Abstract
The effects of CaTiO3 (CT) and BaZrO3 (BZ) modification upon the crystal structure and electromechanical properties of lead‐free Bi0.5Na0.5TiO3–SrTiO3 piezoelectric ceramics were compared within a doping range of 0–4 mol%. The different effects of CT and BZ modification upon the phase transition are clearly observed in the polarization and strain hysteresis loops. The CT‐modified specimens maintain strong ferroelectricity without any abnormal enhancement in the electric field‐induced strain. However, the addition of as little as 1 mol% BZ induces a transition from a nonergodic relaxor phase to an ergodic relaxor phase, thus resulting in disruption of the ferroelectric order and the generation of a high field‐induced strain. The present authors believe that the substitution of large ions (such as Zr4+) into the B‐sites, rather than the A‐sites, of the Bi0.5Na0.5TiO3‐based ceramics plays a significant role in the phase transition behavior.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, lead‐free piezoceramics have become increasingly important due to the requirement for new alternatives to lead‐based ceramics such as Pb(Zr1−xTix)O3 (PZT) in response to environmental issues [1, 2, 3, 4]. Since first discovery by Smolenskii et al. in 1954 [5], the (Bi0.5Na0.5)TiO3 (BNT) ceramics have been recognized among the most important and superior lead‐free materials due to their high Curie temperature (TC) of 320 °C and relatively large remnant polarization (Pr) of about 38 μC/cm2 at room temperature. Nevertheless, the effective poling of pure BNT ceramics is difficult because of their large coercive field (EC = 7.3 kV/mm) and high conductivity, which is a disadvantage for the purpose of application. To overcome these problems, chemical modification (ion substitution or solid solutions) is a useful and widely employed method for improving the electrical performances of BNT materials via the construction of phase boundaries [1, 4, 6].
Two kinds of morphotropic phase boundary (MPB) exist in BNT ceramics, namely the typical MPB, or MPB(I), and the polymorphic phase transition, or MPB(II) [7, 8]. In the MPB(I) boundary, the coexistence of ferroelectric rhombohedral and tetragonal phases results in the significant piezoelectricity which can be obtained in (1 − x)BNT–xBaTiO3 (BNT–BT) and (1 − x)BNT–x(Bi0.5K0.5)TiO3 (BNKT) material systems [9, 10]. By contrast, the MPB(II) boundary involves a phase transition from ferroelectric to antiferroelectric or from ferroelectric to relaxor ferroelectric (FE‐RFE), is temperature‐dependent, and has recently received significant attention due to its high electromechanical strain properties [8, 11, 12, 13, 14, 15, 16, 17, 18]. Currently, the solid solution (1 − x)BNT–xSrTiO3 (BNST) is considered to be an important binary system among BNT‐based ceramics and is reported to form a rhombohedral ferroelectric and pseudocubic MPB at x = 0.24–0.28 that results in an ultra‐high strain of 0.25% and normalized strain (Smax/Emax) of 600 pm/V [14, 15, 19, 20, 21]. The origin of large strain in the BNT‐based systems is known to be associated with the reversible electric field‐induced phase transition from an ergodic relaxor to a ferroelectric state [2, 22, 23]. Furthermore, a similarly large electric field‐induced strain has been achieved in many BNT‐based ceramics via elemental modification at the A‐ and/or B‐sites, e.g., by the substitution of Fe and Nb into the B‐site of BNT‐BT [24], the substitution of Ta into BNKT [25], the co‐doping of BNKT with Li/Nb [26], or Li‐modification of the A‐sites in BNKT‐BT [27]. Interestingly, in previous work on the modification of BNKT with Nb [13], Cu [28], Sn [29], and Ta [30], the present researchers have found that the FE‐RFE phase transition occurs when the dopant modifier leads to a decrease in the Goldschumidt's tolerance factor (t), which is given by the following equation [31]:
where rA, rB, and rO are the ionic radii of A‐ and B‐site cations, and oxygen, respectively.
Similar phenomena are observed in BNKT modified with other ABO3 compounds such as LiTaO3 [32] and CaZrO3 [33]. Hence, it would be interesting to confirm whether a FE‐RFE transition could be induced in the 0.76BNT–0.24ST ceramics via modification with an ABO3‐type compound which leads to a decreasing tolerance factor. In the present work, CaTiO3 (CT) and BaZrO3 (BZ) are selected as two A2+B4+O3‐type end‐members to form two kinds of ternary BNST–A2+B4+O3 solid solution. Using the Shannon ionic radii, the tolerance factors of CT and BZ are calculated to be 0.966 and 1.01, respectively. Thus, in comparison with the tolerance factor of 0.984 for 0.76BNT–0.24ST (abbreviated to BNST hereafter), BZ substitution increases the t‐value, while CT modification decreases the t‐value. This strongly suggests that CT and BZ modifiers exert distinct effects on the ferroelectric properties and could thereby provide further insights into the FE‐RFE phase transition of the BNST‐based ceramic. Hence, the present paper compares the effect of CT and BZ modification upon the crystal structure, dielectric, ferroelectric and field‐induced strain properties of the BNST ceramics.
Results and Discussion
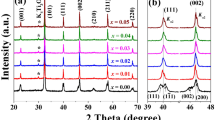
The X‐ray diffraction (XRD) patterns of the CT‐ and BZ‐modified BNST ceramics are presented in Fig. 1. All specimens exhibit a single perovskite structure without any discernible tracks of secondary phase within the resolution limit of the XRD equipment. This implies that the CT and BZ have diffused into the BNST lattice to form complete solid solutions of BNST‐CT and BNST‐BZ. In addition, both CT100x and BZ100y ceramics display the cubic phases, as indicated by the lack of (111) and (200) peak splitting (except for the Kα2 peaks) at 2θ values of approximately 40° and between 46 and 47°, respectively.
In spite of having the same phase, the addition of CT or BZ leads to completely different evolutions of the lattice parameters in the structures of the BNST ceramics. Thus, whereas the (111) and (200) diffraction peaks of the CT100x ceramics are seen to gradually shift toward higher angles with an increasing concentration of CT, a shift toward lower 2θ values is observed in the BZ100y ceramics. This result implies that the lattice parameter decreases with CT substitution but increases with BZ modification. The origin of this contrast is due to the size difference between the substituent and lattice ions. According to a Shannon's report [34], in the case of 12‐fold coordinated A‐site ions, Ba2+ shows a relatively large ionic radius (rBa2+ = 1.61 Å) compared with Ca2+ (rCa2+ = 1.34 Å) and with the average size of the A‐site ions in BNST (rBi3+ = 1.36 Å, rNa+ = 1.39 Å, rSr2+ = 1.44). It is noted that the ion radii of Bi3+ for 12‐fold coordination are obtained by extrapolation of its value for the lower coordination numbers [35]. In the case of the 6‐fold coordinated B‐site ions, the radius of Zr4+ is also bigger than that of Ti4+ (rZr4+ = 0.72 Å, rTi4+ = 0.605 Å).
The FE‐SEM surface micrographs of the polished and thermally etched CT‐ and BZ‐modified BNST ceramics in Fig. 2 reveal clear differences in the effects of each modifier upon the ceramic microstructures. Thus, while the various CT100x ceramics display almost identical microstructures, the addition of CT clearly increases the grain size. According to the linear intercept method, the average grain size drastically increases from 4.4 μm for CT0 to around 6.6 μm for CT4, thus demonstrating that CT modification facilitates the growth of grains in the BNST ceramics. By contrast, BZ addition alters the grain morphology from a faceted to a rounder structure and produces a notable decrease in grain size to around 2.1 μm for BZ4, thus indicating that the dissolution of BZ inhibits the grain growth in the BNST ceramics. Since the solid‐state sintering process occurs via diffusion‐controlled mass transfer through the solid phase, the contrasting effects of CT and BZ upon the ceramic microstructure may be due to the difference in ionic radii of Ca2+, Ba2+, and Zr4+. In the case of BZ modification, the smaller Na+, Bi3+, and Ti4+ ions (1.39, 1.36, and 0.605 Å, respectively) are replaced by the larger Ba2+ and Zr4+ ions (1.61 and 0.72 Å, respectively), leading to a decrease in the overall cationic transport due to their lower ion mobility, thus diminishing the diffusion rate and thereby reducing the grain size [36, 37]. By contrast, the presence of the smaller Ca2+ (1.34 Å) in the CT‐modified ceramics results in greater ion mobility, which promotes a rapid interdiffusion via the grain boundary, speeding up of the grain growth [38, 39]. Similar changes in the microstructures of binary BNT‐CT and BNT‐BZ ceramics have been reported in other studies [40, 41].
In addition, the FE‐SEM images of the pure BNST ceramics (CT0 and BZ0, Fig. 2) display rough surfaces which are indicative of domain structures similar to those observed by the present authors in previous work on BNST ceramics produced by thermal etching [15]. In the present work, the domain patterns are seen to persist and extend through the entire volume of the grains with an increasing CT concentration, but become less pronounced and decrease in size with BZ modification. The difference in the domain structure between the CT‐ and BZ‐modified ceramics influences their electrical and mechanical properties, as described in the following paragraphs.
The temperature‐dependent dielectric properties of the poled and unpoled CT100x and BZ100y ceramics measured at various frequencies are displayed in Fig. 3. Here, two distinct anomalies are observed in the dielectric curve of the unmodified ceramics, including (i) a hump at the ferroelectric–relaxor phase transition temperature (TF–R) of 42 °C and (ii) a peak at the maximum dielectric constant temperature (Tm) of around 180 °C. With the addition of CT, the Tm increases while TF–R decreases slightly from 42 °C for the unmodified ceramic to around 27 °C for the CT4. By contrast, BZ addition has a significant impact upon the TF–R and Tm values. In comparison with CT modification, the dielectric response of the BZ100y ceramics exhibits an increase in the broad of Tm and the frequency dispersion at the low temperature region becomes stronger with an increasing BZ concentration. This indicates that the relaxation characteristic is more evident in the BZ100y rather than in the CT100x system. Moreover, it is reported that the diffuse and frequency‐dependent dielectric constant at Tm is mainly attributed to the distortion of A‐ and B‐sites of the perovskite due to the substitution of isovalent cations [42, 43, 44, 45]. Thus, the different distortion degrees between Ca2+, Ba2+, and Zr4+ ions on the BNST lattice structure are assumed to be the main factor leading to the difference in relaxor behaviors of CT100x and BZ100y ceramics. Furthermore, with the addition of only 1 mol% BZ, no discernable TF–R is observed in the measured temperature range of 25–300 °C. Indeed, all the poled and unpoled BZ100y samples display similar dielectric curves with the absence of TF–R, thus demonstrating that their transition temperatures are below room temperature and, hence, the ergodic relaxor phase will be predominant in the BZ‐modified BNST ceramics. The different effects of CT and BZ modification upon the TF–R of the BNST ceramics could lead to distinct ferroelectric and strain properties, as discussed below.
The current versus electric field (I–E) and polarization hysteresis (P–E) loops of the CT‐ and BZ‐modified BNST ceramics measured at 6 kV/mm and room temperature are presented in Fig. 4. Here, the unmodified BNST ceramic displays a saturated square hysteresis loop indicative of a relatively large remnant polarization and a coercive field. The maximum polarization (Pmax), remnant polarization (Prem), and coercive field (EC) of the unmodified BNST are 38.83 μC/cm2, 31.48 μC/cm2, and 1.87 kV/mm, respectively. Moreover, four current peaks are observed in the I–E curve of the unmodified BNST. The first current peak, E1, appears during the loading of a positive field and relates to a domain switching and field‐induced phase transitions from a weak polar state to a strong polar state [46, 47]. The field‐induced ferroelectric order is irreversible, as evidenced by the absence of a current peak during the unloading, which is characteristic of a nonergodic relaxor material. The second current peak, E2 or depolarization field, appears when a negative field is applied which returns the strong polar state to the initial state. The third and fourth current peaks to appear during the loading and unloading of the negative electric field share similar functions to those of E1 and E2, respectively.
Significant differences in the effects of the CT and BZ modifiers upon the ferroelectric properties of the BNST ceramics can be observed in the Fig. 4. Here, the saturated P–E loop is maintained with the addition of CT even up to 4 mol%, whereas the BZ‐modified samples display a significant slimming of the loop with an increasing BZ concentration. For the CT100x samples, the Pmax and Prem values decreases slightly as the function of CT modification, while EC values remain fairly constant. In addition, the charts in Fig. 5 indicate relatively high Pmax and Prem values of 35.45 and 27.51 μC/cm2, respectively, for the CT4 sample. Furthermore, the CT100x samples display similar current profiles to that of the unmodified BNST ceramics, thus indicating a relatively weak influence of CT upon the ferroelectric properties of the BNST. By contrast, the addition of 1 mol% BZ is seen to result in a pinching of the P–E loop due to a shift of the depolarization field E2 to a positive value (0.67 kV/cm), thus indicating a complete reversion of the electric field‐induced ferroelectric state to the initial relaxor state. This is accompanied by drastic decreases in the Prem and EC values from 38.83 μC/cm2 and 1.87 kV/mm, respectively, for the unmodified sample, to 12.51 μC/cm2 and 1.51 kV/mm, respectively, for the BZ1 sample. Moreover, significant reductions in all the polarization parameters are observed with increasing BZ content, down to Pmax = 23.12 μC/cm2, Prem = 4.16 μC/cm2, and EC = 1.01 kV/cm for the BZ4 ceramic, thus resulting in slimmer P–E hysteresis loops. The changes in polarization hysteresis loops and current curves suggest that BZ substitution strongly induces a transition from the nonergodic relaxor phase to the ergodic relaxor phase in the BNST ceramic, in agreement with previous studies on ABO3‐modified BNT‐based ceramics [41, 45, 48, 49, 50].
The relationship between the bipolar strain and the applied electric field (S–E) hysteresis loops of the CT100x and BZ100y ceramics at 6 kV/mm is presented in Fig. 6, while the maximum strain (Smax) and negative strain (Sneg) values extracted from the S–E curves are plotted as a function of modifier content in Fig. 7. Thus, the undoped BNST ceramic is seen to exhibit a butterfly‐shaped curve (Fig. 6) with an Smax of 0.14% and an Sneg of 0.12% (Fig. 7), as has been typically observed in ferroelectric materials. In addition, a comparison of the S–E curves of CT100x and BZ100y ceramics reveals a similar difference to that noted above in the polarization profiles. Thus, the CT‐modified samples display no change in the butterfly strain curves with an increasing modifier content, along with only slight decreases in the Smax and Sneg values (to 0.12 and 0.08%, respectively) in the CT4. This retention of the Sneg value, which relates to irreversible domain back switching [2, 51, 52, 53], indicates the persistence of ferroelectric phase in the CT100x system within the doping range examined. By contrast, with the addition of 1 mol% BZ, the bipolar strain curve changes from a butterfly shape to a sprout shape and a drastic reduction in Sneg (to 0.02%) is observed, along with a significant increase in Smax (to 0.29%). This means that the entire ferroelectric order in the BNST is disrupted upon the addition of BZ, while the development of the ergodic relaxor phase leads to large strain values, as has been reported in previous studies [2, 16, 53, 54, 55, 56, 57]. Moreover, the disappearance of Sneg and the reduction in Smax with further increases in the BZ content confirm the complete stabilization of the ergodic relaxor state in the BZ100y ceramics.
The piezoelectric coefficient, d33, and the normalized strain, Smax/Emax, of the CT‐ and BZ‐modified BNST ceramics are compared in Fig. 8. Here, the unmodified BNST ceramics display the maximum d33 value of 158 pC/N, with a decrease in d33 being observed in the presence of each modifier. However, the level of decrease in d33 differs between the two modifiers, with a gradual decrease down to 102 pC/N for the CT4 specimen compared with a severe reduction to around 20 pC/N at only 1 mol% BZ content. The relatively high d33 value for the CT100x ceramics can be attributed to the presence of stable electric field‐induced ferroelectric domains, a conclusion which is supported by the high Prem and Sneg values (Figs. 4 and 6). By contrast, the sharp drop in the d33 for the BZ100y ceramics indicates a disruption of the long‐range ferroelectric domains, accompanied by an increase in the degree of ergodicity. For the BZ1 sample, the ratio of ferroelectric and ergodic ralaxor phases reaches a critical range, thus leading to the maximum Smax/Emax value of 498 pm/V [18, 56]. The outcomes are consistent with the above analysis results, in which BZ modification is seen to induce the transition from the nonergodic phase to the ergodic relaxor phase, whereas CT modification does not.
It is noted that the results are inconsistent with the present authors’ predictions regarding the relationship between the tolerance factor and the ferroelectric–relaxor phase transition [13, 28, 29, 30]. Thus, while the CT100x ceramics retain their ferroelectricity with decreasing t and do not undergo a phase transition within the investigated composition range (0–4 mol%), a rapid transition from ferroelectric to relaxor does occur in the BZ100y solid solutions. Indeed, this phenomenon has been observed in other related works. For example, in a study by Tran et al. [58], a similar contrast in the phase transition behavior was obtained for BT‐ and BZ‐modified BNKT ceramics, such that the ferroelectric order of the BNKT‐BZ was strongly destabilized but that of the BNKT‐BT was little affected [58]. Furthermore, a significant enhancement in the electric field‐induced strain relating to the ferroelectric–relaxor transition has previously been revealed with the substitution of 3 mol% CZ into BNKT ceramics [33], which has not been observed in the present work with the CT100x ceramics.
In view of these contrasting results, it is assumed that the FE‐RFE phase transition in A2+B4+O3‐modified BNT‐based ceramics depends strongly on the isovalent cation substitution for the Ti4+ ion on the B‐sites rather than the A‐sites. In the unit cell of BNT, the A‐sites are randomly occupied by the cations Na+ and Bi3+, while the Ti4+ ions are bonded to six O2− to form TiO6 octahedra. Recently, it has been reported that the TiO6 octahedral rotation plays an important role in establishing the relaxor phase in BNT‐based materials [59]. When isovalent cations such as Zr4+ and Sn4+ substitute for Ti4+, they can incorporate into the TiO6 octahedral lattices. The large ion radius and nonferroelectric activity of Zr4+ eventually cause the displacement of Ti4+ and distortion of the TiO6 octahedra, thus leading to disruption of the long‐range ferroelectric order [60, 61, 62, 63, 64, 65, 66, 67]. By contrast, the influence of divalent cations such as Ba2+ and Ca2+ upon the TiO6 octahedral rotation is thought to be less pronounced. Recently, nuclear magnetic resonance (NMR) analysis has been used to investigate the influence of barium addition upon the local structure of the titanium sites in BNT ceramics [68]. It was found that a strongly disordered local structure resulted from the random occupation of the A‐sites by Ba2+, whereas there was no distortion of the oxygen octahedra and no change in the polar displacement of Ti4+ [68]. Accordingly, it is concluded that the substitution of a certain amount of divalent ions into the A‐sites only, without any Zr4+ ions being present on B‐sites, could allow the establishment of a long‐range ferroelectric order under the application of an electric field.
Conclusion
The effects of CT and BZ modifications upon the crystal structure, microstructure, and electromechanical properties of lead‐free BNST ceramics were systematically compared in the present work. Each modifier was shown to exert a distinct influence upon the phase transition behavior in terms of the ferroelectric and strain properties. The CT substitution contributed little to the disruption of ferroelectric order in the BNST ceramics, as evidenced by the stable ferroelectricity and relatively high piezoelectric constant (d33 > 100 pC/N) within the composition range investigated (0–4 mol%). By contrast, the BZ modification was found to strongly induce a transition from the nonergodic relaxor state to the ergodic relaxor state, thus resulting in narrow P–E loops, disappearance of the piezoelectric properties and an abnormal enhancement of the electric field‐induced strain with the addition of 1 mol% BZ. These results suggest that the phase transition is greatly influenced by the substitution of large ions (such as Zr4+) onto B‐sites, rather than the A‐sites, leading to distortion of the TiO6 octahedra in the BNST ceramics. The present work is believed to be meaningful in clarifying the effect of ABO3 modification upon the FE‐RFE phase transition behavior and electromechanical properties of the BNST‐based ceramics.
Experimental Details
A range of ceramic powders conforming to the chemica formulas (0.76−x)Bi0.5Na0.5TiO3–0.24SrTiO3–xCaTiO3 (designated CT100x) and (0.76–y)Bi0.5Na0.5TiO3–0.24SrTiO3–yBaZrO3 (designated BZ100y), with x and y = 0, 0.01, 0.02, 0.03, and 0.04, were synthesized using a conventional solid‐state reaction route. The raw materials Bi2O3 (99.99%), Na2CO3 (99.0%), TiO2 (99.99%), SrCO3 (99.9%), CaCO3 (99.9%), BaCO3 (99.9%), and ZrO2 (99.9%) were weighted according to the nominal compositions after drying at 100 °C for 24 h. After weighing the raw powders according to their stoichiometric formulas, they were mixed and milled thoroughly for 24 h at 400 rpm in a polyethylene jar with ZrO2 balls (3 and 5 mm in diameter) with ethanol as solvent. The wet slurry was then dried at 100 °C for 24 h, followed by calcination at 850 °C for 2 h with a heating rate of 5 °C/min. The resulting powders were ball milled again with the same parameters. After drying, 10 wt% of polyvinyl alcohol (PVA) solution was added as a binder. After that, green bodies of 12 mm diameter and about 1.2 mm thickness were prepared and subsequently sintered in covered alumina crucibles at 1175 °C for 2 h with a heating rate of 5 °C/min.
The samples were characterized by XRD (RAD III, Rigaku, Japan) under monochromatic Cu Kα radiation with the wavelength λKα = 1.54178 Å. The detection range was 20–60° with a step size of 0.02° and a speed of 2°/min. The microstructure was studied by the field emission scanning electron microscope (FE‐SEM, JEOL, JSM‐65OFF, Japan). For measuring the electrical properties, the pellets were coated on both sides with silver paste and sintered at 700 °C for 30 min to form electrodes. The temperature‐ and frequency‐dependent permittivity and dielectric loss of unpoled and poled samples were measured using a high‐ temperature electric prober system (KEYSIGHT‐E4980AL Precision LCR Meter, USA). The electric field‐induced polarization and strain hysteresis loop measurements were performed using the commercial aixPES setup (aixACCT aixPES, Aachen, Germany).
References
J. Rödel, W. Jo, K.T.P. Seifert, E.M. Anton, T. Granzow, and D. Damjanovic: Perspective on the development of lead‐free piezoceramics. J. Am. Ceram. Soc. 92, 1153 (2009).
W. Jo, R. Dittmer, M. Acosta, J. Zang, C. Groh, E. Sapper, K. Wang, and J. Rödel: Giant electric‐field‐induced strains in lead‐free ceramics for actuator applications–status and perspective. J. Electroceram. 29, 71 (2012).
J. Rödel, K.G. Webber, R. Dittmer, W. Jo, M. Kimura, and D. Damjanovic: Transferring lead‐free piezoelectric ceramics into application. J. Eur. Ceram. Soc. 35, 1659 (2015).
J. Hao, W. Li, J. Zhai, and H. Chen: Progress in high‐strain perovskite piezoelectric ceramics. Mater. Sci. Eng., R 135, 1 (2019).
G.A. Smolenskii: New ferroelectrics of complex composition IV. Sov. Phys.‐Solid State 2, 2651 (1961).
T. Zheng, J. Wu, D. Xiao, and J. Zhu: Recent development in lead‐free perovskite piezoelectric bulk materials. Prog. Mater. Sci. 98, 552 (2018).
Y. Hiruma, H. Nagata, and T. Takenaka: Thermal depoling process and piezoelectric properties of bismuth sodium titanate ceramics. J. Appl. Phys. 105, 084112 (2009).
Y. Hiruma, H. Nagata, and T. Takenaka: Detection of morphotropic phase boundary of (Bi1/2Na1/2)TiO3–Ba(Al1/2Sb1/2)O3 solid‐solution ceramics. Appl. Phys. Lett. 95, 052903 (2009).
T. Takenaka, K.I. Maruyama, and K. Sakata: (Bi1/2Na1/2)TiO3‐BaTiO3 system for lead‐free piezoelectric ceramics. Jpn. J. Appl. Phys. 30, 2236 (1991).
A. Sasaki, T. Chiba, Y. Mamiya, and E. Otsuki: Dielectric and piezoelectric properties of (Bi0.5Na0.5)TiO3–(Bi0.5K0.5)TiO3 systems. Jpn. J. Appl. Phys. 38, 5564 (1999).
C.H. Hong, H.P. Kim, B.Y. Choi, H.S. Han, J.S. Son, C.W. Ahn, and W. Jo: Lead‐free piezoceramics–Where to move on? J. Materiomics 2, 1 (2016).
S.Y. Cho, E.Y. Kim, S.Y. Kim, T.L. Pham, J.K. Han, D.S. Song, H.K. Jung, J.S. Lee, K.S. An, J. Lim, and S.D. Bu: Relaxor phase evolution of (Bi0.5Na0.5‐xKx)TiO3 ceramics due to K ion substitution and their corresponding electrical properties. Energies 13, 455 (2020).
K.N. Pham, A. Hussain, C.W. Ahn, W. Kim, S.J. Jeong, and J.S. Lee: Giant strain in Nb‐doped Bi0.5(Na0.82K0.18)0.5TiO3 lead‐free electromechanical ceramics. Mater. Lett. 64, 2219 (2010).
S. Jo, C.H. Hong, D.S. Kim, H.W. Kang, C.W. Ahn, H.G. Lee, S. Nahm, W. Jo, and S.H. Han: Phase transition behavior and mechanical properties of (1‐ x)(Bi1/2Na1/2)TiO3‐xSrTiO3 lead‐free piezoelectric ceramics. Sens. Actuat. A 258, 201 (2017).
T.A. Duong, H.S. Han, Y.H. Hong, Y.S. Park, H.T.K. Nguyen, T.H. Dinh, and J.S. Lee: Dielectric and piezoelectric properties of Bi1/2Na1/2TiO3–SrTiO3 lead‐free ceramics. J. Electroceram. 41, 73 (2018).
G. Wang, Y.H. Hong, H.T.K. Nguyen, B.W. Kim, C.W. Ahn, H.S. Han, and J.‐S. Lee: High electromechanical strain properties in SrTiO‐modified Bi1/2Na1/2TiO3‒KTaO3 lead‐free piezoelectric ceramics under low electric field. Sens. Actuat. A 293, 1 (2019).
Y. Zhang, G. Liang, S. Tang, B. Peng, Q. Zhang, L. Liu, and W. Sun: Phase‐transition induced optimization of electrostrain, electrocaloric refrigeration and energy storage of LiNbO3 doped BNT‐BT ceramics. Ceram. Int. 46, 1343 (2020).
J. Yin, C. Zhao, Y. Zhang, and J. Wu: Ultrahigh strain in site engineering‐independent Bi0.5Na0.5TiO3‐based relaxor‐ferroelectrics. Acta Mater. 147, 70 (2018).
M. Acosta, W. Jo, and J. Rödel: Temperature‐and frequency‐dependent properties of the 0.75Bi1/2Na1/2TiO3–0.25SrTiO3 lead‐free incipient piezoceramic. J. Am. Ceram. Soc. 97, 1937 (2014).
H.L. Li, Q. Liu, J.J. Zhou, K. Wang, J.F. Li, H. Liu, and J.Z. Fang: Grain size dependent electrostrain in Bi1/2Na1/2TiO3‐SrTiO3 incipient piezoceramics. J. Eur. Ceram. Soc. 36, 2849 (2016).
H. He, X. Lu, M. Li, Y. Wang, Z. Li, Z. Lu, and L. Lu: Thermal and compositional driven relaxor ferroelectric behaviours of lead‐free Bi0.5Na0.5TiO3–SrTiO3 ceramics. J. Mater. Chem. C 8, 2411 (2020).
S.K. Gupta, R. McQuade, B. Gibbons, P. Mardilovich, and D.P. Cann: Electric field‐induced strain in Sr(Hf0.5Zr0.5)O3‐modified Bi0.5(Na0.8K0.2)0.5TiO3 piezoelectric ceramics. J. Appl. Phys. 127, 074104 (2020).
M. Habib, M. Munir, S.A. Khan, T.K. Song, M.H. Kim, M.J. Iqbal, I. Qazi, and A. Hussain: Evaluation of high strain response in lead‐free BNBTFS‐xNb ceramics by structure and ferroelectric characterizations. J. Phys. Chem. Solids 138, 109230 (2020).
R. Cheng, Z. Xu, R. Chu, J. Hao, J. Du, and G. Li: Electric field‐induced ultrahigh strain and large piezoelectric effect in Bi1/2Na1/2TiO3‐based lead‐free piezoceramics. J. Eur. Ceram. Soc. 36, 489 (2016).
X. Liu and X. Tan: Giant strains in non‐textured (Bi1/2Na1/2)TiO3‐based lead‐free ceramics. Adv. Mater. 28, 574 (2016).
C. Wang, T. Xia, and X. Lou: Large strain response in Li/Nb co‐doped Bi0.5(Na0.8K0.2)0.5TiO3 lead‐free piezoceramics. Ceram. Int. 44, 7378 (2018).
D.S. Yin, Z.H. Zhao, Y.J. Dai, Z. Zhao, X.W. Zhang, S.H. Wang, and S. Zhang: Electrical properties and relaxor phase evolution of Li‐modified BNT‐BKT‐BT lead‐free ceramics. J. Am. Ceram. Soc. 99, 2354 (2016).
H.S. Han, N.B. Do, K.N. Pham, H.D. Jang, V.D.N. Tran, W.P. Tai, and J.S. Lee: Sintering behaviour and piezoelectric properties of CuO‐added lead‐free Bi(Na,K)TiO3 ceramics. Ferroelectrics 421, 88 (2011).
H.S. Han, C.W. Ahn, I.W. Kim, A. Hussain, and J.S. Lee: Destabilization of ferroelectric order in bismuth perovskite ceramics by A‐site vacancies. Mater. Lett. 70, 98 (2012).
N.B. Do, H.B. Lee, C.H. Yoon, J.K. Kang, J.S. Lee, and I.‐W. Kim: Effect of Ta‐substitution on the ferroelectric and piezoelectric properties of Bi0.5(Na0.82K0.18)0.5TiO3 ceramics. Trans. Electron. Electron. Mater. 12, 64 (2011).
V.M. Goldschmidt: Geochemische verteilungsgesetze der elemente. Skrifter norske videnskaps Akad.(Oslo), I. Math.‐Naturwiss. Kl 8, 5 (1926).
V.Q. Nguyen, H.S. Han, K.J. Kim, D.D. Dang, K.K. Ahn, and J.S. Lee: Strain enhancement in Bi1/2(Na0.82K0.18)1/2TiO3 lead‐free electromechanical ceramics by co‐doping with Li and Ta. J. Alloys Compd. 511, 237 (2012).
I.K. Hong, H.S. Han, C.H. Yoon, H.N. Ji, W.P. Tai, and J.S. Lee: Strain enhancement in lead‐free Bi0.5(Na0.78K0.22)0.5TiO3 ceramics by CaZrO3 substitution. J. Intell. Mater. Syst. Struct. 24, 1343 (2012).
R.D. Shannon: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751 (1976).
C.W. Tai, S.H. Choy, and H.L.W. Chan: Ferroelectric domain morphology evolution and octahedral tilting in lead‐free (Bi1/2Na1/2)TiO3‐(Bi1/2K1/2)TiO3‐(Bi1/2Li1/2)TiO3‐BaTiO3 ceramics at different temperatures. J. Am. Ceram. Soc. 91, 3335 (2008).
C. Ciomaga, M. Viviani, M.T. Buscaglia, V. Buscaglia, L. Mitoseriu, A. Stancu, and P. Nanni: Preparation and characterisation of the Ba(Zr,Ti)O3 ceramics with relaxor properties. J. Eur. Ceram. Soc. 27, 4061 (2007).
S. Zixiong, P. Yongping, and L. Yuwen: Effect of Zr4+ content on the grain growth, dielectric relaxation behavior, and ferroelectric properties of Ba0.4Sr0.6Ti1−xZrxO3 nano‐ceramics prepared by different methods assisted by fast microwave sintering. J. Electron. Mater. 43, 1466 (2014).
T. Badapanda, S.K. Rout, L.S. Cavalcante, J.C. Sczancoski, S. Panigrahi, T.P. Sinha, and E. Longo: Structural and dielectric relaxor properties of Yttrium‐doped Ba(Zr0.25Ti0.75)O3 ceramics. Mater. Chem. Phys. 121, 147 (2010).
F. Guo, W. Cai, R. Gao, C. Fu, G. Chen, X. Deng, Z. Wang, and Q. Zhang: Microstructure, enhanced relaxor‐like behavior and electric properties of (Ba0.85Ca0.15)(Zr0.1−xHfxTi0.9)O3 ceramics. J. Electron. Mater. 48, 3239 (2019).
H.L. Du, X. Du, and H.L. Li: Phase structure and electrical properties of lead‐free Na0.5Bi0.5TiO3–CaTiO3 ceramics. Adv. Appl. Ceram. 112, 277 (2013).
J.U. Rahman, A. Hussain, A. Maqbool, T.K. Song, W.J. Kim, S.S. Kim, and M.H. Kim: Dielectric, ferroelectric and field‐induced strain response of lead‐free BaZrO3‐modified Bi0.5Na0.5TiO3 ceramics. Curr. Appl. Phys. 14, 331 (2014).
L. Gao, Y. Huang, Y. Hu, and H. Du: Dielectric and ferroelectric properties of (1−x)BaTiO3–xBi0.5Na0.5TiO3 ceramics. Ceram. Int. 33, 1041 (2007).
J.U. Rahman, A. Hussain, A. Maqbool, G.H. Ryu, T.K. Song, W.‐J. Kim, and M.H. Kim: Field induced strain response of lead‐free BaZrO3‐modified Bi0.5Na0.5TiO3–BaTiO3 ceramics. J. Alloys Compd. 593, 97 (2014).
A. Hussain, J.U. Rahman, A. Zaman, R.A. Malik, J.S. Kim, T.K. Song, W.J. Kim, and M.H. Kim: Field‐induced strain and polarization response in lead‐free Bi1/2(Na0.80K0.20)1/2TiO3–SrZrO3 ceramics. Mater. Chem. Phys. 143, 1282 (2014).
A. Maqbool, A. Hussain, J. Ur Rahman, T. Kwon Song, W.‐J. Kim, J. Lee, and M.‐H. Kim: Enhanced electric field‐induced strain and ferroelectric behavior of (Bi0.5Na0.5)TiO3–BaTiO3–SrZrO3 lead‐free ceramics. Ceram. Int. 40, 11905 (2014).
F. Li, R. Zuo, D. Zheng, L. Li, and D. Viehland: Phase‐composition‐dependent piezoelectric and electromechanical strain properties in (Bi1/2Na1/2)TiO3‐Ba(Ni1/2Nb1/2)O3 lead‐free ceramics. J. Am. Ceram. Soc. 98, 811 (2015).
S. Huband and P.A. Thomas: Depolarisation of Na0.5Bi0.5TiO3‐based relaxors and the resultant double hysteresis loops. J. Appl. Phys. 121, 184105 (2017).
Y. Zhu, Y. Zhang, B. Xie, P. Fan, M.A. Marwat, W. Ma, C. Wang, B. Yang, J. Xiao, and H. Zhang: Large electric field‐induced strain in AgNbO3‐modified 0.76Bi0.5Na0.5TiO3‐0.24SrTiO3 lead‐free piezoceramics. Ceram. Int. 44, 7851 (2018).
Y.H. Hong, H.S. Han, G.H. Jeong, Y.S. Park, T.H. Dinh, C.W. Ahn, and J.S. Lee: High electromechanical strain properties by the existence of nonergodicity in LiNbO3–modified Bi1/2Na1/2TiO3–SrTiO3 relaxor ceramics. Ceram. Int. 44, 21138 (2018).
M. Ullah, H. Ullah Khan, A. Ullah, A. Ullah, I.W. Kim, I. Qazi, and I. Ahmad: Dielectric, ferroelectric and piezoelectric properties of (1‐x)(Bi0.5Na0.5)0.935Ba0.065Ti‐x(LiSbO3) solid solutions. Ceram. Int. 44, 556 (2018).
W. Jo, T. Granzow, E. Aulbach, J. Rödel, and D. Damjanovic: Origin of the large strain response in (K0.5Na0.5)NbO3‐modified (Bi0.5Na0.5)TiO3–BaTiO3 lead‐free piezoceramics. J. Appl. Phys. 105, 094102 (2009).
H.S. Han, W. Jo, J.K. Kang, C.W. Ahn, I.W. Kim, K.K. Ahn, and J.S. Lee: Incipient piezoelectrics and electrostriction behavior in Sn‐doped Bi1/2(Na0.82K0.18)1/2TiO3 lead‐free ceramics. J. Appl. Phys. 113, 154102 (2013).
T.H. Dinh, V.D.N. Tran, T.T. Nguyen, Q.T.N. Hoang, H.S. Han, and J.S. Lee: The reduced reversible phase transition field of lead‐free Bi‐based ceramic composites by adding nonergodic relaxor. Ceram. Int. 43, 17160 (2017).
N. Mostovych, S.S. Won, I.W. Kim, S.H. Kim, and A.I. Kingon: Understanding the large strain behavior in the lead‐free doped Bi1/2(Na0.78K0.22)1/2TiO3–BiMg1/2Ti1/2O3 (BNKT‐BMT) piezoelectric system. AIP Adv. 10, 045033 (2020).
W. Bai, D. Chen, P. Zheng, B. Shen, J. Zhai, and Z. Ji: Composition‐ and temperature‐driven phase transition characteristics and associated electromechanical properties in Bi0.5Na0.5TiO3‐based lead‐free ceramics. Dalton Trans. 45, 8573 (2016).
N. Ullah Khan, A. Ullah, A. Ullah, M.Y. Khan, T.H. Kim, I.W. Kim, and C.W. Ahn: Boosting electrostriction and strain performance in bismuth sodium titanate‐based ceramics via introducing low tolerance factor chemical modifier. Sens. Actuat. A 291, 156 (2019).
Y. Si, Y. Li, L. Li, H. Li, Z. Zhao, and Y. Dai: Giant electro‐strain in textured Li‐doped 0.852BNT–0.11BKT–0.038BT ternary lead‐free piezoelectric ceramics. J. Am. Ceram. Soc. 103, 1765 (2019).
V.D.N. Tran, A. Hussain, H.S. Han, T.H. Dinh, J.S. Lee, C.W. Ahn, and I.W. Kim: Comparison of ferroelectric and strain properties between BaTiO3‐ and BaZrO3‐modified Bi1/2(Na0.82K0.18)1/2TiO3 ceramics. Jpn. J. Appl. Phys. 51, 09MD02 (2012).
K. Hao, W. Ge, Z. Ren, X. Liu, L. Luo, X. Li, H. Luo, and D. Viehland: Combining effects of TiO6 octahedron rotations and random electric fields on structural and properties in Na0.5Bi0.5TiO3. J. Am. Ceram. Soc. 103, 3349 (2020).
C.W. Ahn, C.H. Hong, B.Y. Choi, H.P. Kim, H.S. Han, Y. Hwang, W. Jo, K. Wang, J.F. Li, and J.S. Lee: A brief review on relaxor ferroelectrics and selected issues in lead‐free relaxors. J. Korean Phys. Soc. 68, 1481 (2016).
C. Laulhé, A. Pasturel, F. Hippert, and J. Kreisel: Random local strain effects in homovalent‐substituted relaxor ferroelectrics: A first‐principles study of BaTi0.74Zr0.26O3. Phys. Rev. B 82, 132102 (2010).
I.K. Jeong, C.Y. Park, J.S. Ahn, S. Park, and D.J. Kim: Ferroelectric‐relaxor crossover in Ba(Ti1−xZrx)O3 studied using neutron total scattering measurements and reverse Monte Carlo modeling. Phys. Rev. B 81, 214119 (2010).
M. Shen, W. Li, M.Y. Li, H. Liu, J. Xu, S. Qiu, G. Zhang, Z. Lu, H. Li, and S. Jiang: High room‐temperature pyroelectric property in lead‐free BNT‐BZT ferroelectric ceramics for thermal energy harvesting. J. Eur. Ceram. Soc. 39, 1810 (2019).
J. Glaum, H. Simons, M. Acosta, M. Hoffman, and A. Feteira: Tailoring the piezoelectric and relaxor properties of (Bi1/2Na1/2)TiO3‐BaTiO3 via zirconium doping. J. Am. Ceram. Soc. 96, 2881 (2013).
P.‐Y. Chen, C.‐S. Chen, C.‐S. Tu, and T.‐L. Chang: Large E‐field induced strain and polar evolution in lead‐free Zr‐doped 92.5%(Bi0.5Na0.5)TiO3–7.5%BaTiO3 ceramics. J. Eur. Ceram. Soc. 34, 4223 (2014).
J. Glaum, M. Zakhozheva, M. Acosta, E. Aksel, H.‐J. Kleebe, M. Hoffman, L.A. Schmitt, and W. Jo: Influence of B‐site disorder on the properties of unpoled Bi1/2Na1/2TiO3‐0.06Ba(ZrxTi1‐x)O3 piezoceramics. J. Am. Ceram. Soc. 99, 2801 (2016).
A. Ullah, M. Ullah, A. Ullah, A. Ullah, G. Saddiq, B. Ullah, A. Zeb, S. Ullah Jan, and I.W. Kim: Dielectric and electromechanical properties of Zr‐doped BNT‐ST lead‐free piezoelectric ceramics. J. Korean Phys. Soc. 74, 589 (2019).
P.B. Groszewicz, H. Breitzke, W. Jo, J. Rödel, and G. Buntkowsky: Local structure of the B‐site in BNT‐xBT investigated by 47,49Ti NMR: Effect of barium content. J. Appl. Phys. 121, 114104 (2017).
Acknowledgment
This study was supported by the National Research Foundation (NRF) Grant (2016R1D1A3B01008169). H.‐S.H. acknowledges financial support from the National Research Foundation (NRF) of Republic of Korea Grant (2020R1C1C1007375).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, H., Duong, T., Lee, S. et al. Comparing the electromechanical properties of CaTiO3‐ and BaZrO3‐modified Bi0.5Na0.5TiO3–SrTiO3 ceramics. Journal of Materials Research 36, 1048–1057 (2021). https://doi.org/10.1557/s43578-020-00080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-020-00080-7