Abstract
Through diversity of composition, sequence, and interfacial structure, hybrid materials greatly expand the palette of materials available to access novel functionality. The NSF Division of Materials Research recently supported a workshop (October 17–18, 2019) aiming to (1) identify fundamental questions and potential solutions common to multiple disciplines within the hybrid materials community; (2) initiate interfield collaborations between hybrid materials researchers; and (3) raise awareness in the wider community about experimental toolsets, simulation capabilities, and shared facilities that can accelerate this research. This article reports on the outcomes of the workshop as a basis for cross-community discussion. The interdisciplinary challenges and opportunities are presented, and followed with a discussion of current areas of progress in subdisciplines including hybrid synthesis, functional surfaces, and functional interfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hybrid materials with tailored interfaces have led to emergent opportunities with exciting future research directions, many of which tie into three of the US National Science Foundation’s (NSF) 10 Big Ideas, including Quantum Leap, hold promise for expanding Understanding of the Rules of Life, and stand poised to benefit from Harnessing the Data Revolution. Hybrid materials combine two or more distinct atomic or chemical phases, and the interface between these phases often plays a crucial role in determining the overall material properties through its effects on modulating flows of charge, mass, and energy. These hybrids can take almost an infinite variety of forms, yet designing, predictively synthesizing, and understanding their function and dynamics is essential if the structure–property relationships are to become known and rendered programmable.
Hybrid materials and interfaces were recently highlighted as a crucial topic for future materials research by the decadal report from the US National Academies on the Frontiers of Materials Research, which devoted two sections to composites and hybrid materials, highlighting recent progress and opportunities.1 The subfields researching hybrid materials often operate in isolation from one another, despite sharing fundamental interfacial questions in different materials contexts. These subfields broadly include soft-matter, self-assembly, additive deposition, epitaxy, and metal–organic frameworks. The translation of knowledge and concepts between these subfields is unfortunately obfuscated by separate conferences and distinct jargon.
The Solid State and Materials Chemistry (SSMC) program within the NSF Division of Materials Research requested and recently supported a workshop (October 17–18, 2019) with the goals of placing a larger umbrella around hybrids and interfaces to foster interfield exchange and dialog, as well as to accelerate ongoing research activities in a similar spirit as the Materials Genome Initiative. Through diversity of composition, sequence, and interfacial structure, hybrid materials greatly expand the palette of materials available to access novel functionality. Approximately 50 invited participants attended the workshop, from a variety of hybrid-related subfields. The goals of the workshop were to (1) identify fundamental questions and potential solutions common to multiple disciplines within the hybrid materials community, (2) initiate interfield collaborations between hybrid materials researchers; and (3) raise awareness in the wider community about experimental toolsets, simulation capabilities, and shared facilities that can accelerate this research. The workshop sought to answer the fundamental question of how combinations of materials with tailored interfaces can be designed to manifest emergent properties and provide functionality (much) more than the sum of their parts. Ultimately, the aim was the translation of knowledge, approaches, and conceptual frameworks between the disparate subdisciplines that are each actively researching different types of hybrids and interfaces with a view toward identifying unifying themes in interfacial chemistry.
This article reports on the outcomes of the workshop, as a basis for cross-community discussion. The interdisciplinary challenges and opportunities are presented first, and followed with a discussion of current areas of progress in particular subdisciplines, including hybrid synthesis (self-assembly, additive deposition, epitaxial interfaces, and metal–organic frameworks) and separately, functional surfaces and functional interfaces.
Challenges and opportunities in hybrid materials chemistry research
Opportunities for hybrid materials synthesis
Opportunities in generating new compositions of hybrid materials require advances in our knowledge of the elementary steps of hybrid assembly. A challenge is that crystal growth and assembly of hard and soft components operate on disparate length scales (nanometer tuning of organic functional groups with inorganic particles having surface defects that span tens of nanometers to microns) and energy scales (covalent bonding on the order of 102–103 kJ/mol to van der Waals forces on the order 1–10 kJ/mol). Precursor selection in materials synthesis is often based on intuitive notions (e.g., solubility in a given solvent, the presence of counterions that are noncoordinating) that can be difficult to translate when preparing new compositions of matter. Even within a given composition, scaling up reactions can be difficult because hybrid assembly is often controlled kinetically.
Conventional energy-intensive synthetic methods that are the mainstay of metallurgy, ceramic science, or solid-state chemistry rely on precursors starting at an initially high-energy state and efficiently making their way toward equilibrium by sampling the energy landscape and dissipating the excess energy without being trapped2 in shallow metastable states (Figure 1).3 In contrast, the shallow energy landscapes of hybrid materials and interfaces offer a richness of functionality matched only in complexity of navigation, as illustrated for crystalline interface in Figure 1a–b. The shallow energy landscapes nevertheless result in materials architectures that are history/processing-dependent in the specifics of their interfacial structure and resulting functionality. Process changes thus result in different observed properties, sometimes a convolution of multiple states, but also sometimes enabling distinct novel states.
(a) Schematic illustration of interfaces between crystalline materials. With permission from J.L. Andrews, Texas A&M University. (b) Free-energy landscape illustrating thermodynamic minimum and shallow local wells corresponding to metastable states. Adapted with permission from Reference 3. © 2018 American Chemical Society. (c) A lattice-resolved high-resolution transmission electron microscope image of a HfO2/V2O3 interface. Reprinted with permission from Reference 2. © 2019 Royal Society of Chemistry.
Two opportunities to address this challenge at the level of synthesis are (1) harness kinetic control by making use of kinetically trapped states2,4–7 (note the shallow wells in Figure 1b) and intermediates, which add new capabilities beyond the equilibrium limits of both composition and morphology control, and (2) harness equilibrium control by constructing composition/structure phase diagrams for complex hybrid materials. Cues from model systems such as hybrid organic/inorganic layered perovskites8 and liquid-crystal phases9 represent a reasonable starting point for other systems (Figure 2). These systems exemplify synergies between synthetic chemists (inorganic and organic for perovskites and liquid crystals, respectively) navigating reaction trajectories to arrive at synthetic targets and physical modeling of structure–property relationships for applications in catalysis, solar cells, and displays.
(Top) Intercalation reaction pathways in layered perovskites. (Bottom) Ternary phase diagram for lamellar (L) versus hexagonal (H) liquid-crystal formation from Reference 9. (Top) Adapted with permission from Reference 8. © 2002 American Chemical Society. (Bottom) Adapted with permission from Reference 9. © 2002 American Chemical Society.
Opportunities for juxtaposing different materials as new types of hybrids
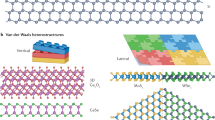
The desire to discover new and emergent phenomena requires consideration of how different parts come together so that the whole is greater than the sum of the parts. This consideration becomes important when juxtaposing different materials to form new hybrids. Different materials brought together to form a hybrid must be synergistic; otherwise, they will work against each other to defeat the original properties that initially imparted functionality to the individual components. One should avoid heterogeneous materials where the heterogeneity detracts from the materials properties (but learn from model systems so that one can extrapolate to the myriad heterogeneous materials found in nature and functional devices). Instead, the community wants to discover new phenomena or improved properties that arise from the juxtaposition of disparate materials. This concept of matching individual parts correctly means that one must consider at what energy and length scale one wants the juxtaposition to occur and the coherence in structure across the interface. Choices of length scale can include anything from the atomic scale as in inorganic–organic hybrids or epitaxially matched interfaces (Figure 1c) to nanometer-sized dimensions in bulk heterojunction solar cells and architected battery electrodes to the micrometer as in biomaterials.
Instead of preparing randomly heterogeneous materials, we aim at building heterostructures such as those shown in Figure 3. The difference between the two words—heterogeneous and heterostructure—implies that we focus on how we structure two different materials and especially the interface between them. A recent effort in this area includes the preparation of heterostructures constructed from layering disparate 2D materials with each other, yielding not just compositional diversity but a vast array of configurations corresponding to the twist angle between overlaid layers.10,11 For example,11 van der Waals heterostructures such as graphene/hexagonal boron nitride/graphene, and MoS2/WSe2 can yield electronic and optoelectronic properties from the composite that do not exist in the individual components, and twisted bilayers of graphene display tunneling signatures dependent on the twist angle of the stack. The juxtaposition of these layers is key toward promoting emergent phenomena, and one area of interest that spans several disciplines is that of quantum materials.
The juxtaposition of two different materials, each with its own crystal structure and chemical composition, coming together to form a heterostructure. The interaction between the two at the appropriate energy and length scales include charge transfer, strain, superexchange, spin–orbit coupling (SOC) among others. These interactions will lead to new, emergent phenomena that will be key to the field of quantum materials, energy conversion, and energy storage, among other applications. Reprinted with permission from Reference 10. © 2019 AAAS.
We broadly categorize quantum materials to include superconductors, topological insulators, Weyl semimetals, highly correlated electron systems, 2D materials, and quantum spin liquids.12 Figure 3 shows a schematic of the juxtaposition of two layered materials to effect new magnetic phenomena in 2D. Likewise, the interfacing of dielectric layers can give rise to a remarkable 2D electron gas. The mechanisms toward achieving new emergent phenomena are numerous and varied, including spin–orbit coupling between the layers, strain engineering, superexchange, charge transfer, and several others. Such juxtapositions can be achieved via physical means such as exfoliation and stamping or through chemical means in solution to scale up production of heterostructures with larger lateral dimensions.13
As in the previously discussed example with heterostructured quantum materials, our motivation for pursuing hybrid materials includes tackling grand scientific challenges. Taking on such multifaceted problems encourages a cross-pollination of different disciplines and also pulls together research groups with different synthetic strategies. Grand challenges include developing materials that enable the quantum or neuromorphic computing revolution, materials for catalysis, and materials for energy and environmental sustainability. The scientific opportunity is in better understanding the interfacial science of constructing the optimal juxtaposition of disparate materials to promote emergent phenomena and deterministically modulate flows of mass, charge, and energy.
Opportunities for mixed hydrophobic–hydrophilic hybrids
Hydrophobic–hydrophilic hybrid materials encompass a wide range of compositions and morphologies, from Janus particles14 to block copolymers15 to hybrid surfaces (Figure 4).16 From a fundamental standpoint, such hybrids bring together highly dissimilar materials into close contact, which can lead to interesting new functionalities. Inspiration can be gleaned from nature’s hydrophobic–hydrophilic hybrids such as lipid bilayers, where self-assembly leads to frustrated confinement of the lipid hydrophobic tails and creates a lateral pressure profile of several hundred atmospheres within just a few nanometers!17 This lateral pressure profile leads to mechanosensitivity and is important for touch, hearing, and osmoregulation in biological organisms.
Examples of hydrophobic/hydrophilic materials and interfaces. (a) Reprinted with permission from Reference 14. © 2011 Royal Society of Chemistry. (b) Adapted with permission from Reference 140. © 1995 American Chemical Society. (c) Adapted with permission from Reference 139. © 2011 Elsevier. (d) Adapted with permission from Reference 16. © 2014 Elsevier.
Synthesis of new hydrophobic–hydrophilic hybrid materials will benefit from improved understanding of the kinetic and thermodynamic factors, as previously noted, to devise new combinations and morphologies. For materials characterization, opportunities exist for fundamental understanding of collective phenomena in hydrophobic–hydrophilic hybrids, understanding the dynamical evolution of structure in these systems in the presence of fluids, and how that structure affects properties such as transport under confinement. Progress in this area will require advanced characterization, model materials, and computation. From a property standpoint, such approaches can lead to self-folding materials, switches, water transport conduits, nanoreactors, and new energy storage concepts.
Opportunities for hard-soft material hybrids
When soft and hard materials are mixed into a hybrid material, the result may be the sum of the parts (i.e., a composite material) or exhibit entirely new and unusual behavior (i.e., emergent behavior). Prototypical hard materials include crystalline periodic solids, covalently bound allotropes such as diamond, and amorphous cross-linked networks. Soft materials include polymers, biological macromolecules, and supramolecular assemblies. In contrast, a number of materials are intermediate between soft and hard, including graphite and carbon nanotubes.
A major inspiration here are the emergent properties from biological hard/soft hybrids (e.g., teeth-tissue, bone regeneration, shells). Soft/hard hybrid materials can be expected to be self-adaptable and dynamic, exhibit cooperative function, and have structure that can be regulated from the atomic to the macroscale maintaining remarkable fidelity of form through the incorporation of self-correcting and replicating motifs. Ultimately, it is desired that one can predict structure–property outcomes of soft-hard hybrid materials.18 Advancing property control in hard-soft hybrids requires chemists and materials scientists to face the challenge of developing a deeper understanding of buried interfacial phenomenon where strain mismatch and related energy terms are connected to diffusive transport, heat transport, and charge transport. Hard-soft materials composed of more than two materials can be regarded as a particularly exciting frontier for materials design.19 Opportunities can be expected to involve the development of new techniques to synthesize unique interfaces, chiral materials, and anisotropic materials with impact in areas such as biology, optics, additive manufacturing, nanoelectronics, energy storage, and three-dimensional (3D) printing.
Opportunities for theory and computation in hybrid materials
Methods covering wide ranging time and length scales are now well-established for computing the electronic structure of solids, providing regular insights into transport, optical, magnetic and dielectric behavior, with more accurate and more expansive methods constantly under development.20 In parallel, the ability of computational methods to predict new solid-state compositions with potentially attractive materials properties is advancing to the point that synthetic efforts aimed at computation-guided targets are increasingly common.21
The importance of interfaces has long been recognized in solid-state chemistry. Interfaces and modified surfaces are regularly used to tune electronic and optical properties. Density functional theory (DFT) methods can predict interface interactions, including lattice mismatches and binding energies, although with some constraints on structure size and environment requiring more accurate treatments of electron correlation. Larger-scale simulations, such as classical molecular dynamics, can be helpful, particularly for dynamically evolving interfaces such as those subjected to electric or strain fields, but reliable interatomic potentials for increasingly specialized and complex systems are required. Current interest in hybrid materials based on low-dimensional structures, such as single layers on surfaces and, ultimately, the promise of designer heterostructures with chemically dissimilar components, will require new computational approaches to inform about increasingly complex materials. In addition to approaches aimed at electronic structures, there is an opportunity for models to predict the hierarchical assembly of hybrid materials and for understanding the individual and collective elastic properties in hybrid materials. The predictive navigation of reaction trajectories as exemplified in Figure 1b necessitates not just static modeling, but an awareness of energy landscapes and their dynamical evolution over the course of a reaction.
Data mining and machine learning methods are advancing rapidly and there is great hope for new models to better understand how solid-state materials perform in complex systems and devices. Hybrid materials, such as the metastable Mo2AlB2-based 2D nanosheet heterostructures discussed in Reference 21, provide a great opportunity for exploring these approaches. However, data sets relevant to materials chemistry are extremely small relative to true Big Data applications, requiring modified machine learning models. Platforms for gathering and making available more experimental data sets, beyond those that make it into published journal articles, can aid these developing tools as well as the adoption of algorithms such as sequential learning methods (e.g., some Bayesian processes) that allow for efficient navigation of design spaces in search of not just singular objectives but that can evaluate the tradeoffs between different physical properties. Last, it is noted that predictive studies to guide the synthesis of hybrid materials are less common and thus an attractive direction of further development.
Infrastructure needs for characterizing hybrid materials
Hybrid materials have electronic states, chemical composition, and mechanical properties that can vary across a wide range of length scales. Ideally, structural data could be obtained in situ with high temporal resolution, allowing researchers to follow phenomena occurring during dynamic processes such as nanocrystal synthesis,22 solar cell function,23 battery operation,24 and quantum state evolution at ultralow temperatures.25 This structural information should be generated and analyzed rapidly enough to provide useful feedback for materials optimization, while being accessible to a wide variety of researchers.
A variety of tools exist for structural characterization on different length scales, as summarized in Table I. Advances in electron microscopy have been especially notable, including improvements in sample preparation and damage minimization,26–29 and new techniques that enable the study of liquids30 and biomaterials.31 But there is a clear need to make these advanced techniques more widely available, and to develop complementary tools with similar spatial resolution. Goals for future infrastructure development to facilitate the study of hybrid materials include: 1.
-
1.
Develop geographically distributed centers for state-of-the-art electron microscopy and ultrafast time-resolved spectroscopy that are accessible to a wide variety of principal investigators, ideally via remote operation.
-
2.
Develop new experimental tools for ultrahigh-resolution structure characterization. Any proposed method should address 3D penetration, buried interfaces, in situ capability, sample preparation requirements, and sample damage.
-
3.
Develop lower cost imaging tools (e.g., tabletop micro-Raman, atomic force microscopy, surface sum frequency generation, and x-ray absorption spectroscopy) that can be used by individual laboratories to rapidly characterize large area (1 mm2) samples and enable probing of local and not just average atomic structure.
In terms of human resources, there is a shortage of people who possess expertise in both state-of-the-art characterization tools (e.g., ultrahigh resolution transmission electron microscopy) and materials synthesis and applications. Suggestions for bridging this gap include: 1.
-
1.
Encourage imaging specialists to work closely with materials researchers.
-
2.
Promote student and principle investigator (PI) travel to microscopy centers.
-
3.
Grow expertise with students and postdoctoral researchers in all facets of sample preparation, instrument operation, and data analysis.
Education and training needs for hybrid materials research
It is obvious that no one person or research group can possess all the expertise required to develop the next generation of functional hybrid materials. Consequently, there is a need to increase participation, train, and educate persons from different disciplinary backgrounds to advance the field. Three different populations are relevant: the general public (K–12 students, teachers, policymakers, and the broader public), higher education communities (administrators, college/university students, academics, and research scientists), and other scientists (in other disciplines and nonacademics). Several goals for training and education are apparent: (1) to take hybrid materials science to members and nonmembers of the hybrid materials community in an accessible way,32 (2) increase the perceived value of hybrid materials science, and (3) to battle misinformation. In an era of technology-driven innovation, the field of hybrid materials should move to digitally transform content, increase the online presence, widely disseminate and create virtual training opportunities, and enable the development of outreach activities with the capacity to have national impact. These efforts to broadly train and educate using digital platforms could meet the previously mentioned goals.
In regard to how to recruit, educate, and train students at various stages, one might argue that there is a lack of visibility of existing resources for learning about hybrid materials. The development of an online repository for existing and newly developed educational resources could mitigate this aspect and widen the audience and interest for hybrid materials research. In developing new pedagogical material, these should provide a personal and sensorial experience to the learner to improve retention, learning and understanding.33–38 There is currently no comprehensive textbook on hybrid materials,39–42 as this is a rapidly evolving field. If we aim to recruit, train, and educate the next generation of research scientists, there is an urgent need to develop a public domain (editable–peer reviewed) textbook within a curated online repository. The textbook could serve to integrate materials science topics at an early stage in the curriculum, enable a deep understanding of the instrumentation used to characterize materials, connect to the broader implications of materials research on improving our quality of life, and increase interest in pursuing research experiences and careers in materials science and related fields. Perhaps taking on a project of this magnitude could also serve to unite key members of the community and propel collaborative projects aimed to reach new frontiers in hybrid materials research.
Perspectives from subdisciplines of hybrid materials research
Hybrid materials synthesis
Self-assembly
Self-assembly is one of the most commonly used bottom-up approaches for hybrid material synthesis.43–46 A self-assembly process typically utilizes noncovalent interactions between the core components being assembled with consideration of both topological structure of molecules/nanoparticles and the nature of functional groups at the interface. While the overall principle of self-assembly processes is thought to still fall into the classical lock-key model, properly and correctly incorporating functional groups that can form cooperative noncovalent interactions to strengthen the overall assemblies is still a significant challenge for hybrid materials synthesis, particularly for soft hybrid materials.
Typical functional groups involved in self-assembling processes include (1) ionic species such as anionic carboxylate, sulfate, and phosphate, as well as cationic ammonium and pyridinium;44 (2) molecules/nanoparticles involving organic ligands and transition-metal ions that form coordination bonds at the interface (e.g., metal–organic frameworks [MOFs]);43,47 (3) hydrogen bonding substituents such as a carboxylic acid, hydroxyl, amine, pyridine, and imidazole; (4) substituents with large dipoles (e.g., carbonyl, cyano, nitro, trifluoromethyl) and quadrupoles (e.g., phenyl, perfluorophenyl); and (5) substituents with large polarizability such as sulfur-containing π systems (e.g., thiophenyl) and bromine- and iodine-containing electron-deficient substituents (e.g., –CF2Br, –CF2I).48–57 These functional groups are used for self-assembling inorganic–inorganic (e.g., nanoparticles), inorganic–organic, and organic–organic hybrid materials. Often these hybrids utilize one of the many types of noncovalent binding forces with limited cases utilizing two or more cooperative binding interactions that were first discovered and used in coordination and supramolecular chemistries.
The challenge of rational design and utilization of such cooperative binding forces in hybrid materials produced via self-assembly processes is due to the complexity of multiple coexisting and weak noncovalent interactions.58 Overcoming such a challenge is expected to provide great opportunities for hybrid material syntheses. A foreseeable strategy to solve this challenge is through well-integrated efforts utilizing the power of both synthetic and modern computational chemistries. A key component of this strategy is to develop model systems that represent major challenges in current hybrid material syntheses, yet are simple enough to allow accurate computational predictions. Successful execution of this strategy is expected to provide opportunities for hybrid materials synthesis under both thermodynamic and kinetic control conditions by self-assembly.
Additive deposition
Apart from the capability of additive deposition (AD) methods to create materials with complex architectures, a unique feature of this fabrication approach is its adaptability in the production of multimaterial systems. The emergence of new AD technologies opens up opportunities in the assembly of novel hybrid materials with interesting physical and chemical properties (Figure 5).
A survey of different additive manufacturing (AD) technologies that can be adapted in the fabrication of hybrid materials: fused deposition modeling (FDM), stereolithography (SLA), two-photon lithography (TPL), reactive extrusion (RE), direct ink writing (DIW), and atomic force microscopy-based nanolithography (AFM-NL).
One area of opportunity for AD technologies is the potential for multi-length scale materials engineering leading to the production of hybrid materials with tunable dimensions that can span across different size regimes—a task that is particularly challenging with other synthetic methods. Three-dimensional printing platforms such as fused deposition modeling (FDM) and stereolithography (SLA) have been successfully utilized in the preparation of hybrid polymer composites with dimensions that can be scaled down from the millimeter to the micrometer-size range.59,60 On the other hand, improvements in resolution to the nanometer-scale region have been achieved using advanced two-photon lithography (TPL)61 and atomic force microscopy-based nanolithography methods.62 Moreover, direct ink writing AD synthetic routes provide location-specific composition and density control, thereby enabling the fabrication of hierarchically porous materials with tunable 3D microstructures that show improved adsorption properties, which is crucial for applications in catalysis, energy storage, and separation processes.63 Furthermore, reactive extrusion-based AD methods offer the capability to make dissimilar materials compatible, resulting in favorable interfacial chemistries that can lead to multifunctional hybrid composites with robust mechanical properties.64
However, while there had been significant progress in multimaterials deposition using different AD technologies, the fabrication of certain types of hybrid material systems using this synthetic approach still poses major challenges. This is particularly the case for fabrication of hybrid composites involving 2D materials that have high surface energy, which make them difficult to de-aggregate and reassemble in a controlled fashion during processing.65 This type of synthetic challenge thus provides prospects for the development of new chemistries that can be adapted with AD manufacturing technologies.
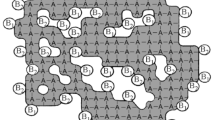
Metal–organic frameworks
MOFs are hybrid materials by design incorporating both inorganic and organic components that have been investigated for a variety of applications, such as in gas separation/storage, drug delivery, catalysis, and energy-storage applications.66–72 The breadth of possible structures (>30,000 known MOFs have been reported thus far) provides a platform for optimization for these and other applications (Figure 6).73–79 However, there is still space for the development of new MOF structures, as well as answering the many open fundamental questions regarding their function. In the area of new MOF structures, there are still numerous nodal elements and linker structures that have yet to be realized. While the chemistries regarding carboxylate and nitrogenous linkers are quite mature, new binding groups (e.g., hydroxamate and thiocarbamate) are in their infancy.80,81 Additionally, the number of MOFs that incorporate second- and third-row transition metals, save for Zr and Hf, are few and far between despite the immense application space for these.82–84 Beyond the component parts, there is also a need to consider alternative MOF architectures, namely single-crystal monolithic thin films.85,86 While various methods exist to prepare polycrystalline films, many applications and fundamental measurements require single-crystal films. The intersection of traditional film preparation techniques (e.g., chemical vapor deposition and vapor transport) and MOFs may result in significant advances by providing access to well-defined hybrid systems with atomically defined interfaces.87
There is space to move beyond one-component systems to MOFs that exhibit a hybrid (collective) function between linker and node. Many publications mention the similarity between enzyme-like environments and MOF structure, however, examples exploiting multifunction, cooperative behavior are rare.88,89 Can a MOF be used to truly mimic protein function, including directed molecular transport, selective, and rapid (single site) catalysis? The topic of transport of molecules, ions, or electrons in MOFs introduces more unanswered questions. What factors control mass transport through MOF structures? How are solvent molecules arranged within MOF pores and how do they affect mass transport? In the case of electronic transport, how can the node and linker be made to “communicate” in three dimensions? These are important questions that remain unanswered. There are also opportunities to understand long-term stability, the roles of defects in reactivity, and for computational/experimental partnerships to elucidate the design rules for desired functions. To address these latter questions, advanced instrumentation and partnerships with national user facilities are needed to provide unprecedented atomic scale information for in situ bridging across decades of time and length scales. The crystalline nature of MOFs also renders them ideal candidates for pushing the limits of techniques (e.g., electron and x-ray spectroscopies, neutron scattering for identification of sites occupied by light elements).
Functional surfaces and interfaces
Functional surfaces
A key driving force of hybrid materials is to integrate dissimilar materials and collectively utilize their complementary properties. This mitigates the limits of single-phase material components and enhances system performance.90 Successful examples are found in energy conversion and storage, catalysis, environmental, and biomedical applications. The characteristics of the material components are discussed in other sections. Frequently, a hybrid system may involve a series of materials interfaces in multiscale hierarchical structures. The functional surfaces generated in a preceding step critically define the assembly and quality of the following layer. Single-junction solar cells made of organo-metal-trichloride perovskites involving six layers of hybrid materials are a good example to demonstrate the effects of the interfaces and intralayer architectures on the cell performance.91,92 The separation and transport of photoexcited electrons and holes, the temperature stability and chemical stability have been dramatically improved in the past decade based on hybrid materials.
Carbon materials are a good example to illustrate the role of functional surfaces in energy applications.93 Figure 7a shows four molecular functional sites in N-doped graphene, which exhibit drastically different electrocatalytic activities for oxygen reduction reaction in fuel cells.94 Proper N sites in the conductive graphitic carbon framework also serve as binding sites to form single metal atom complexes as competing Pt-free catalysts.95 Due to the unique sp2 bonds in carbon materials, their internal microstructure critically determines the surface functionalities.96 As shown in Figure 7b–c, commonly used multiwalled carbon nanotubes present a smooth graphite basal-plane-like structure at the sidewall, which is inert and weakly interacts with other materials. In contrast, carbon nanofibers (CNFs) consisting of conically stacked graphitic cups can be obtained with a plasma-enhanced chemical vapor deposition process.97 The abundant graphitic edge sites at the sidewall enable stronger interface with the added overcoating and facilitate faster electron transfer.98,99 Enhanced lithium-ion battery performance has been demonstrated using the core–shell structure to allow high electron transport in CNF cores and faster Li+ ion transport across the thin Si98 or V2O599 shells, as illustrated in Figure 7d. Fundamental questions of interfacial science pertain to desolvation mechanisms of cations at interfaces, the insertion of solvated/desolvated species within host lattices, the interplay of electron and ion transport, electrode/electrolyte reactivity, and the nucleation of metal clusters onto electrified surfaces.
(a) The molecular functional sites of N-doped graphene. (b) The atomic model of an ideal multiwalled carbon nanotube. (c) The atomic model of a carbon nanofiber consisting of a stack of graphitic cones. Theta represents the angle between the conical graphitic cup and the main axis of the carbon nanofiber. (d) A core–shell hybrid structure consisting of conformal V2O5 shells deposited on the vertically aligned carbon nanofiber cores as a lithium-ion battery cathode. Panel (d) adapted with permission from Reference 99. © 2016 Wiley.
Functional interfaces
Hybrid materials can be designed with interfacial structures that allow for a myriad of functions. Recent examples show how synthesizing functional interfaces advances their application in a variety of disciplines, including catalysis, solar cells, thermal materials, and biotechnology. A theme common to such interface design is the modulation of the flows of charge, mass, and energy in terms of directionality, magnitude, and temporal sequence. For instance, platinum nanoparticles decorated on one-dimensional TiO2 nanorods enhance the electron-transfer rate for the oxygen reduction reaction and engender effective charge interfacial charge separation.100 Conversely, graphene oxide with amphiphilic function forms a dual-functional buffer layer between a hybrid perovskite and Spiro-MeOTAD (N2,N2,N2′,N2′,N7,N7,N7′,N7′-octakis(4-methoxyphenyl)-9,9′-spirobi[9H-fluorene]-2,2′,7,7′-tetramine) hole transport layer imbuing directionality and establishing a charge-transfer cascade that ultimately brings about significant increases in open-circuit voltage and fill factor.101 Interfacial design is critical to mediating ultrafast charge or energy-transfer dynamics that is pivotal to electronic, photonic, and quantum architectures. The interplay between thermodynamic energy offsets and dynamics of charge transfer reflects a challenging problem that requires probes of electronic structure and interfacial charge-transfer dynamics.
In other examples, interfaces facilitate targeted delivery of disparate payloads. Cetyltrimethylammonium bromide (CTAB)-coated gold nanorods can be wrapped with polyacrylic acid in layer-by-layer synthesis to give water-dispersible, negatively charged materials that allow for bioconjugation with positively charged horse heart cytochrome c, a small hemeprotein.102 Such interfaces can enable selective binding of specific molecular fragments enabling a specific response to be elicited that allows for sensitive and selective detection of analytes. For instance, template-directed self-assembly allows for hierarchically ordered graphene sheets-enzyme (cholesterol oxidase and cholesterol esterase)-gold nanoparticle arrays for electrochemical biosensing.103 Multiwalled carbon nanotubes modified with CTAB-poly(sodium-p-styrene-sulfonate) polyelectrolyte-surfactant polymer can be used to trap hemoglobin for electrochemical characterization during H2O2, O2, and NO2– catalysis.104 These examples largely rely on weaker van der Waals interactions to create the functional interface, and creating functional interfaces using stronger, covalent bonds provides opportunities to enhance durability of functional interfaces.
Epitaxial interfaces
Interfaces in hybrids between dissimilar crystalline materials are of interest due to their diverse functionality, which often departs fundamentally from the properties of the constituents. Such hybrids represent an extreme case among the materials considered here since they typically show long-range atomic order and low defect densities but also require complex synthesis processes. Crystalline and epitaxial hybrid systems are uniquely amenable to advanced characterization down to the atomic scale, such as by in situ electron microscopy of their synthesis and processing, either in vacuum105,106 or in solution,107,108 local band-structure measurements,109,110, and atomic-level structural and chemical analysis correlated with nanoscale functional probing (e.g., optoelectronics).111 Such heterogeneous materials also lend themselves well to studying how properties respond to applied stimuli (e.g., intercalation53 or extreme pressure).112,113
Although pristine (e.g., epitaxial) hybrids and heterostructures of crystalline materials have been studied extensively, recent developments highlight challenges as well as sweeping opportunities for new discoveries and applications of these systems. Two-dimensional and layered van der Waals crystals have brought major advances, in part because of their relaxed lattice matching requirements compared to conventional 3D crystals.114 While lateral115,116 and vertical heterostructures117 are widely studied, van der Waals crystals can support much more diverse hybrids and interfaces, exemplified by light harvesting wrap-around core–shell heterostructures of layered cores encapsulated in few-layer shells.118 New isolation methods for 2D crystals continue to emerge,119 and chemical functionalization of intrinsically inert materials (e.g., MoS2) enables tuning and control of interfacial interactions as well as functional properties.120 Unconventional degrees of freedom, such as interlayer twist in van der Waals stacks have brought a surprising wealth of emergent phenomena, including modified light–matter interactions121–124 and flat-band-induced correlated electron physics125,126 arising in moiré superlattices at twisted interfaces. While such effects are still primarily discovered in mechanically stacked heterostructures,127 the challenge of suppressing equilibrium stacking to realize controlled interlayer twists via scalable bottom-up synthesis represents an emerging frontier in van der Waals epitaxy.128,129 In addition to the interest in 2D crystals themselves, ultrathin materials such as graphene have also revolutionized thin-film growth and epitaxy of 3D materials. Remote epitaxy, in which the interaction between a growing film and the substrate is moderated for instance by an atomically thin graphene spacer130 yields single-crystal films that have exceptionally low defect density,131 are flexible and can be transferred to arbitrary supports.132
Significant challenges remain also in the fabrication of new types of monocrystalline materials, either as thin films or lower-dimensional nanostructures, by scalable and inexpensive approaches and on nonideal (“real-world”) substrates. High-quality materials and hybrids can be obtained by chemical transformations of template crystals. Reactions with gas-phase species, for example, can transform nanowires or nanorods into single-crystalline core–shell structures, hollow shells,133 or nanotubes.134 Inexpensive processes such as spin-coating are emerging as scalable alternatives to vacuum deposition for growing monocrystalline thin films.135 Creative approaches are being developed to control polymorphism, placement, and orientation of crystalline materials on amorphous supports.136 And finally, epitaxy-like registry effects are being identified and understood across a much broader family of hybrids, for example, to successfully predict and control epitaxy of soft on hard materials as realized in polymer-wrapping processes used for chirality sorting of carbon nanotubes.137
Conclusions
Hybrid and interface-based materials span all material categories and can take almost an infinite variety, where their design and predictive synthesis as well as the understanding of their emergent properties naturally benefits from the perspectives of multiple disciplines. The broad perspective of the workshop included some research ideas beyond the scope of the NSF’s Solid State and Materials Chemistry program. Wide-ranging scientific opportunities were recognized at the nexus of understanding how the juxtaposition of disparate materials can promote novel phenomena such as the stabilization of entirely new crystal polymorphs, interfacially confined quantum materials with discrete electronic states, and spin/energy-selective filtering of current and heat. Many efforts in hybrid and interface material synthesis are beginning to explore opportunities that are uniquely possible via kinetic control and kinetic entrapment. Deterministic control of reaction trajectories that result in stabilization of specific metastable interfacial configurations remains a grand challenge. Furthermore, the character of buried interfaces is believed to largely determine many recent phenomena and thus a deeper understanding is needed through the development of toolsets that probe the dynamical evolution of interfaces “as they are” in the presence of external fields. Advanced characterization methods will enhance physical understanding, while improved access to established regional tools would accelerate many ongoing activities. Computational modeling and simulations are especially beneficial and important for research areas that either are sample-limited or where direct characterization is challenging or impossible. Workforce development toward these ends should be bolstered by improvements to student training at multiple stages as well as enhanced resources for hybrid materials research and development.
References
L. Greene, T. Lubensky, M. Tirrell, P. Chaikin, H. Ding, K. Faber, P. Hammond, C. Heckle, K. Hemker, J. Heremans, Committee on Frontiers of Materials Research: A Decadal Survey (National Academies Press, Washington, DC, 2019).
N.A. Fleer, M.P. Thomas, J.L. Andrews, G.R. Waetzig, O. Gonzalez, G.W. Liu, B.S. Guiton, S. Banerjee, Nanoscale 11, 21354 (2019).
A. Parija, G.R. Waetzig, J.L. Andrews, S. Banerjee, J. Phys. Chem. C 122, 25709 (2018).
K.A. Lantz, N.B. Clamp, W. van den Bergh, A. Sarkar, M. Stefik, Small 15 e1900393 (2019).
H.N. Lokupitiya, A. Jones, B. Reid, S. Guldin, M. Stefik, Chem. Mater. 28, 1653 (2016).
A. Sarkar, L. Evans, M. Stefik, Langmuir 34, 5738 (2018).
A. Sarkar, M. Stefik, J. Mater. Chem. A 5, 11840 (2017).
R.E. Schaak, T.E. Mallouk, Chem. Mater. 14, 1455 (2002).
F.R. Siperstein, K.E. Gubbins, Langmuir 19, 2049 (2003).
C. Gong, X. Zhang, Science 363 (2019), http://dx.doi.org/10.1126/science.aav4450.
K.S. Novoselov, A. Mishchenko, A. Carvalho, A.H. Castro Neto, Science 353, aac9439 (2016).
B. Keimer, J.E. Moore, Nat. Phys. 13, 1045 (2017).
Z. Lin, A. McCreary, N. Briggs, S. Subramanian, K. Zhang, Y. Sun, X. Li, N.J. Borys, H. Yuan, 2D Mater. 3, 042001 (2016).
B.J. Park, T. Brugarolas, D. Lee, Soft Matter 7, 6413 (2011).
R.-S. Lee, Y.-T. Huang, Polym. J. 42, 304 (2010).
C.-W. Yao, J.L. Alvarado, C.P. Marsh, B.G. Jones, M.K. Collins, Appl. Surf. Sci. 290, 59 (2014).
A. Liljas, L. Liljas, J. Piskur, P. Nissen, M. Kjeldgaard, Textbook of Structural Biology (World Scientific, Singapore, 2009).
A. Ramasubramaniam, R. Selhorst, H. Alon, M.D. Barnes, T. Emrick, D. Naveh, J. Mater. Chem. C 5, 11158 (2017).
W. Xu, D.H. Gracias, ACS Nano 13, 4883 (2019).
L.-Q. Chen, L.-D. Chen, S.V. Kalinin, G. Klimeck, S.K. Kumar, J. Neugebauer, I. Terasaki, NPJ Comput. Mater. 1 (2015)
L.T. Alameda, R.W. Lord, J.A. Barr, P. Moradifar, Z.P. Metzger, B.C. Steimle, C.F. Holder, N. Alem, S.B. Sinnott, R.E. Schaak, J. Am. Chem. Soc. 141, 10852 (2019).
J.M. Yuk, J. Park, P. Ercius, K. Kim, D.J. Hellebusch, M.F. Crommie, J.Y. Lee, A. Zettl, A.P. Alivisatos, Science 336, 61 (2012).
E.M. Tennyson, J.M. Howard, M.S. Leite, ACS Energy Lett. 2, 1825 (2017).
X. Wang, Q. Weng, Y. Yang, Y. Bando, D. Golberg, Chem. Soc. Rev. 45, 4042 (2016).
D. Halbertal, J. Cuppens, M.B. Shalom, L. Embon, N. Shadmi, Y. Anahory, H.R. Naren, J. Sarkar, A. Uri, Y. Ronen, Y. Myasoedov, L.S. Levitov, E. Joselevich, A.K. Geim, E. Zeldov, Nature 539, 407 (2016).
J. Mayer, L.A. Giannuzzi, T. Kamino, J. Michael, MRS Bull. 32, 400 (2007).
Z.J.W.A. Leijten, A.D.A. Keizer, G. de With, H. Friedrich, J. Phys. Chem. C Nanomater. Interfaces, 121, 10552 (2017).
Y. Li, A. Pei, K. Yan, Y. Sun, C.L. Wu, L.M. Joubert, R. Chin, A.L. Koh, Y. Yu, J. Perrino, B. Butz, S. Chu, Y. Cui, Science 358, 506 (2017).
R.E.A. Williams, D.W. McComb, S. Subramaniam, MRS Bull. 44, 929 (2019).
H.G. Liao, H. Zheng, H. Annu. Rev. Phys. Chem. 67, 719 (2016).
L.F. Kourkoutis, J.M. Plitzko, W. Baumeister, Annu. Rev. Mater. Res. 42, 33 (2012).
R. Burks, K.D. Deards, E. DeFrain, J. Chem. Educ. 94, 1918 (2017).
A. Kondinski, T.N. Parac-Vogt, J. Chem. Educ. 96, 601 (2019).
S. Houben, G. Quintens, L.M. Pitet, J. Chem. Educ. (2020), http://dx.doi.org/10.1021/acs.jchemed.0c00190.
J.A. Rood, K.W. Henderson, J. Chem. Educ. 90, 379 (2013).
A.N. Corpus-Mendoza, P.M. Moreno-Romero, H. Hu, J. Chem. Educ. 96, 974 (2019).
J.M. Ting, R.G. Ricarte, D.K. Schneiderman, S.A. Saba, Y. Jiang, M.A. Hillmyer, F.S. Bates, T.M. Reineke, C.W. Macosko, T.P. Lodge, J. Chem. Educ. 94, 1629 (2017).
P.P. Rodenbough, W.B. Vanti, S.-W. Chan, J. Chem. Educ. 92, 1960 (2015).
G. Kickelbick, Hybrid Materials (Wiley, Hoboken, NJ, 2019).
B.P.S. Chauhan, Novel Nanoscale Hybrid Materials (Wiley, Hoboken, NJ, 2018).
P. Gomez-Romero, C. Sanchez. Functional Hybrid Materials (Wiley, Hoboken, NJ, 2004).
B. Yan, Photofunctional Rare Earth Hybrid Materials (Springer, New York, 2017).
T.R. Cook, Y.R. Zheng, P.J. Stang, Chem. Rev. 113, 734 (2013).
C.F.J. Faul, M. Antonietti, Adv. Mater. 15, 673 (2003).
E. Katz, I. Willner, Angew. Chem. Int. Ed. 43, 6042 (2004).
S. Mann, Nat. Mater. 8, 781 (2009).
Y. Cui, B. Li, H. He, W. Zhou, B. Chen, G. Qian, Acc. Chem. Res. 49, 483 (2016).
A. Putta, J.D. Mottishaw, Z. Wang, H. Sun, Cryst. Growth Des. 14, 350 (2014).
H. Sun, U.K. Tottempudi, J.D. Mottishaw, P.N. Basa, A. Putta, A.G. Sykes, Cryst. Growth Des. 12, 5655 (2012).
R.G. Surbella, L.C. Ducati, K.L. Pellegrini, B.K. McNamara, J. Autschbach, J.M. Schwantes, C.L. Cahill, J. Am. Chem. Soc. 139, 10843 (2017).
L. Mei, C.-Z. Wang, L. Wang, Y.-L. Zhao, Z.-F. Chai, W.-Q. Shi, Cryst. Growth Des. 15, 1395 (2015).
M. Boterashvili, M. Lahav, S. Shankar, A. Facchetti, M.E. van der Boom, J. Am. Chem. Soc. 136, 11926 (2014).
C. Wang, Q. He, U. Halim, Y. Liu, E. Zhu, Z. Lin, H. Xiao, X. Duan, Z. Feng, R. Cheng, N.O. Weiss, G. Ye, Y.C. Huang, H. Wu, H.C. Cheng, I. Shakir, L. Liao, X. Chen, W.A. Goddard, Y. Huang, X. Duan, Nature 555, 231 (2018).
T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F.X. Llabrés, I. Xamena, J. Gascon, Nat. Mater. 14, 48 (2015).
P. Politzer, J.S. Murray, M.C. Concha, J. Mol. Model. 13, 643 (2007).
G. Gattuso, R. Liantonio, P. Metrangolo, F. Meyer, A. Pappalardo, M.F. Parisi, T. Pilati, I. Pisagatti, G. Resnati, Supramol. Chem. 18, 235 (2006).
D.B. Fox, R. Liantonio, P. Metrangolo, T. Pilati, G. Resnati, J. Fluor. Chem. 125, 271 (2004).
Z.M. Marsh, D.A. Blom, M. Stefik, Adv. Mater. Interfaces 7, 1901691 (2020).
F.A. Chávez, P.A. Quiñonez, D.A. Roberson, J. Thermoplast. Compos. Mater. 089270571986415 (2019).
R. Yu, X. Yang, Y. Zhang, X. Zhao, X. Wu, T. Zhao, Y. Zhao, W. Huang, ACS Appl. Mater. Interfaces 9, 1820 (2017).
A. Vyatskikh, S. Delalande, A. Kudo, X. Zhang, C.M. Portela, J.R. Greer, Nat. Commun. 9, 593 (2018).
S. Biswas, F. Brinkmann, M. Hirtz, H. Fuchs, Nanofabrication 2, 19 (2015).
M. Zhang, L. Li, Q. Lin, M. Tang, Y. Wu, C. Ke, J. Am. Chem. Soc. 141, 5154 (2019).
S.F. Situ, J. Cao, C. Chen, E.C. Abenojar, J.M. Maia, A.C.S. Samia, Macromol. Mater. Eng. 301, 1525 (2016).
X. Cai, Y. Luo, B. Liu, H.-M. Cheng, Chem. Soc. Rev. 47, 6224 (2018).
J. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, S.T. Nguyen, J.T. Hupp, Chem. Soc. Rev. 38, 1450 (2009).
L.E. Kreno, K. Leong, O.K. Farha, M. Allendorf, R.P. Van Duyne, J.T. Hupp, Chem. Rev. 112, 1105 (2012).
L.J. Murray, M. Dincă, J.R. Long, Chem. Soc. Rev. 38, 1294 (2009).
K. Sumida, D.L. Rogow, J.A. Mason, T.M. McDonald, E.D. Bloch, Z.R. Herm, T.H. Bae, J.R. Long, Chem. Rev. 112, 724 (2012).
S.M. Cohen, Chem. Rev. 112, 970 (2012).
S.T. Meek, J.A. Greathouse, M.D. Allendorf, Adv. Mater. 2, 249 (2011).
A.J. Matzger, K. Suresh, V. López-Mejías, S. Roy, D.F. Camacho, Synlett (2020), http://dx.doi.org/10.1055/s-0040-1707139.
S. Yuan, L. Huang, Z. Huang, D. Sun, J.S. Qin, L. Feng, J. Li, X. Zou, T. Cagin, H.C. Zhou, J. Am. Chem. Soc. 142, 4732 (2020).
O.M. Yaghi, M. O'Keeffe, N.W. Ockwig, H.K. Chae, M. Eddaoudi, J. Kim, Nature 423, 705 (2003).
H.C. Zhou, S. Kitagawa, Chem. Soc. Rev. 43, 5415 (2014).
K.F. White, B.F. Abrahams, R. Babarao, A.D. Dharma, T.A. Hudson, H.E. Maynard-Casely, R. Robson, Chemistry 21, 18057 (2015).
F. Nouar, J.F. Eubank, T. Bousquet, L. Wojtas, M.J. Zaworotko, M. Eddaoudi, J. Am. Chem. Soc. 130, 1833 (2008).
M. Kandiah, M.H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E.A. Quadrelli, F. Bonino, K.P. Lillerud, Chem. Mater. 22, 6632 (2010).
Férey, G. Chem. Soc. Rev. 37, 191 (2008).
J.A. Chiong, J. Zhu, J.B. Bailey, M. Kalaj, R.H. Subramanian, W. Xu, S.M. Cohen, F.A. Tezcan, J. Am. Chem. Soc. 142, 6907 (2020).
R. Haldar, T.K. Maji, CrystEngComm 15, 9276 (2013).
W. Zhang, K. Freitag, S. Wannapaiboon, C. Schneider, K. Epp, G. Kieslich, R.A. Fischer, Inorg. Chem. 55, 12492 (2016).
J.A. Navarro, E. Barea, J.M. Salas, N. Masciocchi, S. Galli, A. Sironi, C.O. Ania, J.B. Parra, Inorg. Chem. 45, 2397 (2006).
M.E. Ziebel, J.C. Ondry, J.R. Long, Chem. Sci. 11, 6690 (2020).
J. Liu, C. Wöll, Chem. Soc. Rev. 46, 5730 (2017).
O. Shekhah, J. Liu, R.A. Fischer, C. Wöll, Chem. Soc. Rev. 40, 1081 (2011).
M.A. Solomos, F.J. Claire, T.J. Kempa, J. Mater. Chem. A 7, 23537 (2019).
J. Zhu, P.M. Usov, W. Xu, P.J. Celis-Salazar, S. Lin, M.C. Kessinger, C. Landaverde-Alvarado, M. Cai, A.M. May, C. Slebodnick, D. Zhu, S.D. Senanayake, A.J. Morris, J. Am. Chem. Soc. 140, 93 (2018).
Y. Song, X. Feng, J.S. Chen, C. Brzezinski, Z. Xu, W. Lin, J. Am. Chem. Soc. 142, 4872 (2020).
National Academies of Science, Engineering, and Medicine, Frontiers of Materials Research: A Decadal Survey (National Academies Press, Washington, DC, 2019).
J. Seo, J.H. Noh, S.I. Seok, Acc. Chem. Res. 49, 562 (2016).
M. Grätzel, Acc. Chem. Res. 50, 487 (2017).
M.P. Thomas, N. Wanninayake, M. De Alwis Goonatilleke, D.Y. Kim, B.S. Guiton, Nanoscale 12, 6144 (2020).
L. Lai, J.R. Potts, D. Zhan, L. Wang, C.K. Poh, C. Tang, H. Gong, Z. Shen, J. Lin, R.S. Ruoff, Energy Environ. Sci. 5, 7936 (2012).
X.X. Wang, M.T. Swihart, G. Wu, Nat. Catal. 2, 578 (2019).
J. Li, G.P. Pandey, Annu. Rev. Phys. Chem. 66, 331 (2015).
A.V. Melechko, V.I. Merkulov, T.E. McKnight, M.A. Guillorn, K.L. Klein, D.H. Lowndes, M.L. Simpson, J. Appl. Phys. 97, 041301 (2005).
S.A. Klankowski, R.A. Rojeski, B.A. Cruden, J. Liu, J. Wu, J. Li, J. Mater. Chem. A 1, 1055 (2013).
E. Brown, J. Acharya, G.P. Pandey, J. Wu, J. Li, Adv. Mater. Interfaces 3, 1600824 (2016).
P.S. Murphin Kumar, V.K. Ponnusamy, K.R. Deepthi, G. Kumar, A. Pugazhendhi, H. Abe, S. Thiripuranthagan, U. Pal, S.K. Krishnan, J. Mater. Chem. A, 6, 23435 (2018).
W. Li, H. Dong, X. Guo, N. Li, J. Li, G. Niu, L. Wang, J. Mater. Chem. A 2, 20105 (2014).
T. Placido, L. Tognaccini, B.D. Howes, A. Montrone, V. Laquintana, R. Comparelli, M.L. Curri, G. Smulevich, A. Agostiano, ACS Omega 3, 4959 (2018).
O. Parlak, A. Tiwari, A.P. Turner, A. Tiwari, Biosens. Bioelectron. 49, 53 (2013).
L. Chen, G. Lu, J. Electroanal. Chem. 597, 51 (2006).
P. Sutter, Y. Huang, E. Sutter, Nano Lett. 14, 4846 (2014).
L. Yu, B.M. Hudak, A. Ullah, M.P. Thomas, C.C. Porter, A. Thisera, R.H. Pham, M. De Alwis Goonatilleke, B.S. Guiton, Chem. Mater. 32, 639 (2020).
C. Zhu, S. Liang, E. Song, Y. Zhou, W. Wang, F. Shan, Y. Shi, C. Hao, K. Yin, T. Zhang, J. Liu, H. Zheng, L. Sun, Nat. Commun. 9, 421 (2018).
E. Sutter, P. Sutter, A.V. Tkachenko, R. Krahne, J. de Graaf, M. Arciniegas, L. Manna, Nat. Commun. 7, 11213 (2016).
W. Jin, P.C. Yeh, N. Zaki, D. Zhang, J.T. Sadowski, A. Al-Mahboob, A.M. van der Zande, D.A. Chenet, J.I. Dadap, I.P. Herman, P. Sutter, J. Hone, R.M. Osgood, Phys. Rev. Lett. 111, 06801 (2013).
P.V. Nguyen, N.C. Teutsch, N.P. Wilson, J. Kahn, X. Xia, A.J. Graham, V. Kandyba, A. Giampietri, A. Barinov, G.C. Constantinescu, N. Yeung, N.D.M. Hine, X. Xu, D.H. Cobden, N.R. Wilson, Nature 572, 220 (2019).
P. Sutter, C. Argyropoulos, E. Sutter, Nano Lett. 18, 4576 (2018).
M. Yankowitz, S. Chen, H. Polshyn, Y. Zhang, K. Watanabe, T. Taniguchi, D. Graf, A.F. Young, C.R. Dean, Science 363, 1059 (2019).
M.J. Crane, A. Petrone, R.A. Beck, M.B. Lim, X. Zhou, X. Li, Sci. Adv. 5, eaau6073 (2019).
A.K. Geim, I.V. Grigorieva, Nature 499, 419 (2013).
P.K. Sahoo, S. Memaran, Y. Xin, L. Balicas, H.R. Gutiérrez, Nature 553, 63 (2018).
S. Xie, L. Tu, Y. Han, L. Huang, K. Kang, K.U. Lao, P. Poddar, C. Park, D.A. Muller, R.A. DiStasio, J. Park, Science 359, 1131 (2018).
K. Kang, K.H. Lee, Y. Han, H. Gao, S. Xie, D.A. Muller, J. Park, Nature 550, 229 (2017).
P. Sutter, J. Wang, E. Sutter, Adv. Mater. 31, e1902166 (2019).
B.J. Ryan, M.P. Hanrahan, Y. Wang, U. Ramesh, C.K.A. Nyamekye, R.D. Nelson, Z. Liu, C. Huang, B. Whitehead, J. Wang, L.T. Roling, E.A. Smith, A.J. Rossini, M.G. Panthani, Chem. Mater. 32, 795 (2020).
D.O. Li, X.S. Chu, Q.H. Wang, Langmuir 35, 5693 (2019).
E.M. Alexeev, D.A. Ruiz-Tijerina, M. Danovich, M.J. Hamer, D.J. Terry, P.K. Nayak, S. Ahn, S. Pak, J. Lee, J.I. Sohn, M.R. Molas, M. Koperski, K. Watanabe, T. Taniguchi, K.S. Novoselov, R.V. Gorbachev, H.S. Shin, V.I. Fal'ko, A.I. Tartakovskii, Nature 567, 81 (2019).
C. Jin, E.C. Regan, A. Yan, M.I.B. Utama, D. Wang, S. Zhao, Y. Qin, S. Yang, Z. Zheng, S. Shi, K. Watanabe, T. Taniguchi, S. Tongay, A. Zettl, F. Wang, Nature 567, 76 (2019).
K.L. Seyler, P. Rivera, H. Yu, N.P. Wilson, E.L. Ray, D.G. Mandrus, J. Yan, W. Yao, X. Xu, Nature 567, 66 (2019).
K. Tran, G. Moody, F. Wu, X. Lu, J. Choi, K. Kim, A. Rai, D. Sanchez, D.J. Quan, A. Singh, J. Embley, A. Zepeda, M. Campbell, T. Autry, T. Taniguchi, K. Watanabe, N. Lu, S.K. Banerjee, K.L. Silverman, S. Kim, E. Tutuc, L. Yang, A.H. MacDonald, X. Li, Nature 567, 71 (2019).
Y. Cao, V. Fatemi, S. Fang, K. Watanabe, T. Taniguchi, E. Kaxiras, P. Jarillo-Herrero, Nature 556, 43 (2018).
Y. Cao, V. Fatemi, A. Demir, S. Fang, S.L. Tomarken, J.Y. Luo, J.D. Sanchez-Yamagishi, K. Watanabe, T. Taniguchi, E. Kaxiras, R.C. Ashoori, P. Jarillo-Herrero, Nature 556, 80 (2018).
R. Ribeiro-Palau, C. Zhang, K. Watanabe, T. Taniguchi, J. Hone, C.R. Dean, Science 361, 690 (2018).
P. Sutter, S. Wimer, E. Sutter, Nature 570, 354 (2019).
P. Sutter, R. Ibragimova, H.-P. Komsa, B.A. Parkinson, E. Sutter, Nat. Commun. 10, 5528 (2019).
Y. Kim, S.S. Cruz, K. Lee, B.O. Alawode, C. Choi, Y. Song, J.M. Johnson, C. Heidelberger, W. Kong, S. Choi, K. Qiao, I. Almansouri, E.A. Fitzgerald, J. Kong, A.M. Kolpak, J. Hwang, J. Kim, Nature 544, 340 (2017).
S.-H. Bae, K. Lu, Y. Han, S. Kim, K. Qiao, C. Choi, Y. Nie, H. Kim, H.S. Kum, P. Chen, W. Kong, B.-S. Kang, C. Kim, J. Lee, Y. Baek, J. Shim, J. Park, M. Joo, D.A. Muller, K. Lee, J. Kim, Nat. Nanotechnol. 15, 272 (2020).
H.S. Kum, H. Lee, S. Kim, S. Lindemann, W. Kong, K. Qiao, P. Chen, J. Irwin, J.H. Lee, S. Xie, S. Subramanian, J. Shim, S.-H. Bae, C. Choi, L. Ranno, S. Seo, S. Lee, J. Bauer, H. Li, K. Lee, J.A. Robinson, C.A. Ross, D.G. Schlom, M.S. Rzchowski, C.-B. Eom, J. Kim, Nature 578, 75 (2020).
L. Yu, R. Han, X. Sang, J. Liu, M.P. Thomas, B.M. Hudak, A. Patel, K. Page, B.S. Guiton, ACS Nano 12, 9051 (2018).
E. Sutter, J.S. French, A. Balgarkashi, N. Tappy, A. Fontcuberta, I. Morral, J.C. Idrobo, P. Sutter, Nano Lett. 19, 8903 (2019).
M.V. Kelso, N.K. Mahenderkar, Q. Chen, J.Z. Tubbesing, J.A. Switzer, Science 12, 166 (2019).
V. López-Mejías, J.L. Knight, C.L. Brooks, A.J. Matzger, Langmuir 27, 7575 (2011).
Y. Joo, G.J. Brady, M.S. Arnold, P. Gopalan, Langmuir 30, 3460 (2014).
D. Chen, T. Li, L. Yin, X. Hou, X. Yu, Y. Zhang, B. Fan, H. Wang, X. Li, R. Zhang, T. Hou, H. Lu, H. Xu, J. Sun, L. Gao, Mater. Chem. Phys. 125 (3), 838 (2011).
C. Amiel, M. Sikka, J.W. Schneider Jr., Y.H. Tsao, M. Tirrell, J.W. Mays, Macromolecules 28 (9), 3125 (1995).
Acknowledgments
The workshop and collaborative work in generating this article were supported by the National Science Foundation DMR-1940540.
Author information
Authors and Affiliations
Acknowledgments
Acknowledgments
 Beth S. Guiton is an associate professor at the University of Kentucky (UK). She received her BA and MSci degrees from the University of Cambridge, UK, her AM degree from Harvard University, and her PhD degree from the University of Pennsylvania. Prior to joining the UK faculty, Guiton was a Eugene P. Wigner Fellow at Oak Ridge National Laboratory. Her research focuses on local probe techniques such as in situ heating in the transmission electron microscope to address questions regarding solid-state synthetic mechanisms, and the characteristics of phase transformations, on the atomic and nanoscale. She is a recipient of the National Science Foundation CAREER Award, and a Research Corporation for Science Advancement Scialog Award. Guiton can be reached by email at beth.guiton@uky.edu.
Beth S. Guiton is an associate professor at the University of Kentucky (UK). She received her BA and MSci degrees from the University of Cambridge, UK, her AM degree from Harvard University, and her PhD degree from the University of Pennsylvania. Prior to joining the UK faculty, Guiton was a Eugene P. Wigner Fellow at Oak Ridge National Laboratory. Her research focuses on local probe techniques such as in situ heating in the transmission electron microscope to address questions regarding solid-state synthetic mechanisms, and the characteristics of phase transformations, on the atomic and nanoscale. She is a recipient of the National Science Foundation CAREER Award, and a Research Corporation for Science Advancement Scialog Award. Guiton can be reached by email at beth.guiton@uky.edu.
 Morgan Stefik is an associate professor of chemistry and biochemistry at the University of South Carolina, and is the founding director of the South Carolina SAXS Collaborative. He obtained his BE degree in materials engineering from California Polytechnic State University in 2005, and his PhD degree in materials science from Cornell University in 2010. He then completed postdoctoral research at École Polytechnique Fédérale de Lausanne. His awards include a National Science Foundation CAREER Award in 2018, recognition as a “Rising Star of Materials Chemistry” by the Royal Society of Chemistry in 2017, a Breakthrough Star Award from the University of South Carolina in 2018, election to the council of the International Mesostructured Materials Association in 2018, and selection as an American Chemical Society Division of Polymeric Materials: Science and Engineering division Young Investigator in 2020. Stefik can be reached by email at morgan@stefikgroup.com.
Morgan Stefik is an associate professor of chemistry and biochemistry at the University of South Carolina, and is the founding director of the South Carolina SAXS Collaborative. He obtained his BE degree in materials engineering from California Polytechnic State University in 2005, and his PhD degree in materials science from Cornell University in 2010. He then completed postdoctoral research at École Polytechnique Fédérale de Lausanne. His awards include a National Science Foundation CAREER Award in 2018, recognition as a “Rising Star of Materials Chemistry” by the Royal Society of Chemistry in 2017, a Breakthrough Star Award from the University of South Carolina in 2018, election to the council of the International Mesostructured Materials Association in 2018, and selection as an American Chemical Society Division of Polymeric Materials: Science and Engineering division Young Investigator in 2020. Stefik can be reached by email at morgan@stefikgroup.com.
 Veronica Augustyn is an assistant professor of materials science and engineering, and University Faculty Scholar at North Carolina State University. She received her BS degree from The University of Arizona and her PhD degree from the University of California, Los Angeles, in materials science and engineering. She was a postdoctoral fellow at The University of Texas at Austin. Her research focuses on the behavior of materials in electrochemical environments, and includes the synthesis of hybrid layered materials. Her awards include a 2017 National Science Foundation CAREER Award, a 2019 US Department of Energy Early Career Award, and a 2019 Alfred P. Sloan Research Fellowship in Chemistry. Augustyn can be reached by email at vaugust@ncsu.edu.
Veronica Augustyn is an assistant professor of materials science and engineering, and University Faculty Scholar at North Carolina State University. She received her BS degree from The University of Arizona and her PhD degree from the University of California, Los Angeles, in materials science and engineering. She was a postdoctoral fellow at The University of Texas at Austin. Her research focuses on the behavior of materials in electrochemical environments, and includes the synthesis of hybrid layered materials. Her awards include a 2017 National Science Foundation CAREER Award, a 2019 US Department of Energy Early Career Award, and a 2019 Alfred P. Sloan Research Fellowship in Chemistry. Augustyn can be reached by email at vaugust@ncsu.edu.
 Sarbajit Banerjee is the Davidson Professor of Chemistry, Chancellor EDGES Fellow, and a professor of materials science and engineering at Texas A&M University. His research interests are focused on electron correlated solids, electronic structure studies at interfaces, metastable materials, energy conversion and storage, energy-efficient computation, and the development of synchrotron spectroscopy and imaging methods. His awards include a National Science Foundation CAREER Award, the ExxonMobil Solid-State Chemistry Faculty Fellowship, the Massachusetts Institute of Technology Review TR35 Award, Cottrell Scholar Award, Rosenhain Medal and Prize from The Institute of Materials, Minerals and Mining, and the Beilby Medal and Prize from the Royal Society of Chemistry. Banerjee can be reached by email at banerjee@chem.tamu.edu.
Sarbajit Banerjee is the Davidson Professor of Chemistry, Chancellor EDGES Fellow, and a professor of materials science and engineering at Texas A&M University. His research interests are focused on electron correlated solids, electronic structure studies at interfaces, metastable materials, energy conversion and storage, energy-efficient computation, and the development of synchrotron spectroscopy and imaging methods. His awards include a National Science Foundation CAREER Award, the ExxonMobil Solid-State Chemistry Faculty Fellowship, the Massachusetts Institute of Technology Review TR35 Award, Cottrell Scholar Award, Rosenhain Medal and Prize from The Institute of Materials, Minerals and Mining, and the Beilby Medal and Prize from the Royal Society of Chemistry. Banerjee can be reached by email at banerjee@chem.tamu.edu.
 Christopher J. Bardeen is a professor in the Department of Chemistry and Materials Science, and Department of Engineering at the University of California, Riverside. He received his BS degree in chemistry from Yale University in 1989, and his PhD degree in chemistry from the University of California, Berkeley, in 1995. His awards include the 3M Non-Tenured Faculty Award, the National Science Foundation CAREER Award, and the Alfred P. Sloan Research Fellowship. His research focuses on the photochemical and photophysical properties of organic molecular crystals, including exciton fission and photomechanical actuation. Bardeen can be reached by email at christopher.bardeen@ucr.edu.
Christopher J. Bardeen is a professor in the Department of Chemistry and Materials Science, and Department of Engineering at the University of California, Riverside. He received his BS degree in chemistry from Yale University in 1989, and his PhD degree in chemistry from the University of California, Berkeley, in 1995. His awards include the 3M Non-Tenured Faculty Award, the National Science Foundation CAREER Award, and the Alfred P. Sloan Research Fellowship. His research focuses on the photochemical and photophysical properties of organic molecular crystals, including exciton fission and photomechanical actuation. Bardeen can be reached by email at christopher.bardeen@ucr.edu.
 Bart M. Bartlett is a professor of chemistry at the University of Michigan. He received his AB degree in chemistry from Washington University in 2000, and his PhD degree in inorganic chemistry from the Massachusetts Institute of Technology in 2005. He was a postdoctoral fellow at the University of California, Berkeley, from 2005 to 2008. His research includes developing synthesis methods to prepare compositionally complex materials in which the interplay between structure, composition, phase, and morphology tunes relevant physical properties such as optical absorptivity and electrical conductivity needed for applications in chemical catalysis, energy conversion, and energy storage. His awards include the National Science Foundation CAREER Award in 2013, the College of Literature, Science, and the Arts Excellence in Education Award in 2013, the Seyhan N. Eğe Faculty Development Award in 2013, and the LSA Class of 1923 Memorial Teaching Award in 2014. Bartlett can be reached by email at bartmb@umich.edu.
Bart M. Bartlett is a professor of chemistry at the University of Michigan. He received his AB degree in chemistry from Washington University in 2000, and his PhD degree in inorganic chemistry from the Massachusetts Institute of Technology in 2005. He was a postdoctoral fellow at the University of California, Berkeley, from 2005 to 2008. His research includes developing synthesis methods to prepare compositionally complex materials in which the interplay between structure, composition, phase, and morphology tunes relevant physical properties such as optical absorptivity and electrical conductivity needed for applications in chemical catalysis, energy conversion, and energy storage. His awards include the National Science Foundation CAREER Award in 2013, the College of Literature, Science, and the Arts Excellence in Education Award in 2013, the Seyhan N. Eğe Faculty Development Award in 2013, and the LSA Class of 1923 Memorial Teaching Award in 2014. Bartlett can be reached by email at bartmb@umich.edu.
 Jun Li is a professor in the Department of Chemistry at Kansas State University. He received his BS degree from Wuhan University, China, in 1987, his PhD degree from Princeton University in 1995, and completed postdoctoral training at Cornell University. He was a GS15 scientist at NASA Ames Research Center before joining Kansas State University. His research is focused on nanomaterials for energy, biosensors, and nanoelectronics. He was elected as a Fellow of the National Academy of Inventors in 2019. Li can be reached by email at junli@ksu.edu.
Jun Li is a professor in the Department of Chemistry at Kansas State University. He received his BS degree from Wuhan University, China, in 1987, his PhD degree from Princeton University in 1995, and completed postdoctoral training at Cornell University. He was a GS15 scientist at NASA Ames Research Center before joining Kansas State University. His research is focused on nanomaterials for energy, biosensors, and nanoelectronics. He was elected as a Fellow of the National Academy of Inventors in 2019. Li can be reached by email at junli@ksu.edu.
 Vilmalí López-Mejías is an assistant professor at the University of Puerto Rico. She received her BS degree in chemistry from the University of Puerto Rico and her MS and PhD degrees from the University of Michigan. She completed postdoctoral research at the Massachusetts Institute of Technology (MIT) where she worked on projects related to the MIT-Novartis Center for Continuous Manufacturing. Her research focuses on the application and mechanistic understanding of polymer–crystal interfaces for the control and discovery of polymorphism in organic and hybrid materials and their polymer-based formulations. López-Mejías can be reached by email at vilmali.lopez@upr.edu.
Vilmalí López-Mejías is an assistant professor at the University of Puerto Rico. She received her BS degree in chemistry from the University of Puerto Rico and her MS and PhD degrees from the University of Michigan. She completed postdoctoral research at the Massachusetts Institute of Technology (MIT) where she worked on projects related to the MIT-Novartis Center for Continuous Manufacturing. Her research focuses on the application and mechanistic understanding of polymer–crystal interfaces for the control and discovery of polymorphism in organic and hybrid materials and their polymer-based formulations. López-Mejías can be reached by email at vilmali.lopez@upr.edu.
 Leonard R. MacGillivray is a professor in the Department of Chemistry at The University of Iowa. He obtained his BSc degree at Saint Mary’s University in Halifax, Canada, in 1994, and his PhD degree in chemistry as a NSERC 1967 Science and Engineering Scholarship recipient at the University of Missouri-Columbia in 1998. He was a research associate at the Steacie Institute for Molecular Sciences at the National Research Council of Canada from 1998 to 2000. His research focuses on applications of supramolecular chemistry to the field of crystal engineering. His awards include the American Association for the Advancement of Science (2012), and the American Chemical Society (2015). He has published more than 230 manuscripts and is currently a co-editor of the International Union of Crystallography. MacGillivray can be reached by email at len-macgillivray@uiowa.edu.
Leonard R. MacGillivray is a professor in the Department of Chemistry at The University of Iowa. He obtained his BSc degree at Saint Mary’s University in Halifax, Canada, in 1994, and his PhD degree in chemistry as a NSERC 1967 Science and Engineering Scholarship recipient at the University of Missouri-Columbia in 1998. He was a research associate at the Steacie Institute for Molecular Sciences at the National Research Council of Canada from 1998 to 2000. His research focuses on applications of supramolecular chemistry to the field of crystal engineering. His awards include the American Association for the Advancement of Science (2012), and the American Chemical Society (2015). He has published more than 230 manuscripts and is currently a co-editor of the International Union of Crystallography. MacGillivray can be reached by email at len-macgillivray@uiowa.edu.
 Amanda Morris is a Patricia Caldwell Faculty Fellow of the College of Science and professor of inorganic and energy chemistry at the Virginia Polytechnic Institute and State University. Her research focuses on light–matter interactions, electron-transfer reactivity, and catalysis in porous three-dimensional architectures, namely metal–organic frameworks. Her awards include a National Science Foundation CAREER Award, an Alfred P. Sloan Fellowship, the Camille Dreyfus Teacher-Scholar Award, and the Inter-American Photochemical Society Young Investigator Award. Morris can be reached by email at ajmorris@vt.edu.
Amanda Morris is a Patricia Caldwell Faculty Fellow of the College of Science and professor of inorganic and energy chemistry at the Virginia Polytechnic Institute and State University. Her research focuses on light–matter interactions, electron-transfer reactivity, and catalysis in porous three-dimensional architectures, namely metal–organic frameworks. Her awards include a National Science Foundation CAREER Award, an Alfred P. Sloan Fellowship, the Camille Dreyfus Teacher-Scholar Award, and the Inter-American Photochemical Society Young Investigator Award. Morris can be reached by email at ajmorris@vt.edu.
 Efrain E. Rodriguez is an associate professor in the Department of Chemistry and Biochemistry at the University of Maryland, College Park. He received his BS degree from the Massachusetts Institute of Technology and his PhD degree from the University of California, Santa Barbara. He completed a National Research Council postdoctoral fellowship at the National Institute of Standards and Technology. He is the recipient of the National Science Foundation CAREER Award and the Margaret C. Etter Early Career Award from the American Crystallographic Association. Rodriguez can be reached by email at efrain@umd.edu.
Efrain E. Rodriguez is an associate professor in the Department of Chemistry and Biochemistry at the University of Maryland, College Park. He received his BS degree from the Massachusetts Institute of Technology and his PhD degree from the University of California, Santa Barbara. He completed a National Research Council postdoctoral fellowship at the National Institute of Standards and Technology. He is the recipient of the National Science Foundation CAREER Award and the Margaret C. Etter Early Career Award from the American Crystallographic Association. Rodriguez can be reached by email at efrain@umd.edu.
 Anna Cristina S. Samia is an associate professor in the Department of Chemistry at Case Western Reserve University. Her research interests focus on the synthesis and study of the magneto-opto-electronic properties of intermetallic and metal oxide nanostructures with an emphasis on chemical design to achieve desired properties and function. The main applications are in the areas of magnetic imaging and therapy, nanosensor development, and environmental nanotechnology. Samia can be reached by email at axs232@case.edu.
Anna Cristina S. Samia is an associate professor in the Department of Chemistry at Case Western Reserve University. Her research interests focus on the synthesis and study of the magneto-opto-electronic properties of intermetallic and metal oxide nanostructures with an emphasis on chemical design to achieve desired properties and function. The main applications are in the areas of magnetic imaging and therapy, nanosensor development, and environmental nanotechnology. Samia can be reached by email at axs232@case.edu.
 Haoran Sun is an associate professor of chemistry and director of the Center for Fluorinated Functional Materials at the University of South Dakota. He received his BS and PhD degrees in chemistry from Jilin University, China. His research focuses on fluorine chemistry and fluorinated functional materials with emphasis on the development of safe fluorination methods and high-performance organic semiconductor and battery materials. His awards include the National Science Foundation CAREER Award and the University of South Dakota President’s Award for Research Excellence. Sun can be reached by email at Haoran.Sun@usd.edu.
Haoran Sun is an associate professor of chemistry and director of the Center for Fluorinated Functional Materials at the University of South Dakota. He received his BS and PhD degrees in chemistry from Jilin University, China. His research focuses on fluorine chemistry and fluorinated functional materials with emphasis on the development of safe fluorination methods and high-performance organic semiconductor and battery materials. His awards include the National Science Foundation CAREER Award and the University of South Dakota President’s Award for Research Excellence. Sun can be reached by email at Haoran.Sun@usd.edu.
 Peter Sutter has been a professor of electrical and computer engineering at the University of Nebraska–Lincoln since 2015. He received his PhD degree in physics from the Swiss Federal Institute of Technology (ETH Zürich), Switzerland. He was an assistant/associate professor of physics at the Colorado School of Mines and a staff scientist and group leader in the Center for Functional Nanomaterials at Brookhaven National Laboratory. His research focuses on synthesis, electronic, and optoelectronic properties of two-dimensional materials and nanomaterials, studied by in situ microscopy and advanced spectroscopy techniques. His awards include the National Science Foundation CAREER Award, the Scientific American 50 Award, and the Sapphire Prize. He has authored more than 180 peer-reviewed publications, presented 100 invited talks, and holds 8 US patents. Sutter can be reached by email at psutter@unl.edu.
Peter Sutter has been a professor of electrical and computer engineering at the University of Nebraska–Lincoln since 2015. He received his PhD degree in physics from the Swiss Federal Institute of Technology (ETH Zürich), Switzerland. He was an assistant/associate professor of physics at the Colorado School of Mines and a staff scientist and group leader in the Center for Functional Nanomaterials at Brookhaven National Laboratory. His research focuses on synthesis, electronic, and optoelectronic properties of two-dimensional materials and nanomaterials, studied by in situ microscopy and advanced spectroscopy techniques. His awards include the National Science Foundation CAREER Award, the Scientific American 50 Award, and the Sapphire Prize. He has authored more than 180 peer-reviewed publications, presented 100 invited talks, and holds 8 US patents. Sutter can be reached by email at psutter@unl.edu.
 Daniel R. Talham is a professor of chemistry at the University of Florida. He received his PhD degree in inorganic chemistry from Johns Hopkins University in 1985. He held postdoctoral positions at the University of Oxford, UK, Université Paris-Sud, France, and the Massachusetts Institute of Technology. From 2012 to 2013, he held the Pierre de Fermat Chair d'Excellence at the Université de Toulouse. His research interests include materials chemistry, and focus on light-switchable magnetism, MRI contrast, mesoscale interface effects, and phosphate adsorption at interfaces for analytical and environmental applications. Talham can be reached by email at talham@chem.ufl.edu.
Daniel R. Talham is a professor of chemistry at the University of Florida. He received his PhD degree in inorganic chemistry from Johns Hopkins University in 1985. He held postdoctoral positions at the University of Oxford, UK, Université Paris-Sud, France, and the Massachusetts Institute of Technology. From 2012 to 2013, he held the Pierre de Fermat Chair d'Excellence at the Université de Toulouse. His research interests include materials chemistry, and focus on light-switchable magnetism, MRI contrast, mesoscale interface effects, and phosphate adsorption at interfaces for analytical and environmental applications. Talham can be reached by email at talham@chem.ufl.edu.
Rights and permissions
About this article
Cite this article
Guiton, B.S., Stefik, M., Augustyn, V. et al. Frontiers in hybrid and interfacial materials chemistry research. MRS Bulletin 45, 951–964 (2020). https://doi.org/10.1557/mrs.2020.271
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2020.271