Abstract
Electronic performance in semiconducting polymers has improved dramatically in recent years owing to a host of novel materials and processing techniques. Our understanding of the factors governing charge transport in these materials has also been enhanced through advancements in both experimental and computational techniques, with disorder appearing to play a central role. In this prospective, we propose that disorder is an inextricable aspect of polymer morphology which need not be highly detrimental to charge transport if it is embraced and planned for. We discuss emerging guidelines for the synthesis of polymers which are resilient to disorder and present our vision for how future advances in processing and molecular design will provide a path toward further increases in charge-carrier mobility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semiconducting polymers demonstrate great potential in enabling the production of low-cost, light-weight, and flexible electronic devices such as transistors, solar cells, sensors, and light-emitting diodes (LEDs).[1–4] In recent years, many of these applications have come closer to commercial reality due to continual and rapid improvement in materials properties. Dramatic increases in charge-carrier mobility, one of the most important figures of merit, have been achieved, with polymers now regularly being synthesized with performances exceeding that of amorphous silicon (>1 cm2/Vs).[5–9] In fact, new high-performance materials have mobilities comparable with those found in commercial metal-oxide transistors (10–30 cm2/Vs) when processed in a special manner to achieve high degrees of chain alignment,[10] suggesting that polymer semiconductors have potential for use in backplanes for next-generation organic light-emitting diode (OLED) displays. Indeed, when coupled with the other advantages provided by semiconducting polymers (low-temperature solution processing, low embodied energy, mechanical flexibility, etc.), it seems clear that the future is bright for these materials to find a commercial role.
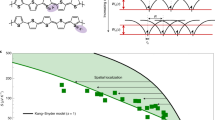
State-of-the-art polymer mobilities have improved by over six orders of magnitude in the last 30 years, owing primarily to major advancements in molecular design (Fig. 1).[11,12] Over time, design rules have emerged and evolved as our understanding offeatures needed for high mobility has improved. Owing to the complexity of polymer microstructure and its importance on the resulting electronic properties of the material, many of these rules involve structural features of the polymer. A major milestone in the history of semiconducting polymers was reached with the development of highly regioregular poly(3-hexylthiophene) (P3HT), which was the first undoped polymer with a mobility near 0.1 cm2/Vs.[13] As one of the most crystalline semiconducting polymers synthesized to that point in time, the success of P3HT led to the logical conclusion that long-range order is necessary for efficient charge transport. This idea was reinforced by the record-setting mobility of highly crystalline poly-2,5-bis (3-tetradecylthiophen-2-yl)thieno[3,2-b]thiophene] (PBTTT).[14] However, in recent years this design guideline has been disrupted by the emergence of weakly ordered donor-acceptor polymers which have pushed mobilities higher still.
Evolution of semiconducting polymer mobility. Polymer mobility has improved by over six orders of magnitude in the last 30 years. Surprisingly, most of the recent gains have been made by weakly ordered donor-acceptor copolymers. The asterisk denotes materials aligned using a special technique.[15]
Indeed, the last several years have witnessed the emergence of many new design rules for achieving high-performance polymers. Some of these rules have already been applied in the synthesis of new molecules (reduced π-stacking distance,[7,16] large conjugated core[17,18]), while others are only just beginning to be developed thanks to careful structure–property studies (nearly torsion-free backbone,[19–21] electronic resilience[22]). Advances in processing will also clearly play a role in continuing to push performances further still.[9,10,15] However, owing to the complexity of polymer microstructure and the difficulty in predicting a priori electronic properties from molecular structure, the combination of all the above factors together has not yet been realized in a single material. As such, we believe that there is significant room for further performance improvements based solely on design rules that have already been discovered.
In this prospective, we summarize our current understanding of the relationship between microstructure and charge transport in semiconducting polymers and review the design guidelines which have emerged for high-performance materials. We also synthesize results obtained by both experimentalists and theoreticians in order to reconcile the findings of both communities and provide design rules that are general and applicable to all semiconducting polymers. We propose that disorder is an inextricable aspect of semiconducting polymer microstructure and suggest that rather than attempting to get rid of disorder, future efforts should focus on designing materials which will be resilient to it. Several materials already unwittingly exhibit aspects of this strategy; however, here we lay out the guidelines which should be intentionally incorporated into the design of future materials. Finally, we present our vision for how future advances in processing and molecular design can push performances further still.
Polymers are inevitably disordered, leading to electronic traps
Despite being a topic of intense study for more than 50 years, polymer microstructure remains an area of research where our phology is exceptionally complex with chains displaying conformations that contain a multitude of kinks, bends, folds, and twists as well as exhibiting a high degree of entanglement owing to the macromolecular nature of the polymer chains (Fig. 2). Intermolecular interactions are driven by weak van der Waals forces and the resulting structure of a material is often more strongly affected by processing conditions and polymer-substrate interactions than the inherent properties of the material itself.[23–25] Further, polymer properties can change dramatically based solely on the molecular weight[26–29]and additional complexity is introduced by the fact that nearly all polymer samples are polydisperse and contain a distribution of chain lengths.[27,30]
Schematic microstructures of semiconducting polymers. Representative of microstructure of (a) semicrystalline, (b) weakly ordered, and (c) amorphous polymer films. Polymers need a minimal amount of aggregation to allow intermolecular transport, while large-scale crystallinity is not necessary for efficient transport. Fast charge transport pathways are highlighted in red. Reproduced from Ref. 31 with permission from Macmillan Publishers Limited.
The highest mobility semiconducting polymers are all semicrystalline, containing both ordered and disordered regions. In these materials, aggregates, groups of π-stacked conjugated segments typically ranging from a few to tens of nanometers in size, are surrounded by amorphous regions.[32] As will be discussed in more detail below, the ordered regions play an integral role in charge transport in the highest performing materials; charges move from aggregate to aggregate, often by traveling along tie-molecules, single polymer chains that bridge neighboring aggregates.[31] Although much is still unknown about the nature of how aggregates form and the mechanism by which charge carriers travel between them, our understanding of charge transport in polymer semiconductors has improved dramatically in recent years, resulting in many new design guidelines for high-mobility materials.
A significant portion of this improved understanding has been the result of enhanced modeling and computational techniques. As a single polymer chain can often contain thousands of atoms, and many chains are involved in charge transport, conventional physical and electronic structure modeling techniques such as molecular dynamics (MD) and density functional theory (DFT) struggle to provide an accurate picture of polymer microstructure combined with charge transport on length scales relevant to devices. These techniques do however excel in calculations involving single or several polymer chains, length scales which are difficult to probe with most conventional experimental techniques,[33–35] and can provide important insight into what features are important at the molecular level for efficient charge transport. Hence, there is still a disconnect between computational studies that largely address the microscopic length scales, and transport at the macroscopic/device level.
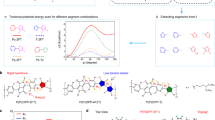
Fornari et al. studied charge transport in a single disordered polymer chain using a model Hamiltonian of the system, demonstrating that static disorder plays a major role in determining intrachain transport properties.[36] For average levels of disorder found in many semiconducting polymers, the typical hopping distance along a chain is approximately ten monomers and is relatively unaffected by further increases in disorder. However, for minor reductions in disorder the hopping range increases rapidly as electronic states become delocalized and accessible from many more initial states (Fig. 3). These delocalized states facilitate more frequent long-range hopping events and allow intrachain charge-carrier mobility to increase rapidly with only small reductions in disorder. In similar work, Qin and Troisi utilized MD calculations to simulate the structure of the prototypical amorphous polymer poly [2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV) and calculate its resulting electronic properties using DFT.[37] They found that the electronic structure was determined largely by the conformational disorder of individual chains, with little contribution from electrostatic disorder or interchain coupling, suggesting that control of molecular configuration is of paramount importance for improved electronic performance in disordered materials. It was also demonstrated that the highest occupied molecular orbital (HOMO) is preferentially located in the planar regions of the polymer chain, away from folds in the backbone. The same conclusion was reached for the semicrystalline polymer P3HT, where the lowest energy states involved in charge transport were located in regions of the chain away from kinks or folds.[38] Disorder along the chain backbone clearly plays an important role in determining the efficiency of intramolecular charge transport.
Effect of intrachain disorder on hopping distance. (a) Distribution of the hopping distances for different values of static disorder (σββ0) where β0 is the inter-monomer coupling (of the order of 1.0 eV for many semiconducting polymers) and σβ is the variance in inter-monomer coupling. (b) Dependence of the hopping range (R0/d) (defined from fitting the hopping range distribution) on the static disorder of the chain. R0 is the typical hopping distance and d is the inter-monomer spacing. Decreased disorder below what is typical in many semiconducting polymers (σβ/β0 = 0.1) leads to many more long-range hopping events. Reproduced from Ref. 36 with permission from the PCCP Owner Societies.
Addressing transport over longer length scales, Noriega et al. employed Marcus theory to construct a model of a disordered semiconducting polymer which could reproduce experimental observations of the dependence of mobility on the electric field, temperature, and the length scale of charge transport.[35,39,40] Two distinctly different time scales for charge transport are observed (Fig. 4). At short times, charges move rapidly along the polymer backbone over short distances, whereas at longer times charges are able to traverse longer distances with a significantly lower mobility limited by the rate of intermolecular hopping. The transition between these two regimes corresponds to a charge moving a distance of the order of the persistence length, which increases with chain stiffness, along the polymer backbone. Thus, in disordered systems in which intermolecular charge transport is many orders of magnitude slower than transport along the chain, mobility on the device length scale is limited by the rate at which charges can hop from chain to chain.
Time dependence of mobility for a model disordered semiconducting polymer. A Marcus theory-based model reveals two different regimes of mobility as a function of observation time, showing the different time and length scales for transport in an amorphous polymer. The model is in good qualitative agreement with experimental results from time-resolved electric-field-induced second-harmonic generation measurements (inset).[34] Reproduced from Ref. 39 with permission from the National Academy of Sciences.
In state-of-the-art high-mobility polymers, the picture of charge transport is considerably more complex.[31] Charges must traverse a heterogeneous microstructure consisting of ordered aggregates surrounded by disordered polymer chains [Fig. 2(b)]. The disordered fraction exhibits a reduced conjugation length, resulting in a wider band gap than the ordered regions and providing an energetic driving force for charge carriers to remain in the aggregates. Thus, transport occurs primarily through the ordered regions of the material, moving through amorphous fractions only when necessary to travel between aggregates and doing so on single polymer chains known as tie-molecules. This description of charge transport was confirmed experimentally in semicrystalline P3HT by mixing the controlled amounts of regioregular P3HT (RR-P3HT) nanofibrils[41] in a regiorandom P3HT (RRa-P3HT) matrix and measuring the electroluminescence (EL) under various current densities.[31] At current densities comparable with or larger than those found in devices, the EL spectra of the blends closely resemble the spectra of pure RR-P3HT, indicating that the charges are traveling and recombining in the ordered regions of the film. This observation is in agreement with local mobility[42] and scanning-probe field-effect measurements[43] demonstrating that mobility is not rate-limited by the electrical connection between aggregates, but rather by transport in these nanoscale-ordered regions.
Although the polymer chains in an aggregate are physically close enough to promote photophysical processes that depend on the interaction of adjacent molecules, in reality these assemblies of chains contain significant amounts of disorder. This disorder arises from non-ideal packing, chain backbone twists, dislocations, and impurities and can result in significant long-range distortions in the aggregate. The main contribution to this static disorder, termed paracrystalline disorder, can be measured via x-ray diffraction (XRD) peak shape analysis.[44] An experimentally determined paracrystallinity parameter g describes the average fluctuation in lattice spacing in the ordered regions of the film and provides an appropriate measure of disorder in the aggregates. In most materials g ranges from 0% to 15%, where <1% represents a highly crystalline material, 1%–10% represents a paracrystalline material, and 10%–15% represents a glass ormelt.[45] Disorderinpolymers is highlyanisotropic. Well-known materials such as P3HT and PBTTT, which are typically thought of as highly crystalline, owe their reputation for being very ordered to the low paracrystallinity parameters, of the order of 2%, in the alkyl stacking direction. Charges cannot travel in this direction however because of the insulating side-chains. In the direction more relevant to transport, the π-stacking direction, the paracrystalline parameter has a much higher value of 7%–8%, and many of the newer high-mobility polymers have even higher values.[44] While disorder is reduced in low-molecular weight materials,[26] in polymers which have a sufficiently high molecular weight to ensure good electrical connectivity, paracrystalline disorder is consistently large. Indeed, for all high-mobility semiconducting polymers, a significant amount of aggregate disorder appears to be universally observed and inevitable.
Optoelectronic properties of semiconducting polymers are also strongly affected by the molecular orbital overlap between nearby molecules. Thus, aggregate disorder not only affects atomic positions, but also impacts the electronic transport and charge-trapping properties of the material as well. Rivnay et al. utilized DFT and a simple tight-binding model to demonstrate that the primary electronic effect of paracrystalline disorder is to induce a tail of trap states into the band gap of the polymer[46] (Fig. 5). The depth of these traps is highly dependent on the level of disorder in the aggregates, with more disorder resulting in deeper and more localized trap states. Although difficult to measure experimentally, significant disorder along the polymer backbone is present in polymer chains embedded in aggregates.[36] This disorder induces localization and broadening of electronic states, resulting in typical localization lengths below ten monomers in most semiconducting polymers.[37,38]
Calculated DOS in PBTTT for different values of paracrystalline disorder (g). A Hamiltonian was constructed for a crystallite containing 50 π-stacked PBTTT chains. For each value of g, the energy eigenvalues and eigenfunctions were solved for and averaged over an ensemble of 104 crystallites to obtain the DOS in the tail region. Increasing levels of disorder lead to a greater number of deeper and more localized trap states which are detrimental to charge transport. The energy EVBM corresponds to the valence band maximum in a perfectly ordered crystalline region. Reproduced from Ref. 46 with permission from the American Physical Society.
It now becomes clear why charge transport is limited by the aggregated regions of the film. It is in these aggregates that charge carriers must undergo the slow charge transport step of moving from chain to chain, and do so in an energetic landscape that contains significant numbers of traps. Modeling indicates that for levels of disorder typically found in semiconducting polymers, the breadth of this trap distribution is of the order of 100 meV, suggesting that at room temperature these traps will serve as a significant impediment to charge transport.[46] The trap states are also highly localized, significantly reducing the probability of a long-range hop between chains, and potentially forcing charge carriers to make many such hops while traversing a single aggregate.[36,46]
In field-effect transistors (FETs) the difference in energy between occupied traps and mobile states manifests itself experimentally as the activation energy for charge transport, with higher activation energies corresponding to the Fermi level being pinned in deeper traps. In classic semicrystalline materials such as P3HT, PBTTT, and poly[5,5′-bis(3-alkyl-2-thienyl)-2,2′-bithiophene] (PQT), activation energies of the order of 70 meV are consistently measured.[31] On the other hand, in completely amorphous polymers that possess no aggregated regions, measured activation energies of ~230 meV are consistent with the presence of more disorder. New materials, despite seemingly exhibiting structural features more characteristic of amorphous polymers (e.g., diffuse diffraction peaks), possess electronic properties similar to the semicrystalline materials and also display ~70 meV activation energies. In the remainder of this paper, we summarize recently proposed explanations as to why these weakly ordered polymers are able to transport charge so efficiently and extract lessons that can be applied in the design of future high-performance materials.
High mobility is obtained by making the polymer resilient to disorder
Recent years have witnessed a revolution in the development of high-mobility polymers. While previous advances have primarily been the result of a single material breakthroughs (P3HT, PBTTT, etc.),[14,47] there are now a host of new materials with a wide variety of chemical structures yielding excellent electrical performance.[5-7] The performance of these new polymers is especially impressive in light of the structural features they exhibit. By one of the most well-accepted and commonly used morphological characterization techniques, XRD, many of the highest mobility materials appear nearly amorphous, failing to display significant diffraction intensity in either the alkyl- or π-stacking directions.[5,48,49] Despite this lack of significant crystallinity, these polymers are able to transport charge efficiently and have trap distributions similar to the highly crystalline materials, evidenced by their low activation energies and sharp band-edge absorption.[19] UV–vis absorption measurements on these same high-mobility materials however, display strong vibronic features characteristic of significant aggregation.[48,50] Taken together, these measurements indicate that although high-mobility polymers often lack the long-range periodic order necessary to yield significant diffracted x-ray intensity, they do aggregate on an extremely local scale, perhaps only a few chains, and they do so in solution. This local aggregation is sufficient to give rise to the vibronic features observed in absorption measurements as well as to permit charge carriers to jump from molecule to molecule. Based on electrical measurements, we can also conclude that this interchain transport is equally efficient to that in materials which possess long-range order. This observation is not entirely unexpected since the mean-free path of charges in the π-stacking direction is of the order of a few molecules. These observations lead us to conclude that only local aggregation is needed for efficient charge transport and that the trap distribution that charge carriers traverse is dictated only by the chain backbone and local interchain transport, not long-range order.
Many different strategies have been employed in recent years to engineer polymers for high mobility, and explanations proposed for their improved performance are wide ranging. In the following sections, we aim to combine the learning gained from this new host of materials and present our best understanding of the features that are necessary for efficient charge transport. We summarize these guidelines in four points: improve intermolecular coupling, improve structural resilience, improve electronic resilience, and enhance molecular weight.
Improve Intermolecular coupling
As transport is limited by the rate of transfer between chains in locally aggregated regions of the film, strategies which can improve intermolecular coupling are especially effective at improving the charge-carrier mobility of semiconducting polymers. Engineering of the polymer backbone and side chains to enable closer π-stacking is one of the most widely used and successful such technique.[7,51,52] Mei et al. employed siloxane-terminated side chains to reduce the steric hindrance between neighboring molecules and achieved a nearly 5% reduction in π-stacking distance.[16] This increased coupling resulted in an improvement in the hole mobility by a factor of six to 2.00cm2/Vs. Similarly, Kang et al. moved the side-chain branching point further from the backbone in a diketopyrrolopyrrole (DPP)-based polymer, also enabling a reduced intermolecular distance and yielding a reported high mobility of 12cm2/Vs.[7] While reducing the π-stacking distance is an effective technique to improve intermolecular coupling, it should be noted that doing so while maintaining the same level of intermolecular disorder will result in traps moving deeper into the band gap (Fig. 5), negating some of the benefits of the improved coupling.
Owing to the different and non-uniform shapes of the HOMO and lowest unoccupied molecular orbital (LUMO) along a polymer backbone, the wavefunction overlap between adjacent chains is highly dependent on their relative longitudinal shift with respect to one another.[53,54] A shift of as little as 1.0 Å was shown to result in a more than one order of magnitude change in the hole charge-transfer integral in a cyclopentadithiophene-benzothiadiazole (CDT-BTZ) based polymer, with the coupling being maximized when the acceptor units are face to face.[55] Molecular design strategies which preferentially align particular functional units on adjacent chains may be an effective way to overcome this issue, although this adds another design constraint to the already challenging task of semiconducting polymer synthesis. Instead, increased tolerance to unpredictable backbone shifts and conformational disorder can be engineered by increasing the overlap area of molecular orbital wavefunctions on neighboring chains. This can be achieved through larger conjugated cores and orbitals which are spread along the chain backbone rather than localized on a particular functional unit. These large cores are a feature of many of the new donor-acceptor polymers with the best performance[56,57] and DFT simulations demonstrate that in many cases the HOMO and LUMO are highly delocalized in these types of structures.[5,22] It should be noted that while such techniques may be utilized to improve charge-carrier mobility in some materials, structure-property relationships in semiconducting polymers are complex, and structural modifications which work for one material may have undesirable effects on others. As improvements in computational methods continue to increase the accessibility of orbital structure calculations, we suggest that there is the potential to utilize these techniques in a more predictive fashion in the future to determine which structures will display intermolecular coupling most resilient to the unpredictable nature of polymer packing.
There is a trade-off in molecular structure between process-ability and ability to deliver high mobilities. On the one hand, completely amorphous polymers have outstanding processability, but exhibit intrinsically limited mobility.[58] On the other hand, while long-range order is not necessary for efficient charge transport, sufficient aggregation is needed to enable enough high-mobility pathways for intermolecular hopping. Polymers aggregating so strongly that the gel in solution however cannot be processed in a practical manner. Liu et al. demonstrated this trade-off recently in a series of benzothiadiazole-based materials in which the branching point of the alkyl side chain was moved progressively further from the polymer backbone.[59] Branching at the first carbon led to significant steric hindrance between the side chain and the backbone, preventing polymer aggregation, while branching at the third carbon resulted in too little steric hindrance and significant aggregation in solution even at elevated temperatures. The intermediate, second-carbon branched variant yielded a material that was both easily processable and possessed a favorable microstructure, being soluble at 85 °C but aggregating strongly in the solid state. A similar side-chain engineering technique was utilized in a DPP-based polymer to achieve a material which balances adequate solubility and a strong tendency to aggregate. This combination resulted in a well-connected film and a high charge-carrier mobility of 2.25 cm2/Vs.[60]
Enhance structural resilience
Structural resilience to the inevitably large amounts of disorder present in semiconducting polymers can be further enhanced by increasing chain rigidity. Owing to mobility being limited by intermolecular charge transport in current materials, intramolecular transport rates, and in particular the impact of chain rigidity, are not considered in most design rules for highmobility polymers.[61,62] However, coarse grain modeling of a model semiconducting polymer has shown that hopping between chains need not always be the rate-limiting step.[63] Carbone and Troisi demonstrated that in the absence of an external electric field, both inter- and intramolecular rates are important for charge transport, and that transport was enhanced as chains became more rigid (Fig. 6). In realistic microstructures, kinks in the polymer backbone in the direction of an applied field as well as chain ends may act as traps for charge carriers, increasing the relative importance of intermolecular charge transport. However, this modeling suggests that maximum mobility can be achieved if a polymer can be engineered to retain its favorable aggregation and intermolecular charge-transport properties while being made sufficiently long and more rigid. In this event, carrier transport along the chain backbone becomes increasingly important as charges can travel further on a single chain before being forced to make a slow intermolecular hop.
Impact of persistence length on mobility for an infinitely long chain. The mobility, as measured by the diffusion coefficient, increases as persistence length increases and the relative importance of intermolecular transport decreases with increasing chain stiffness. Eventually the intermolecular transport rate becomes irrelevant in the rigid rod limit. Reproduced from Ref. 63 with permission from the American Chemical Society.
Evidence of the benefits of backbone rigidity has recently been reported in an indacenothiophene-benzothiadiazole (IDTBT) copolymer.[64,65] Temperature-dependent transistor measurements and field-effect-modulated Seebeck measurements were used to show that all molecular sites are thermally accessible in IDTBT.[19] Quite counterintuitively, this weakly ordered polymer[64] exhibits significantly lower energetic disorder than the highly crystalline polymers P3HT and PBTTT. Quantum chemical and MD calculations were used to demonstrate that IDTBT exhibits a planar conformation in the solid state with a remarkably torsion-free backbone (Fig. 7). This finding is further supported by pressure-dependent Raman spectroscopy measurements which show no pressure dependence of the Raman intensity ratio between the ring-stretching modes of the IDT and BT units, suggesting that the IDTBT backbone is already planar in as-deposited films. IDTBT is uniquely able to accommodate significant side-chain disorder through bends in the polymer backbone while retaining a near planar conformation. Thus, the density of states (DOS) is relatively similar for chains in both aggregated and disordered states, with even the highest energy states within a couple kT’s of the band edge. In contrast, two other high-performing polymers, P(NDI2OD-T2) and PBTTT, adopt more twisted backbone conformations in the amorphous phase, resulting in significant disorder-induced broadening in the DOS compared with their crystalline phase.
Resilience of torsion-free polymer backbone conformation to side-chain disorder. (a) Simulations of the backbone conformation of IDTBT and PBTTT in side-chain-disordered and non-interdigitated structures. (b) Simulation of the backbone conformation of IDTBT in the amorphous phase. A single chain from the simulated unit cell has been highlighted in yellow. (c) Calculated gas-phase torsion potentials of IDTBT and PBTTT. For PBTTT, the potential for torsion between the thiophene and thienothiophene units is shown. A significantly larger barrier to torsion is present in IDTBT than in PBTTT. Reproduced from Ref. 19 with permission from Macmillan Publishers Limited.
Significant amounts of side-chain disorder will inevitably result from the fast-drying speeds necessary for the high-throughput solution-based deposition techniques desirable for organic electronics. Thus, designing materials which, such as IDTBT, exhibit structural resilience in the presence of large amounts of disorder is crucial. Venkateshvaran et al. suggest several techniques for designing new molecules which display similar backbone resilience: (1) reduce the number of torsion-susceptible linkages (possibly through the use of larger conjugated units); (2) design materials with deep potential energy minima favoring the planar structure; (3) include side chains on both sides of the molecule to enable space filling without significant backbone torsion. Backbone planarity does not appear to be the major factor-limiting mobility in semiconducting polymers, providing sufficient π–π interactions can be achieved, as materials which exhibit a larger degree of backbone torsion exhibit similar or better mobilities to IDTBT.[6,19,66] However, planar molecules are likely to have an easier time aggregating and, as intermolecular transfer rates improve, engineering materials with planar backbones that are resilient to disorder may prove to be an important design attribute that will enable not only more ideal device characteristics, but greater charge-carrier mobilities as well.
Enhance electronic resilience
In addition to designing molecules with favorable microstructural characteristics, engineering materials with electronic resilience to structural disorder is emerging as a complementary technique to achieve high mobilities. The disorder common to all semiconducting polymers can have dramatic effects on the optoelectronic properties of the material. This is clearly demonstrated in P3HT, for which the aggregating regioregular form exhibits a ~250 meV smaller band gap and five order of magnitude higher mobility than its amorphous regiorandom counterpart.[31,67] As a certain amount of microstructural disorder is inevitable, it is preferable to design materials which exhibit minimal amounts of disorder-dependent variation in their electronic properties thereby decoupling the electronic structure from structural disorder.
PBTTT is a model example of a material that exhibits such favorable electronic characteristics.[14] While it was initially believed that the high mobility of PBTTT (~1cm2 /Vs) resulted primarily from its increased structural ordering, Rivnay et al. demonstrated that in the π-stacking direction, the rate limiting direction for charge transport, PBTTT is just as disordered as many other less crystalline semiconducting polymers.[44] Liu and Troisi performed a comprehensive computational study of PBTTT and presented several alternate explanations for its high performance: (1) its molecular orbitals become rapidly de-localized within a few tens of eV from the valence band edge; that is, the density of trap states is low and, in the language of a classic mobility edge transport model, the mobility edge is very close to the valence band edge; (2) it exhibits a delocalization of states across multiple chains, facilitating faster intermolecular charge transport; (3) the traps, determined by local distortions of the polymer backbone, are short lived with lifetimes comparable with the time scale of intramolecular transport (Fig. 8). The last of these points is critical. DFT modeling shows trap lifetimes in PBTTT to be of the order of ~ 0.1 ns, comparable with the time scale of intramolecular charge transport, indicating that conformational changes in the polymer due to its thermal fluctuations are sufficient to introduce a detrapping of charge carriers. The traps are thus in a sense “self-healing” and as a consequence, these results suggest that the activation energy for detrapping in this material is instead an activation energy for conformational changes. In MEH-PPV, on the other hand, conformational changes in the polymer backbone sufficient to change the position of the HOMO occur on the time scale of ~100 ns, which is much too slow to aid in charge transport.[37] In that system, conformational disorder can be considered static from the perspective of charge carriers, resulting in long-lived traps detrimental to mobility.
Molecular orbital temporal evolution in PBTTT. (a) The orbital density positions and shapes of HOMO and HOMO-1 at different MD simulation times between 80.0 and 80.5 ns. (b) The energy level of orbital HOMO-n (π =0–4) as afunction of MD simulation time in a lamella between 80.0 and 80.5 ns. PBTTT trap states are extremely shortlived (of the order of transport time) and thus are likely not an impediment to charge transport. This property may explain the high mobility measured in PBTTT. (c) The orbital density positions and shapes, with energies of selected orbitals of PBTTT at t = 60 ns of the MD simulations. DFT calculations show that trap states delocalize rapidly as one moves away from the valence band edge into the band. Reproduced from Ref. 22 with permission from Wiley.
These beneficial features of PBTTT arise in part due to the relatively narrow bandwidth that comes as a result of being a copolymer with alternating sites of thieno(3,2-b)thiophene and 4,4-dialkyl 2,2-bithiophene, possessing different energies.[22] From this point of view, PBTTT appears to be part of a recent trend of high-mobility copolymers. The number of new semiconducting copolymers has exploded in recent years, primarily driven by chemists seeking to design lower-band gap materials to enhance light collection in organic photovoltaics. This approach has been widely successful in creating materials that yield high efficiency, pushing single junction device efficiencies over 10%,[59] but has also yielded a large host of polymers which exhibit high charge-carrier mobilities in excess of 1 cm2/Vs. Most of these new materials are based on push-pull, or donor-acceptor monomers. Such efficient charge transport at first appears counterintuitive as the use of a donor-acceptor structure also narrows the bandwidth of the polymer, a feature which is detrimental to mobility in the most commonly used models of charge transport.[68] However, narrower bands also result in a reduction of the activation energy required to promote charge carriers from localized states in the band tails to delocalized states deeper in the band. In other words, the tail states are energetically close to the transport states (Fig. 9). While this effect is countered by a reduction in the intrachain coupling due to the energy difference between adjacent monomers, the decreased activation energy results in a sizeable net improvement in charge-carrier mobility. Additionally, disorder has a smaller detrimental effect on intrachain transport in materials with larger differences in the on-site energy between donor and acceptor units. Together, these results suggest a compelling reason for the recent flood of new donor-acceptor materials which exhibit high mobility despite significant amounts of disorder.
Effect of difference in on-site energy on band structure for a donor-acceptor copolymer. Diagram representing the energy levels (horizontal red segments) and their delocalization (length of the segment) for differences of on-site energy (Δ/β = 0,1,2) (left, center, right panel), where Δ and β are the energy difference and coupling between adjacent sites in the copolymer, respectively. The blue segments connect sites with particle currents (charge-transport events) exceeding a common threshold. The larger total particle current and increase in long-range hopping for Δ/β=2is due to the presence of more delocalized states near the bottom of the band. Reproduced from Ref. 68 with permission from Wiley.
Increase molecular weight
Molecular weight is an extremely important, but often overlooked, material parameter that significantly affects the charge-transport properties of semiconducting polymers. A strong molecular-weight dependence of charge-carrier mobility has been reported in several systems,[9,8,29,69] yet for many of the highest performing materials it is a topic that often receives little or no quantitative attention. The reasons for this molecular weight dependence are well known; charge transport along the chain backbone is far faster than charge transport between chains. Thus, the further charges can travel on a single chain, the better. If the molecular weight is too low, then charges will be forced to make frequent hops between chains in an extremely disordered energetic landscape. Very low molecular-weight polymers also form well-defined extended chain crystals that are poorly connected, resulting in slow charge transport between crystalline regions. High molecular-weight polymers, on the other hand, form defected crystals but with an abundance of chains which bridge multiple crystallites, resulting in high mobilities. In order to achieve efficient transport then, the molecular weight must be high enough that single chains can bridge aggregated regions of the film.[29] In P3HT, mobility increases continuously with molecular weight until ~20 kDa before leveling off; at this point the measured level of disorder in the film saturates as well and it is thought that the polymer microstructure has become fully entangled and the aggregates are well connected (Fig. 10).[26] Chain folding against the direction of the electric field prohibits charge carriers from traveling further and further along single chains with increasing molecular weight, resulting in the saturation in mobility. However, similar detailed studies as a function of molecular weight have not been performed for newer high-mobility donor-acceptor polymers. These materials possess backbones which are considerably more rigid than P3HT and therefore will not fold back on themselves as easily. It stands to reason that the “saturation point” for mobility in these materials will lie at much higher molecular weights than P3HT and the enhancements in performance can be achieved by increasing the often modest molecular weights reported for state-of-the-art materials. This was demonstrated in a DPP-based polymer in which the mobility was shown to increase continuously with molecular weight, even past 500 kDa.[9]
Disorder and mobility for P3HT as a function of molecular weight. (a) The paracrystalline disorder parameter g is found to level off above 20 kDa. (b) Charge-carrier mobility plateaus at roughly the same point after the polymer microstructure is well connected and fully entangled. Reproduced from Ref. 26 with permission from Elsevier.
The case for higher molecular weights is supported by the theoretical work of Carbone and Troisi.[63] Their coarse grain simulations demonstrate that the diffusion coefficient (correlated to mobility) rises with increasing chain rigidity as carriers are able to travel further on each individual chain, provided the chains are sufficiently long. The intermolecular transport rate is most important for short and flexible chains but becomes less so as the polymer persistence length increases, eventually becoming irrelevant in the infinitely long and totally rigid chain limit (Fig. 6). An early analytical model developed by Pearson et al. came to similar conclusions regarding the effects of molecular weight and chain extension.[70] Their results suggest that conductivity (proportional to mobility) should rise linearly with molecular weight before saturating at high molecular weights when the intermolecular transport rate becomes less relevant. Additionally, the model indicates that conductivity can be greatly enhanced through chain alignment or other polymer extension techniques. While experimental results show that most materials are currently limited by transport between chains, as increasingly long and rigid polymers are developed, this modeling indicates that designing materials with efficient intermolecular transport may become less important. Provided challenges associated with the solubility and processability of very rigid and high molecular weight polymers can be overcome without significantly impacting the microstructure of the material in the solid state, there is potential room for improvement in device performance simply by synthesizing longer chains.
Conclusions
A certain amount of structural disorder is inevitable in semiconducting polymers. Even the most crystalline of these materials have been shown to exhibit microstructures in the π-stacking direction closer to an amorphous melt than to a perfect crystal. New, highly disordered materials are pushing mobility records higher and higher, proving that disorder need not be extremely detrimental to device performance. Instead, this disorder must be embraced and planned for. Rather than focusing on strategies to reduce disorder, we suggest that efforts be aimed at designing molecules that can be resilient to it. We have presented several emerging strategies to achieve this: (1) improve inter-molecular coupling; (2) enhance structural resilience to disorder; (3) enhance electronic resilience to disorder; and (4) increase molecular weight. While examples exist where each of these strategies has been utilized to some extent in existing materials, to the best of our knowledge, no polymer has yet been consciously designed and synthesized which employs all of these strategies simultaneously.
Although the synthesis of new molecules has accounted for the bulk of the improvement in charge-carrier mobility in the past and we believe is likely to continue in the future, there are other avenues which will help push performance further still. Processing clearly plays an important role, with the effects of processing solvents[71,72] and post-deposition treatments being well documented.[25,73] Owing to the anisotropic nature of charge transport in polymer semiconductors, chain alignment appears to be one of the most promising processing techniques to enhance mobilities, although achieving this goal using production-worthy techniques remains challenging.[10,15,74] Dielectrics can also significantly impact polymer performance in transistor architectures,[75,76] yet they receive less attention. There are likely still performance gains to be had with improved dielectrics and considerably more work is needed to reliably enable the high-mobility, low-voltage operation of devices.[77,78] Although all semiconducting polymers measured to date possess significant amounts of disorder in the π-stacking direction, efforts to reduce this disorder should not be totally abandoned. Should this disorder be reduced through either improved processing techniques or changes to molecular structure, significant performance gains are possible. Here we have presented a series of design guidelines based on the latest understanding of charge transport to appear in the field. The continual improvement in polymer mobility, however, suggests that new design guidelines may emerge at any time. Indeed, the future looks bright for semiconducting polymers.
References
C.-C. Chen, W.-H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong, and Y. Yang: An efficient triple-junction polymer solar cell having a power conversion efficiency exceeding 11%. Adv. Mater. 26, 5670–5677 (2014).
O. Knopfmacher, M.L. Hammock, A.L. Appleton, G. Schwartz, J. Mei, T. Lei, J. Pei, and Z. Bao: Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 5, (2014).
M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz, S. Bauer-Gogonea, S. Bauer, and T. Someya: An ultra-lightweight design for imperceptible plastic electronics. Nature 499, 458–463 (2013).
M.S. White, M. Kaltenbrunner, E.D. Głowacki, K. Gutnichenko, G. Kettlgruber, I. Graz, S. Aazou, C. Ulbricht, D.A.M. Egbe, M.C. Miron, Z. Major, M.C. Scharber, T. Sekitani, T. Someya, S. Bauer, and N.S. Sariciftci: Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 7, 811–816 (2013).
H.-J. Yun, S.-J. Kang, Y. Xu, S.O. Kim, Y.-H. Kim, Y.-Y. Noh, and S.-K. Kwon: Dramatic inversion of charge polarity in diketopyrrolopyrrole-based organic field-effect transistors via a simple nitrile group substitution. Adv. Mater. 26, 7300–7307 (2014).
G. Kim, S.-J. Kang, G.K. Dutta, Y.-K. Han, T.J. Shin, Y.-Y. Noh, and C. Yang: A thienoisoindigo-naphthalene polymer with ultrahigh mobility of 14.4 cm2/Vs that substantially exceeds benchmark values for amorphous silicon semiconductors. J. Am. Chem. Soc. 136, 9477–9483 (2014).
I. Kang, H.-J. Yun, D.S. Chung, S.-K. Kwon, and Y.-H. Kim: Record high hole mobility in polymer semiconductors via side-chain engineering. J. Am. Chem. Soc. 135, 14896–14899 (2013).
J. Lee, A.-R. Han, H. Yu, T.J. Shin, C. Yang, and J.H. Oh: Boosting the ambipolar performance of solution-processable polymer semiconductors via hybrid side-chain engineering. J. Am. Chem. Soc. 135, 9540–9547 (2013).
J. Li, Y. Zhao, H.S. Tan, Y. Guo, C.-A. Di, G. Yu, Y. Liu, M. Lin, S.H. Lim, Y. Zhou, H. Su, and B.S. Ong: A stable solution-processed polymer semiconductor with record high-mobility for printed transistors. Sci. Rep. 2 (2012).
C. Luo, A.K.K. Kyaw, L.A. Perez, S. Patel, M. Wang, B. Grimm, G.C. Bazan, E.J. Kramer, and A.J. Heeger: General strategy for self-assembly of highly oriented nanocrystalline semiconducting polymers with high mobility. Nano Lett. 14, 2764–2771 (2014).
A. Tsumura, H. Koezuka, and T. Ando: Macromolecular electronic device: field-effect transistor with a polythiophene thin film. Appl. Phys. Lett. 49, 1210–1212 (1986).
H. Dong, X. Fu, J. Liu, Z. Wang, and W. Hu: 25th anniversary article: key points for high-mobility organic field-effect transistors. Adv. Mater. 25, 6158–6183 (2013).
Z. Bao, A. Dodabalapur, and A.J. Lovinger: Soluble and processable regioregular poly(3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 69, 4108–4110 (1996).
I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. MacDonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M.L. Chabinyc, R.J. Kline, M.D. McGehee, and M.F. Toney: Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 5, 328–333 (2006).
H.-R. Tseng, H. Phan, C. Luo, M. Wang, L.A. Perez, S.N. Patel, L. Ying, E.J. Kramer, T.-Q. Nguyen, G.C. Bazan, and A.J. Heeger: High-mobility field-effect transistors fabricated with macroscopic aligned semiconducting polymers. Adv. Mater. 26, 2993–2998 (2014).
J. Mei, D.H. Kim, A.L. Ayzner, M.F. Toney, and Z. Bao: Siloxane-terminated solubilizing side chains: bringing conjugated polymer backbones closer and boosting hole mobilities in thin-film transistors. J. Am. Chem. Soc. 133, 20130–20133 (2011).
H. Yan, Z. Chen, Y. Zheng, C. Newman, J.R. Quinn, F. Dötz, M. Kastler, and A. Facchetti: A high-mobility electron-transporting polymer for printed transistors. Nature 457, 679–686 (2009).
H. Li, F.S. Kim, G. Ren, and S.A. Jenekhe: High-mobility n-type conjugated polymers based on electron-deficient tetraazabenzodifluoranthene diimide for organic electronics. J. Am. Chem. Soc. 135, 14920–14923 (2013).
D. Venkateshvaran, M. Nikolka, A. Sadhanala, V. Lemaur, M. Zelazny, M. Kepa, M. Hurhangee, A.J. Kronemeijer, V. Pecunia, I. Nasrallah, I. Romanov, K. Broch, I. McCulloch, D. Emin, Y. Olivier, J. Cornil, D. Beljonne, and H. Sirringhaus: Approaching disorder-free transport in high-mobility conjugated polymers. Nature 515, 384–388 (2014).
H. Sirringhaus: 25th anniversary article: organic field-effect transistors: the path beyond amorphous silicon. Adv. Mater. 26, 1319–1335 (2014).
C.B. Nielsen, M. Turbiez, and I. McCulloch: Recent advances in the development of semiconducting DPP-containing polymers for transistor applications. Adv. Mater. 25, 1859–1880 (2013).
T. Liu and A. Troisi: Understanding the microscopic origin of the very high charge mobility in PBTTT: tolerance of thermal disorder. Adv. Funct. Mater. 24, 925–933 (2014).
A. Salleo, M.L. Chabinyc, M.S. Yang, and R.A. Street: Polymer thin-film transistors with chemically modified dielectric interfaces. Appl. Phys. Lett. 81, 4383–4385 (2002).
L.H. Jimison, S. Himmelberger, D.T. Duong, J. Rivnay, M.F. Toney, and A. Salleo: Vertical confinement and interface effects on the microstructure and charge transport of P3HT thin films. J. Polym. Sci. B: Polym. Phys. 51, 611–620 (2013).
R.J. Kline, M.D. McGehee, and M.F. Toney: Highly oriented crystals at the buried interface in polythiophene thin-film transistors. Nat. Mater. 5, 222–228 (2006).
F.P.V. Koch, J. Rivnay, S. Foster, C. Müller, J.M. Downing, E. Buchaca-Domingo, P. Westacott, L. Yu, M. Yuan, M. Baklar, Z. Fei, C. Luscombe, M.A. McLachlan, M. Heeney, G. Rumbles, C. Silva, A. Salleo, J. Nelson, P. Smith, and N. Stingelin: The impact of molecular weight on microstructure and charge transport in semicrystalline polymer semiconductors-poly(3-hexylthiophene), a model study. Prog. Polym. Sci. 38, 1978–1989 (2013).
S. Himmelberger, K. Vandewal, Z. Fei, M. Heeney, and A. Salleo: Role of molecular weight distribution on charge transport in semiconducting polymers. Macromolecules 47, 7151–7157 (2014).
A. Zen, J. Pflaum, S. Hirschmann, W. Zhuang, F. Jaiser, U. Asawapirom, J.P. Rabe, U. Scherf, and D. Neher: Effect of molecular weight and annealing of poly(3-hexylthiophene)s on the performance of organic field-effect transistors. Adv. Funct. Mater. 14, 757–764 (2004).
R.J. Kline, M.D. McGehee, E.N. Kadnikova, J. Liu, and J.M.J. Fréchet: Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight. Adv. Mater. 15, 1519–1522 (2003).
W. Li, L. Yang, J.R. Tumbleston, L. Yan, H. Ade, and W. You: Controlling molecular weight of a high efficiency donor-acceptor conjugated polymer and understanding its significant impact on photovoltaic properties. Adv. Mater. 26, 4456–4462 (2014).
R. Noriega, J. Rivnay, K. Vandewal, F.P.V. Koch, N. Stingelin, P. Smith, M.F. Toney, and A. Salleo: A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 12, 1038–1044 (2013).
D.T. Duong, M.F. Toney, and A. Salleo: Role of confinement and aggregation in charge transport in semicrystalline polythiophene thin films. Phys. Rev.. 86, 205205 (2012).
A. Devižis, A. Serbenta, K. Meerholz, D. Hertel, and V. Gulbinas: Ultrafast dynamics of carrier mobility in a conjugated polymer probed at molecular and microscopic length scales. Phys. Rev. Lett. 103, 027404 (2009).
A. Devizis, K. Meerholz, D. Hertel, and V. Gulbinas: Ultrafast charge carrier mobility dynamics in poly(spirobifluorene-co-benzothiadiazole): influence of temperature on initial transport. Phys. Rev. B 82, 155204 (2010).
A. Devižis, K. Meerholz, D. Hertel, and V. Gulbinas: Hierarchical charge carrier motion in conjugated polymers. Chem. Phys. Lett. 498, 302–306 (2010).
R.P. Fornari and A. Troisi: Theory of charge hopping along a disordered polymer chain. Phys. Chem. Chem. Phys. 16, 9997–10007 (2014).
T. Qin and A. Troisi: Relation between structure and electronic properties of amorphous MEH-PPV polymers. J. Am. Chem. Soc. 135, 11247–11256 (2013).
D.P. McMahon, D.L. Cheung, L. Goris, J. Dacuña, A. Salleo, A. Troisi:Relation between microstructure and charge transport in polymers of different regioregularity. J. Phys. Chem. C 115, 19386–19393 (2011).
R. Noriega, A. Salleo, and A.J. Spakowitz: Chain conformations dictate multiscale charge transport phenomena in disordered semiconducting polymers. Proc. Natl. Acad. Sci. USA 110, 16315–16320 (2013).
F. Laquai, G. Wegner, and H. Bässler: What determines the mobility of charge carriers in conjugated polymers?Phil. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 365, 1473–1487 (2007).
C. Scharsich, R.H. Lohwasser, M. Sommer, U. Asawapirom, U. Scherf, M. Thelakkat, D. Neher, and A. Köhler: Control of aggregate formation in poly(3-hexylthiophene) by solvent, molecular weight, and synthetic method. J. Polym. Sci. B: Polym. Phys. 50, 442–453 (2012).
P. Pingel, A. Zen, R.D. Abellón, F.C. Grozema, L.D.A. Siebbeles, and D. Neher: Temperature-resolved local and macroscopic charge carrier transport in thin P3HT layers. Adv. Funct. Mater. 20, 2286–2295 (2010).
J.-C. Bolsée, W.D. Oosterbaan, L. Lutsen, D. Vanderzande, and J. Manca: The importance of bridging points for charge transport in webs of conjugated polymer nanofibers. Adv. Funct. Mater. 23, 862–869 (2013).
J. Rivnay, R. Noriega, R.J. Kline, A. Salleo, and M.F. Toney: Quantitative analysis of lattice disorder and crystallite size in organic semiconductor thin films. Phys. Rev. B 84, 045203 (2011).
A.M. Hindeleh and R. Hosemann: Microparacrystals: the intermediate stage between crystalline and amorphous. J. Mater. Sci. 26, 5127–5133 (1991).
J. Rivnay, R. Noriega, J.E. Northrup, R.J. Kline, M.F. Toney, and A. Salleo: Structural origin of gap states in semicrystalline polymers and the implications for charge transport. Phys. Rev. B 83, 121306 (2011).
A. Assadi, C. Svensson, M. Willander, and O. Inganäs: Field-effect mobility of poly(3-hexylthiophene). Appl. Phys. Lett. 53, 195–197 (1988).
J.D. Yuen, J. Fan, J. Seifter, B. Lim, R. Hufschmid, A.J. Heeger, and F. Wudl: High performance weak donor-acceptor polymers in thin film transistors: effect of the acceptor on electronic properties, ambipolar conductivity, mobility, and thermal stability. J. Am. Chem. Soc. 133, 20799–20807 (2011).
I. Kang, T.K. An, J. Hong, H.-J. Yun, R. Kim, D.S. Chung, C.E. Park, Y.-H. Kim, and S.-K. Kwon: Effect of selenophene in a DPP copolymer incorporating a vinyl group for high-performance organic field-effect transistors. Adv. Mater. 25, 524–528 (2013).
J.E. Donaghey, E.-H. Sohn, R.S. Ashraf, T.D. Anthopoulos, S.E. Watkins, K. Song, C.K. Williams, and I. McCulloch: Pyrroloindacenodithiophene polymers: the effect of molecular structure on OFET performance. Polym. Chem. 4, 3537–3544 (2013).
A.T. Yiu, P.M. Beaujuge, O.P. Lee, C.H. Woo, M.F. Toney, and J.M.J. Fréchet: Side-chain tunability of furan-containing low-band-gap polymers provides control of structural order in efficient solar cells. J. Am. Chem. Soc. 134, 2180–2185 (2012).
J. Mei and Z. Bao: Side chain engineering in solution-processable conjugated polymers. Chem. Mater. 26, 604–615 (2014).
V. Coropceanu, J. Cornil, D.A. da Silva Filho, Y. Olivier, R. Silbey, and J.-L. Brédas: Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
J.L. Brédas, J.P. Calbert, D.A. da Silva Filho, and J. Cornil: Organic semiconductors: a theoretical characterization of the basic parameters governing charge transport. Proc. Natl. Acad. Sci. USA 99, 5804–5809 (2002).
Y. Olivier, D. Niedzialek, V. Lemaur, W. Pisula, K. Müllen, U. Koldemir, J.R. Reynolds, R. Lazzaroni, J. Cornil, and D. Beljonne: 25th anniversary article: high-mobility hole and electron transport conjugated polymers: how structure defines function. Adv. Mater. 26, 2119–2136 (2014).
T. Lei, J.-Y. Wang, and J. Pei: Design, synthesis, and structure-property relationships of isoindigo-based conjugated polymers. Acc. Chem. Res. 47, 1117–1126 (2014).
Y. Deng, Y. Chen, X. Zhang, H. Tian, C. Bao, D. Yan, Y. Geng, and F. Wang: Donor-acceptor conjugated polymers with dithienocarbazoles as donor units: effect of structure on semiconducting properties. Macromolecules 45, 8621–8627 (2012).
A. Troisi: The speed limit for sequential charge hopping in molecular materials. Org. Electron. 12, 1988–1991 (2011).
Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade, and H. Yan: Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 5 (2014)..
M.S. Chen, O.P. Lee, J.R. Niskala, A.T. Yiu, C.J. Tassone, K. Schmidt, P.M. Beaujuge, S.S. Onishi, M.F. Toney, A. Zettl, and J.M.J. Fréchet: Enhanced solid-state order and field-effect hole mobility through control of nanoscale polymer aggregation. J. Am. Chem. Soc. 135, 19229–19236 (2013).
A. Facchetti: π-conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 23, 733–758 (2010).
I. McCulloch, R.S. Ashraf, L. Biniek, H. Bronstein, C. Combe, J.E. Donaghey, D.I. James, C.B. Nielsen, B.C. Schroeder, and W. Zhang: Design of semiconducting indacenodithiophene polymers for high performance transistors and solar cells. Acc. Chem. Res. 45, 714–722 (2012).
P. Carbone and A. Troisi: Charge diffusion in semiconducting polymers: analytical relation between polymer rigidity and time scales for intrachain and interchain hopping. J. Phys. Chem. Lett. 5, 2637–2641 (2014).
W. Zhang, J. Smith, S.E. Watkins, R. Gysel, M. McGehee, A. Salleo, J. Kirkpatrick, S. Ashraf, T. Anthopoulos, M. Heeney, and I. McCulloch: Indacenodithiophene semiconducting polymers for high-performance, air-stable transistors. J. Am. Chem. Soc. 132, 11437–11439 (2010).
X. Zhang, H. Bronstein, A.J. Kronemeijer, J. Smith, Y. Kim, R.J. Kline, L.J. Richter, T.D. Anthopoulos, H. Sirringhaus, K. Song, M. Heeney, W. Zhang, I. McCulloch, and D.M. DeLongchamp: Molecular origin of high field-effect mobility in an indacenodithiophene-benzothiadiazole copolymer. Nat. Commun. 4 (2013).
T. Schuettfort, S. Huettner, S. Lilliu, J.E. Macdonald, L. Thomsen, and C.R. McNeill: Surface and bulk structural characterization of a high-mobility electron-transporting polymer. Macromolecules 44, 1530–1539 (2011).
C. Wang, J. Rivnay, S. Himmelberger, K. Vakhshouri, M.F. Toney, E.D. Gomez, and A. Salleo: Ultrathin body poly(3-hexylthiophene) transistors with improved short-channel performance. ACS Appl. Mater. Interfaces 5, 2342–2346 (2013).
R.P. Fornari and A. Troisi: Narrower bands with better charge transport: the counterintuitive behavior of semiconducting copolymers. Adv. Mater. 26, 7627–7631 (2014).
H.N. Tsao, D.M. Cho, I. Park, M.R. Hansen, A. Mavrinskiy, D.Y. Yoon, R. Graf, W. Pisula, H.W. Spiess, and K. Müllen: Ultrahigh mobility in polymer field-effect transistors by design. J. Am. Chem. Soc. 133, 2605–2612 (2011).
D.S. Pearson, P.A. Pincus, G.W. Heffner, and S.J. Dahman: Effect of molecular weight and orientation on the conductivity of conjugated polymers. Macromolecules 26, 1570–1575 (1993).
J.-F. Chang, B. Sun, D.W. Breiby, M.M. Nielsen, T.I. Sölling, M. Giles, I. McCulloch, and H. Sirringhaus: Enhanced mobility of poly(3-hexylthiophene) transistors by spin-coating from high-boiling-point solvents. Chem. Mater. 16, 4772–4776 (2004).
P.K.-H. Ho, L.-L. Chua, M. Dipankar, X.Y. Gao, D.C. Qi, A.T.-S. Wee, J.-F. Chang, and R.H. Friend: Solvent effects on chain orientation and interchain π-interaction in conjugated polymer thin films: direct measurements of the air and substrate interfaces by near-edge x-ray absorption spectroscopy. Adv. Mater. 19, 215–221 (2007).
J. Rivnay, R. Steyrleuthner, L.H. Jimison, A. Casadei, Z. Chen, M.F. Toney, A. Facchetti, D. Neher, and A. Salleo: Drastic control of texture in a high performance n-type polymeric semiconductor and implications for charge transport. Macromolecules 44, 5246–5255 (2011).
L.H. Jimison, M.F. Toney, I. McCulloch, M. Heeney, and A. Salleo: Charge-transport anisotropy due to grain boundaries in directionally crystallized thin films of regioregular poly(3-hexylthiophene). Adv. Mater. 21, 1568–1572 (2009).
J. Li, J. Du, J. Xu, H.L.W. Chan, and F. Yan: The influence of gate dielectrics on a high-mobility n-type conjugated polymer in organic thin-film transistors. Appl. Phys. Lett. 100, 033301 (2012).
J. Veres, S.D. Ogier, S.W. Leeming, D.C. Cupertino, and S. Mohialdin Khaffaf: Low-k insulators as the choice of dielectrics in organic field-effect transistors. Adv. Funct. Mater. 13, 199–204 (2003).
F. Pettersson, R. Österbacka, J. Koskela, A. Kilpelä, T. Remonen, Y. Zhang, S. Inkinen, C.-E. Wilén, R. Bollström, M. Toivakka, A. Määttänen, P. Ihalainen, and J. Peltonen: Ion-modulated transistors on paper using phase-separated semiconductor/insulator blends. MRS Commun. 4, 51–55 (2014).
J.H. Cho, J. Lee, Y. Xia, B. Kim, Y. He, M.J. Renn, T.P. Lodge, and C. Daniel Frisbie: Printable ion-gel gate dielectrics for low-voltage polymer thin-film transistors on plastic. Nat. Mater. 7, 900–906 (2008).
Acknowledgments
S. H. and A. S. acknowledge financial support from the National Science Foundation (Award no. DMR 1205752).
Author information
Authors and Affiliations
Corresponding author
Additional information
This author was an editor of this journal during the review and decision stage. For the MRC policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/mrc-editor-information/
Rights and permissions
About this article
Cite this article
Himmelberger, S., Salleo, A. Engineering semiconducting polymers for efficient charge transport. MRS Communications 5, 383–395 (2015). https://doi.org/10.1557/mrc.2015.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2015.44