000. 2012;59:158–84.
Moraschini V, Calasans-Maia MD, Dias AT, de Carvalho Formiga M, Sartoretto SC, Sculean A, et al. Effectiveness of connective tissue graft substitutes for the treatment of gingival recessions compared with coronally advanced flap: a network meta-analysis. Clin Oral Investig. 2020;24:3395–406.
Balderrama ÍF, Ferreira R, Rezende DRB, Nogueira ALRN, Greghi SLA, Zangrando MSR. Root coverage stability with acellular dermal matrix in multiple gingival recessions in esthetic zone: a clinical case report with 12-year follow-up. J Indian Soc Periodontol. 2019;23:584–8.
Barootchi S, Tavelli L, Gianfilippo RD, Eber R, Stefanini M, Zucchelli G, et al. Acellular dermal matrix for root coverage procedures: 9-year assessment of treated isolated gingival recessions and their adjacent untreated sites. J Periodontol. 2021;92:254–62.
Shi Y, Segelnick SL, El Chaar ES. A modified technique of tacking acellular dermal matrix to increase keratinized mucosa around dental implants as an alternative to a free gingival graft: a case report. Clin Adv Periodontics. 2020;10:175–80.
Tavelli L, Barootchi S, Di Gianfilippo R, Modarressi M, Cairo F, Rasperini G, et al. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12-year follow-up from a randomized clinical trial. J Clin Periodontol. 2019;46:937–48.
Ye WM, Zhu HG, Zheng JW, Wang XD, Zhao W, Zhong LP, et al. Use of allogenic acellular dermal matrix in prevention of Frey’s syndrome after parotidectomy. Br J Oral Maxillofac Surg. 2008;46:649–52.
Al-Aroomi MA, Mashrah MA, Al-Aroomi OA, Al-Worafi NA, Al-Sharani HM, Sun C, et al. Acellular dermal matrix for prevention of Frey’s syndrome after superficial parotidectomy of benign tumors. Am J Otolaryngol. 2021;42:102893.
Choi J, Park SI, Rha EY, Seo BF, Kwon H, Jung SN. Acellular dermal matrix (Insuregraf) in the prevention of Frey’s syndrome and surgical site depression after parotidectomy. Arch Craniofac Surg. 2019;20:176–80.
Luo W, Zheng X, Chen L, Jing W, Tang W, Long J, et al. The use of human acellular dermal matrix in the prevention of infra-auricular depressed deformities and Frey’s syndrome following total parotidectomy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e9-13.
Tse RW, Siebold B. Cleft palate repair: description of an approach, its evolution, and analysis of postoperative fistulas. Plast Reconstr Surg. 2018;141:1201–14.
Smyth AG, Wu J. Cleft palate outcomes and prognostic impact of palatal fistula on subsequent velopharyngeal function-A retrospective cohort study. Cleft Palate Craniofac J. 2019;56:1008–12.
Saralaya S, Desai AK, Kumar N. Difficulty index-based management of palatal fistula after primary cleft palate repair: an institutional experience. J Oral Maxillofac Surg. 2019;77:851.e1–7.
Kim JY, Kim SG, Park YW, Hwang DS, Paeng JY, Seok H. The effect of buccal fat pad graft in the palatoplasty and the risk factor of postoperative palatal fistula. J Craniofac Surg. 2020;31:658–61.
Aziz SR, Rhee ST, Ziccardi VB. Acellular dermal graft augmentation of primary palatoplasty: case report and review of the literature. J Oral Maxillofac Surg. 2011;69:1221–4.
Yi CR, Jeon DN, Choi JW, Oh TS. Primary palatoplasty with intravelar veloplasty using acellular dermal matrix interpositional graft. J Craniofac Surg. 2021;32:252–6.
Calì Cassi L, Massei A. The use of acellular dermal matrix in the closure of oronasal fistulae after cleft palate repair. Plast Reconstr Surg Glob Open. 2015;3:e341.
Cole P, Horn TW, Thaller S. The use of decellularized dermal grafting (AlloDerm) in persistent oro-nasal fistulas after tertiary cleft palate repair. J Craniofac Surg. 2006;17:636–41.
Agir H, Eren GG, Yasar EK. Acellular dermal matrix use in cleft palate and palatal fistula repair: a potential benefit? J Craniofac Surg. 2015;26:1517–22.
Aldekhayel SA, Sinno H, Gilardino MS. Acellular dermal matrix in cleft palate repair: an evidence-based review. Plast Reconstr Surg. 2012;130:177–82.
Canellas JVDS, Fraga SRG, Santoro MF, Netto JNS, Tinoco EMB. Intrasocket interventions to prevent alveolar osteitis after mandibular third molar surgery: a systematic review and network meta-analysis. J Craniomaxillofac Surg. 2020;48:902–13.
Alsini AY, Sayed S, Alkaf HH, Abdelmonim SK, Alessa MA. Tongue reconstruction post partial glossectomy during the COVID-19 pandemic. A case report. Ann Med Surg (Lond). 2020;59:53–6.
Wang CW, Ashnagar S, Gianflippo RD, Arnett M, Kinney J, Wang HL. Laser-assisted regenerative surgical therapy for peri-implantitis: a randomized controlled clinical trial. J Periodontol. 2020;92:378–88.
Nica C, Lin Z, Sculean A, Asparuhova MB. Adsorption and release of growth factors from four different porcine-derived collagen matrices. Materials (Basel). 2013;13:2635.
Patel MH, Kim RY, Aronovich S, Skouteris CA. Clinical assessment of acellular dermal matrix (AlloDerm©) as an option in the replacement of the temporomandibular joint disc: a pilot study. J Stomatol Oral Maxillofac Surg. 2020;121:496–500.
Wang C, Wu D, Mao C, Lu M, Cai Z, Lai Y, et al. The preventive effect of decellularized pericardial patch against Frey’s syndrome following the superficial parotidectomy. J Craniomaxillofac Surg. 2019;47:832–6.
Shanti RM, Ziccardi VB. Use of decellularized nerve allograft for inferior alveolar nerve reconstruction: a case report. J Oral Maxillofac Surg. 2011;69:550–3.
Acknowledgements
This study was supported by the Construction Projects of Medical Biomaterial Research & Development Talent Base in Guizhou Province and Zunyi City (No. [2018]3, and No. [2019] 69).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, A., Li, H., Liu, J. et al. The Progress of Decellularized Scaffold in Stomatology. Tissue Eng Regen Med 19, 451–461 (2022). https://doi.org/10.1007/s13770-022-00432-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00432-w