Abstract
This study investigated the effects of CaTiO3, BaTiO3, and BaZrO3 doping on the phase transition and strain properties of lead-free 0.76Bi1/2Na1/2TiO3-0.24SrTiO3 (BNT-24ST) piezoceramics. The nonergodicity of the BNT-24ST ceramic was stabilized as a function of CaTiO3 doping, corresponding to the existence of the ferroelectric-to-relaxor phase transition temperature (TF-R) peak in the dielectric permittivity curves of the samples. However, the BaTiO3- or BaZrO3- doped NBT-24ST samples promote the transition from a nonergodic to an ergodic relaxor phase. The 0.01 mol BaTiO3 or 0.01 mol BaZrO3 doping decreases the TF-R peak of the NBT-24ST sample to below room temperature. Interestingly, it is noted that the nonergodic-to-ergodic relaxor phase transition of the BaTiO3-doped BNT-24ST ceramics was faster than that of the BaZrO3-doped BNT-24ST ceramics. The 0.01 mol BaTiO3-doped BNT-24ST sample presents a maximum dielectric constant of ~ 8000. The maximum piezoelectric actuator coefficient (uni-Smax/Emax) of ~ 525 pm/V was observed for the 0.01 mol BaTiO3-doped BNT-24ST ceramic. The effect of the tolerance factor on the phase transition and electrical properties of the BNT-24ST-ABO3 ceramics is suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Piezoelectric materials have been extensively used in various applications such as generators, energy harvestings, sensors, actuators, and many others [1,2,3,4,5]. The commercially available piezoelectric materials are dominated by Pb-based ceramics such as Lead Zirconate Titanate (PZT) due to their excellent electrical properties [2, 3, 5]. However, PZT-based piezoelectric ceramics contain more than 60 wt. % of Pb, which is toxic to humans and harmful to the environment. Therefore, Pb and its compounds have been restricted by RoHS (Restriction of Hazardous Substances) legislation [6], which greatly promotes the research on Pb-free piezoelectric ceramics [7,8,9]. However, the performance and reliability of Pb-free piezoelectric ceramics are relatively worse than Pb-based piezoelectric ceramics.
There are some Pb-free materials such as BaTiO3 (BT), BiFeO3 (BFO), Bi1/2Na1/2TiO3 (BNT), K0.5Na0.5NbO3 (KNN), and their solid solution. Among some Pb-free systems, BNT-based piezoelectric ceramic is a promising candidate to replace Pb-based piezoelectric ceramic materials [7,8,9]. BNT-based piezoelectric ceramics have been widely studied due to their superior electrical properties, especially their excellent electromechanical strain properties [10]. The large electric field-induced strain (EFIS) properties of BNT-based ceramics were ascribed from the ergodic relaxor (ER)-to-ferroelectric (FE) reversible phase transition [11], the core–shell structures [12] or the relaxor (RE)/ferroelectric (FE) composite structures [13]. However, the high required electric field (≥ 6 kV/mm) is still too difficult for BNT-based ceramics to be implanted in practical applications [7,8,9,10].
Recently, ABO3 doping is an effective solution to increase the electromechanical strain properties of lead-free BNT-based piezoelectric ceramics [10]. The SrTiO3-modified BNT (BNT-ST) ceramics exhibit a high electromechanical strain of ~ 0.25% under a relatively low electric field of 4 kV/mm [14]. Some previous studies on ternary BNT-ST-ABO3 ceramics showed electromechanical strain enhancement. For example, a unipolar large strain (Smax) of 0.36% in BNT-ST-(Bi,K)TiO3 ceramic [15], a Smax of 0.42% in BNT-ST-BaTiO3 ceramic [16], a Smax of 0.27% at 4 kV/mm in BNT-ST-AgNbO3 ceramic [17], a Smax of 0.239% in BNT-ST-KTaO3 ceramic [18], a large electrostrain of 405 pm/V at 1.7 kV/mm in BNT-ST-LaFeO3 ceramic [19], a Smax of 0.299% in BNT-ST-BaZrO3 ceramic [20], or a large electrostrain of 995 pm/V at 2 kV/mm in BNT-ST-LiNbO3 ceramic [21].
Besides, our previous studies reported that the change of tolerance factor (t) by impurities [22, 23] or ABO3 [20, 24] modified BNT-based ceramics is strongly related to their phase transition behaviors. Interestingly, the nonergodic-to-ergodic relaxor (NER-ER) phase transition in BNT-(Bi,K)TiO3 (BNT-BKT) ceramics is induced by decreasing the t factors [22,23,24]. In contrast, the NER-ER phase transition behavior in BNT-ST-based ceramics (more precisely, a comparison between BaZrO3 and CaTiO3 modified BNT-ST ceramics) was inconsistent with BNT-BKT-based ceramics [20]. Intuitively, the NER-ER phase transition in BNT-ST-based is induced by increasing the t factor. Therefore, this study compares the electrical properties and phase transition behaviors of the BaTiO3-modified BNT-ST ceramics with the BaZrO3-/ and CaTiO3-modified BNT-ST ceramics. The surface morphologies, crystal structures, dielectric, ferroelectric, and strain properties of the samples are studied. The results are expected to clarify the effects of Ba2+ ions on the properties of the A-sited modified BNT-ST ceramics.
2 Experimental procedure
Lead-free piezoceramic compositions of (0.76-x)Bi1/2Na1/2TiO3-0.24SrTiO3-xABO3 (x = 0, 0.01, 0.02, 0.03, and 0.04) with ABO3 = CaTiO3 [20], BaTiO3, and BaZrO3 [20] are synthesized using a conventional solid-state reaction. The commercial powders of Bi2O3 (99.9%), Na2CO3 (99.8%), SrCO3 (99.0%), TiO2 (99.0%), CaCO3 (99.0%), BaCO3 (99.0%), and ZrO2 (99.0%) are used as raw materials (High Purity Chemicals, Japan). The raw materials are mixed by their stoichiometry fraction, ball-milled in ethanol as solvent using a zirconia ball, and dried at 100 °C for 24 h. The dried powders are calcined at 850 °C for 2 h before the second time ball-milling process. The calcined powders are mixed with polyvinyl alcohol (PVA) as a binder and pressed into green body discs of diameter 12 mm under the uniaxial pressure of 98 MPa. Finally, the discs are placed in a sealed alumina crucible and sintered at 1175 °C for 2 h.
The surfaces of the sintered samples are polished and thermally etched before analyzing their microstructure using field-emission scanning electron microscopy (FE–SEM, JEOL JSM–65OFF, Japan). The crystal structures of sintered samples are characterized using X-ray diffractometry (XRD, RAD III, Rigaku, Japan). For electrical measurements, sintered samples are screen printed with silver paste onto both sides and then burned at 700 °C for 30 min. The piezoelectric constant (d33) is measured using a Quasi-Static Piezo d33/d31 Meter (ZJ-6B, Institute of Acoustics Chinese Academy of Sciences, Beijing, China). The temperature-dependent dielectric constant and dielectric loss are recorded by using a high-temperature electric prober system (LABSYS HTEP-8000, NEXTRON, Korea). Electric field-induced strain (S-E) and electric field-induced polarization (P-E) hysteresis loops are measured in a silicon oil bath and a modified Sawyer–Tower circuit (LVDT, MCH-331 & M401, Mitutoyo, Japan).
3 Results and discussion
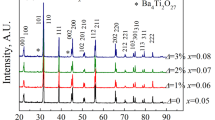
Figure 1 shows the XRD patterns of (0.76-x)Bi1/2Na1/2TiO3-0.24SrTiO3-xCaTiO3 (CT100x), (0.76x)Bi1/2Na1/2TiO3-0.24SrTiO3-xBaTiO3 (BT100x), and (0.76-x)Bi1/2Na1/2TiO3-0.24SrTiO3-xBaZrO3 (BZ100x) samples in the 2θ range of 39.5°-47.2°. All the samples exhibited a single perovskite structure without any secondary phases except Kα2 peaks, indicating the development of homogenous solid solutions as a function of ABO3 doping in 0.76Bi1/2Na1/2TiO3-0.24SrTiO3 (BNT-24ST) ceramic. Two different peaks around 40° and 46.5o of 2θ were indexed as each of (111) and (002) peaks, suggesting the pseudo-cubic phases for all samples. It found that 2θ of both (111) and (002) peaks of BNT-24ST ceramic were gradually changed by the ABO3 doping, even though crystallographic phase transitions were not detected in all samples. In the case of CT100x ceramics, the (111) and (200) diffraction peaks were slightly shifted to higher 2θ angles with increasing CaTiO3 content. In contrast, the (111) and (200) diffraction peaks of BT100x and BZ100x were drastically shifted to lower 2θ angles as a function of x. According to Shannon’s report [25], the ionic radii are 1.03 Å for Bi3+, 1.02 Å for Na+, 1.18 Å for Sr2+, 1.00 Å for Ca2+, 1.35 Å for Ba2+ as the A-site doping elements and 0.67 Å for Ti4+, 0.72 Å for Zr4+ as the B-site doping elements. The differences in shifting peaks of the ceramics could be originated from the different ionic radii of CaTiO3, BaTiO3, and BaZrO3 doping.
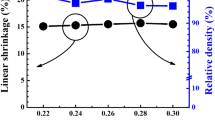
Figure 2 shows the surface FE-SEM images of CT100x, BT100x, and BZ100x ceramics. All samples present a dense microstructure, which agrees well with their sinter ability at 1175 °C for 2 h (relative densities ≥ 96% and linear shrinkage ≥ 15%). The different ABO3-modified BNT-24ST samples show different surface morphologies. Figure 3 presents the average grain size (AGS) of the BNT-24ST- ABO3 ceramics as a function of doping content. The AGS of the undoped BNT-24ST sample was ~ 4.7 μm. The CaTiO3 doping increases the average grain size (AGS) of CT100x ceramic. The AGS of CT4 ceramic is ~ 6.6 μm. In contrast, the BaTiO3 or BaZrO3 doping decrease the AGS of BT100x or BZ100x ceramics. The AGS of BT4 and BZ4 ceramics are ~ 1.0 μm and ~ 2.1 μm for BZ4 ceramic, respectively. It implies that the grain growth of BNT-24ST ceramic was suppressed by the addition of BaTiO3 and BaZrO3 doping.
We have suggested that the different morphologies of CaTiO3-/ and BaZrO3-modified BNT-24ST ceramics originated from their difference in the ionic radius size [20]. The previous study on BaZrO3-modified BNT-24ST ceramics reported that the replacing of the small Na+, Bi3+, and Ti4+ ions of A-/B-site by the large Ba2+ and Zr4+ ions could decrease their ion mobility or their overall cationic transport [18]. Therefore, the diffusion rate of the ionic in the BaZrO3-modified BNT-24ST ceramics during the sintering process is decreased. As a result, the AGS of the BaZrO3-modified BNT-24ST ceramics is decreased, as seen in previous studies [20, 21, 26, 27]. Besides, the small ionic radius of Ca2+ (1.34 Å) in the CaTiO3-modified BNT-24ST ceramics could increase the cationic transport as well as the AGS of the CaTiO3-modified BNT-24ST ceramics [20, 28, 29]. As seen in Fig. 3, the BT100x ceramics show a smaller AGS than the BZ100x ceramics, which might belong to the replacement of the single large Ba2+ ions to the small ions in the A-site of BT100x ceramic. Based on the previous study, it is suggested that the grain growth of BT100x ceramics is more strongly suppressed by the single Ba2+ ions [20, 30, 31].
Figure 4 depicts the temperature dependence of the dielectric constant (εr) and dielectric loss (tan δ) of the CT100x, BT100x, and BZ100x ceramics. Two distinct anomalies peaks are observed in the dielectric constant (εr) and dielectric loss (tan δ) curves of undoped BNT-24ST ceramic. The first peak at 42 °C is the ferroelectric-to-relaxor phase transition temperature (TF-R) and the second peak at 180 °C is the maximum dielectric constant temperature (Tm). The addition of CaTiO3 slightly decreases the TF-R of CT100x ceramics and finally reaches 27 °C for the CT4 ceramic. Besides, the Tm of CT100x ceramics is increased from 180 °C for undoped BNT-24ST ceramic to 202 °C for CT4 ceramic. In comparison, the dielectric responses of BT100x, and BZ100x ceramics exhibit more stable Tm and strong frequency-dependent dielectric responses at the low-temperature than the CT100x ceramics. Furthermore, the invisible TF–R peak in the dielectric properties of BT100x, and BZ100x ceramics implies the NER-to-ER phase transitions in the ceramics as a function of BaTiO3 and BaZrO3 dopants. The stabilized relaxor in BaTiO3-/ and BaZrO3-modified BNT-24ST ceramics agrees well with previous studies on Ba2+ ion-modified BNT-based ceramics [10, 16, 32, 33].
Figure 5 shows the polarization hysteresis loops (P-E) and bipolar strain curves (S-E) of CT100x, BT100x, and BZ100z ceramics under an applied electric field of 4 kV/mm. The undoped BNT-24ST sample shows a NER phase with a squared P-E loop and a butterfly S-E curve. The CT100x samples show the same saturated P-E loop and a similar butterfly S-E curve, resulting in a NER phase. Besides, the BaTiO3- or BaZrO3-modified BNT-24ST samples display a significant change in their P-E loop and S-E curves. The sprout S-E shape along with a slim P-E loop is observed for all BT100x and BZ100x samples when x ≥ 0.01, showing a NER-to-ER phase transition in BT100x and BZ100x ceramics. The corresponding maximum polarization (Pmax), remanent polarization (Pr), coercive field (Ec), bipolar maximum strain (Smax), and negative strain (Sneg) of the samples are presented in Fig. 6. As seen in Fig. 6, the undoped BNT-24ST ceramic shows a typical NER phase with high Pmax, Pr, Ec, Smax, and Sneg values of 34.43 µC/cm2, 27.73 µC/cm2, 1.99 kV/mm. 0.91%, and -0.106%. The addition of CaTiO3 maintained the constant S-E curves of CT100x ceramics, resulting in a decrease of the values to Pmax = 30.34 µC/cm2, Pr = 22.78 µC/cm2, Ec = 1.71 kV/mm, Smax = 0.084%, and Sneg = -0.075%, respectively. In contrast, the addition of 0.01 mol BaTiO3 and BaZrO3 to BNT-24ST ceramic shows an increase in the Smax values of 0.17% and 0.157%, whereas the Pmax, Pr, Ec, and Sneg values are decreased. The further increase of BaTiO3 and BaZrO3 content to over 0.01 mol in the BT100x and BZ100x ceramics shows a decrease in Pmax, Pr, Ec, Smax, and Sneg values. The results confirm the stability of the NER phase in the BNT-24ST ceramic by the CaTiO3. It implies that the A-site modification has better effects on the phase transition than the B-site modification of ternary BNT-24ST-ABO3 ceramics [20, 30].
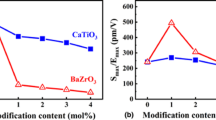
Figure 7 shows unipolar strain curves of CT100x, BT100x, and BZ100x ceramics at 4 kV/mm. The corresponding uni-Smax and the uni-Smax/Emax values of the ceramics are plotted in Fig. 8. The undoped BNT-24ST ceramic shows a uni-Smax of 0.1%, corresponding to the uni-Smax/Emax values of 250 pm/V. Similar to the bipolar S-E curves, the unipolar S-E curves of CT100x ceramics show almost similar shapes. The highest uni-Smax values of 0.21% and 0.20% are observed for BT1 and BZ1 ceramics. The BT1 ceramic presents the highest piezoelectric actuator coefficient (uni-Smax/Emax)of 525 pm/V under an applied field of 4 kV/mm. The strain properties enhancement in BT1 ceramic may be related to the disruption of the long-range ferroelectric order during the NER-ER phase transition of BNT-24ST ceramics by the Ba2+ ion doping.
Figure 9 shows the Goldschmidt tolerance factor and the phase diagram of the CT100x, BT100x, and BZ100x ceramics. The Goldschmidt tolerance factor (t) of the sample was calculated as the Eq. (1) [34]:
where rA and rB are the average ionic radii of the A- and B-site cations in the ternary ceramics; rO is the ionic radii of oxygen, respectively. The ionic radii of each element in the materials are collected from Shannon’s report [25]. Interestingly, the addition of CaTiO3 decreases the t of the CT100x ceramics. However, the t of the BT100x and BZ100x was increased as a function of doping concentration (x). The results confirm the NER-ER phase transition in ternary BNT-24ST-ABO3 ceramics by enhancement of the tolerance factor.
4 Conclusion
Lead-free (0.76-x)BNT-24ST-xABO3 piezoceramics with ABO3 = CaTiO3, BaTiO3, and BaZrO3 were studied in the composition range of x = 0–0.04. All samples were fabricated by a conventional solid-state reaction method. The relationship between structures, electrical properties, doping level, type of ABO3, and tolerance factor of the samples was investigated. The addition of Ba2+ ion into BNT-24ST ceramic presents significant effects on the phase structure as well as the dielectric and strain properties of the ceramics. A large piezoelectric actuator coefficient of 525 pm/V was achieved in the 0.01 mol BaTiO3-modified BNT-24ST ceramic. This work suggests a phase diagram of BNT-24ST-ABO3 ceramics as a function of the ABO3 doping level and the tolerance factor.
Availability of data
The authors confirm that the data supporting the findings of this study are available within the article.
References
G.H. Haertling, J. Am. Ceram. Soc. 82, 797 (1999)
K. Uchino, Ferroelectric Devices (CRC Press, 2000)
M.E. Lines, A.M. Glass, Principles and Applications of Ferroelectric and Related Materials (Oxford University Press, 2001)
M. Safaei, H.A. Sadano, S.R. Anton, Smart Mater. Struct. 28, 113001 (2019)
X. Gao, J. Yang, J. Wu, X. Xin, Z. Li, X. Yuan, X. Shen, S. Dong, Adv. Mater. Technol. 5, 1900716 (2020)
E.U. Directive, Restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS). Official J. Eur. Union 46, 19 (2013)
J. Rödel, K.G. Webber, R. Dittmer, W. Jo, M. Kimura, D. Damjanovic, J. Eur. Ceram. Soc. 35, 1659 (2015)
C.H. Hong, H.P. Kim, B.Y. Choi, H.S. Han, J.S. Lee, C.W. Ahn, W. Jo, J. Materiomics. 2, 1 (2016)
J. Koruza, A.J. Bell, T. Frömling, K.G. Webber, K. Wang, J. Rödel, J. Materiomics. 4, 13 (2018)
J. Hao, W. Li, J. Zhai, H. Chen, Mater. Sci. Eng. R. 135, 1 (2019)
W. Jo, T. Granzow, E. Aulbach, J. Rödel, D. Damjanovic, J. Appl. Phys. 105, 094102 (2009)
M. Acosta, L.A. Schmitt, L. Moina-Luna, M.C. Scherrer, M. Brilz, K.G. Webber, M. Deluca, H.J. Kleebe, J. Rödel, J. Am. Ceram. Soc. 98, 3405 (2015)
T.H. Dinh, J.K. Kang, J.S. Lee, N.H. Khansur, J. Daniels, H.Y. Lee, F.Z. Yao, K. Wang, J.F. Li, H.S. Han, W. Jo, J. Eur. Ceram. Soc. 36, 3401 (2016)
T.A. Duong, H.S. Han, Y.H. Hong, Y.S. Park, H.T.K. Nguyen, T.H. Dinh, J.S. Lee, J. Electrochem. 41, 73 (2018)
K. Wang, A. Hussain, W. Jo, J. Rödel, J. Am. Ceram. Soc. 95, 2241 (2012)
S. Praharaj, D. Rout, S.J.L. Kang, I.W. Kim, Mater. Lett. 184, 197 (2016)
Y. Zhu, Y. Zhang, B. Xie, P. Fan, M.A. Marwat, W. Ma, C. Wang, B. Yang, J. Xiao, H. Zhang, Ceram. Int. 44, 7851 (2018)
G. Wang, Y.H. Hong, H.T.K. Nguyen, B.W. Kim, C.W. Ahn, H.S. Han, J.S. Lee, Sens. Actuators A 293, 1 (2019)
T.H. Dinh, H.S. Han, J.S. Lee, Mater. Lett. 258, 126793 (2020)
H.T.K. Nguyen, T.A. Duong, S.S. Lee, C.W. Ahn, H.S. Han, J.S. Lee, J. Mater. Res. 36, 1048 (2021)
T.H. Dinh, J.S. Lee, Mater. Lett. 313, 131772 (2022)
H.S. Han, C.W. Ahn, I.W. Kim, A. Hussain, J.S. Lee, Mater. Lett. 70, 98 (2012)
T.H. Dinh, H.Y. Lee, C.H. Yoon, R.A. Malik, Y.M. Kong, J.S. Lee, V.D.N. Tran, J. Korean Phys. Soc. 62, 1004 (2013)
I.K. Hong, H.S. Han, C.H. Yoon, H.N. Ji, W.P. Tai, J.S. Lee, J. Intell. Mater. Syst. Struct. 24, 1343 (2012)
R.D. Shannon, Acta Crystallogr. Sect. A: Found. Crystallogr. 32, 751 (1976)
C. Ciomaga, M. Viviani, M.T. Buscaglia, V. Buscaglia, L. Mitoseriu, A. Stancu, P. Nanni, J. Eur. Ceram. Soc. 27, 4061 (2007)
S. Zixiong, P. Yongping, L. Yuwen, J. Electron. Mater. 43, 1466 (2014)
T. Badapanda, S.K. Rout, L.S. Cavalcante, J.C. Sczancoski, S. Panigrahi, T.P. Sinha, E. Longo, J. Mater. Chem. Phys. 121, 147 (2010)
F. Guo, W. Cai, R. Gao, C. Fu, G. Chen, X. Deng, Z. Wang, Q. Zhang, J. Electron. Mater. 48, 3239 (2019)
V.D.N. Tran, A. Hussain, H.S. Han, T.H. Dinh, C.W. Ahn, I.W. Kim, J.S. Lee, Jpn. J. Appl. Phys. 51, 09MD02 (2012)
W. Jo, S. Schaab, E. Sapper, L.A. Schmitt, H.J. Kleebe, A.J. Bell, J. Rödel, J. Appl. Phys. 110, 074106 (2011)
V.D.N. Tran, T.H. Dinh, H.S. Han, W. Jo, J.S. Lee, Ceram. Int. 39, S119 (2013)
T.H. Dinh, H.S. Han, V.D.N. Tran, V.L. Van, J.S. Lee, J. Electro. Mater. (2023). https://doi.org/10.1007/s11664-023-10263-7
V.M. Goldschmidt, Naturwissenschaften 14, 477 (1926)
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.02-2020.28.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukhlishah, A.D., Dinh, T.H., Han, HS. et al. Effects of CaTiO3, BaTiO3, and BaZrO3 on the crystal structures and electrical properties of Bi1/2Na1/2TiO3–SrTiO3 piezoelectric ceramics. J Electroceram 51, 192–198 (2023). https://doi.org/10.1007/s10832-023-00326-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-023-00326-w