Abstract
Reduced graphene oxide supported titanium dioxide (GO/TiO2) heterojunction composites as highly active photocatalysts were synthesized via simple ultrasonic mixing and hydrothermal reaction using TiCl3 and GO as precursors. Their structure and morphology were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, Raman spectra, UV-vis spectroscopy, and thermogravimetic analysis. The GO/TiO2 heterojunction composites were used to degrade methyl orange (MO). The adsorption and photocatalytic degradation rate of the prepared GO/TiO2 composites increased by nearly three times compared with that of pristine TiO2 or GO, which reached up 90%, to degrade MO after 4 h, which provides a simple method to obtain photocatalytic materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photocatalytic technology is one of the most effective methods for wastewater treatment because of its low investment cost, mild reaction conditions, and negligible secondary pollution to the environment [1, 2, 3, 4, 5, 6]. Preparation of photocatalysts with high photocatalytic activity and photochemical stability is the key factor to boost practical applications of semiconductor photocatalysts [7, 8, 9, 10, 11, 12]. Among well-known photocatalysts, titanium dioxide (TiO2) exhibits excellent photocatalytic properties, long-term stability, nontoxicity, chemical inertness, and low cost [13]. Therefore, TiO2 has been widely used in photocatalytic studies [14, 15, 16, 17, 18, 19, 20]. However, some serious shortcomings still need to be overcome. For example, TiO2 has a large band gap (the rutile and anatase phases are 3.03 and 3.20 eV, respectively), which can absorb only ultraviolet light (approximately 5% of solar light) [21]. Meanwhile, its photogenerated electron–hole pairs are easy to recombine. Many researchers have focused on the modification of TiO2 to obtain new types of highly active photocatalysts that can work under visible light [22, 23, 24, 25, 26, 27]. Many attempts have been performed to enhance the visible light photocatalytic efficiency of TiO2, including metal or nonmetal doping [28, 29, 30, 31], dye sensitization [32], surface modification [33, 34], and coupling with other semiconductor materials [35, 36, 37]. Among these methods, the coupling of TiO2 with guest semiconductors is an effective way to improve its photocatalytic activity under visible light [21].
Graphene is a two-dimensional allotrope of crystalline carbon formed by hexagonally arranging sp2-bonded carbon atoms, which presents excellent optical properties, transparency, mechanical flexibility, and good thermal and chemical stability [38]. Graphene oxide (GO), an oxidized derivative of graphene, is a promising carbon material that has attracted significant interest over the last decade. When graphene is heavily oxygenated to generate GO, a number of functional groups on its basal planes, such as hydroxyl and epoxies, in addition to carbonyl and carboxyl groups located at the sheet edges, are formed [39]. The presence of these functional groups makes GO sheets strongly hydrophilic, allowing GO to readily exfoliate in water or solvents to produce stable dispersions [40, 41]. GO sheets possessing oxygenated functional groups anchor active materials easily, and large surface areas focus some potential supported materials on nanomaterials to prevent their aggregation [42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52]. TiO2-RGO nanocomposites were fabricated using either isopropyl (IsoprOH) or ethyl (EtOH) alcohol. The photocatalytic properties of the prepared materials using IsoprOH had smaller crystallite size, narrower apparent band gap, smaller isoelectric point, larger adsorption capacity, and higher photocatalytic activity [53]. In Ag3VO4/C3N4/reduced TiO2 microsphere ternary composites, the introduction of Ag3VO4 into g-C3N4/r-TiO2 can effectively improve the photocatalytic activity of degradation of methylene blue, compared with single component and C3N4/reduced TiO2 binary composites, due to a synergetic effect including the formation of a heterojunction, large surface area, improved light absorption, matched energy band structure, and improved separation efficiency of photogenerated charges coming from the dual Z-scheme structure [54].
In this study, GO/TiO2 composites were synthesized using TiCl3 and graphite as raw materials via hydrothermal reaction. The phase composition, morphology, and thermal stability of the prepared GO/TiO2 were investigated by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and thermogravimetic analysis (TGA). In addition, their photocatalytic activities were evaluated via ultraviolet-visible (UV-vis) spectroscopy based on degraded methyl orange (MO) dye. The GO/TiO2 composites evidently degraded 90% of MO.
Results and discussion
The characterization of GO/TiO2 composites
Fig. 1(a) shows the FTIR spectra of GO and GO/TiO2 composites. In the GO spectrum, a broad band appears at around 3423 cm−1, which is due to the O–H stretching vibration, and a peak at 1730 cm−1, which could be assigned to the carbonyl C=O stretching vibration. The peaks at 1620 cm−1 and 1226 cm−1 are attributed to C=C vibrations and C–O–C stretching vibrations, respectively. The peak at 1049 cm−1 for the C–O stretching vibration appears due to the presence of epoxide groups on the GO layer. However, the strength of the vibration peaks of the main oxygen functional groups of GO in GO/TiO2 composites is obviously weakened, which indicates that GO has a certain degree of reduction during the hydrothermal process.
For the Raman spectra of GO and GO/TiO2 composites [Fig. 1(b)], it can be seen that the GO and GO/TiO2 have D and G peaks at 1348 and 1586 cm−1, respectively. The peak intensities of ID/IG for GO and GO/TiO2 are 0.925 and 0.921, respectively. So, the GO/TiO2 exhibits a higher regularity than GO due to the reduction of GO during the hydrothermal process to decrease the oxygen content on the surface of GO. It further indicates that the number of sp2 hybrid carbon atoms in GO is more than that of sp3 hybrid carbon atoms in the GO/TiO2 composites [55].
The TGA curves of GO and GO/TiO2 composites are shown in Fig. 1(d) to evaluate their thermal stability. For the two samples, the TGA curves have three weight loss stages. For the GO, the first weight loss at about 25–120 °C is caused by the volatilization of absorbed water in materials. The content of combined water is about 12 wt% due to hydrophilic groups. The second weight loss at about 120–210 °C is sharp and caused by the decomposition of the oxygen groups present such as C=O, C–O, and epoxy groups, which reaches up to 14 wt%. The second weight loss at about 220–1000 °C is due to the decomposition of the carbon and carbon skeleton in GO. Their decomposition is serious due to the carbonization of a great amount of sp2 and sp3 hybrid carbon atoms even after 1000 °C. In contrast, the GO/TiO2 composites exhibit obvious thermal stability. The content of absorbed water is about 5 wt%. The oxygen groups present only cause about 5 wt% of decomposition. Decomposition of the carbon and carbon skeleton is faint, and the GO/TiO2 composites remain about 90 wt% after 1000 °C. These indicate that the thermal stability of GO/TiO2 composites is higher than that of GO. The decrease in the oxygen groups present results from the loading TiO2 consuming oxygen groups and reduction of some containing oxygen groups after the hydrothermal treatment.
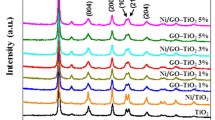
Fig. 1(c) shows the XRD spectra of GO and GO/TiO2 composites. There is a strong peak near 2θ = 32°, which is the diffraction peak of graphite surface (002). The sharp peak near 2θ = 12° indicates that the structure of GO (001) contains a lot of defects and containing oxygen groups. The oxygen groups are beneficial to further load TiO2 nanoparticles. For the GO/TiO2 composites, the sharp peak near 2θ = 125° comes from the (002) face of anatase TiO2. So, the prepared sample is anatase TiO2 with high photocatalytic ability. In addition, the diffraction peaks corresponding to the crystal faces of TiO2 (003), (004), (005), (006), (007), and (008) are also found in the vicinity of 35–40°, 49–55°, 62–65°, 68–70°, and 75°, respectively. Therefore, TiO2 was formed on the surface of GO by redox reaction with TiCl3 in solution. However, the peaks of GO cannot be found because the intensity of TiO2 is obviously higher than that of GO [56, 57, 58, 59, 60, 61, 62].
Fig. 2 is the scanning electron microscope (SEM) images of GO and GO/TiO2. It can be seen from Figs. 2(a) and 2(c) that the GO sheet has the obvious folds. A large number of spherical particles are formed on the surface of partially reduced GO [Figs 2(b) and 2(d)]. Combining with GO [Figs 2(a) and 2(c)], it can be concluded that the addition of graphene effectively inhibits the agglomeration of TiO2 particles, promoting uniform dispersion of TiO2 on the surface of GO sheets.
The adsorption–desorption isotherm and the corresponding BET pore diameter distribution curves of the sample are shown in Fig. 3. It can be seen from Fig. 3(a) that the adsorption increases with the increase in pressure. As shown in Fig. 3(b), all the isothermal adsorption–desorption curves show type-IV isotherms shape at a relative pressure range of 0.42–0.95. This clearly indicates that the samples are mesoporous materials with multilayer adsorption.
Photocatalytic activity of GO/TiO2 composites
UV-vis spectroscopy is implemented to estimate the optical absorption ability of GO/TiO2 nanocomposites. The UV-vis spectra of GO and GO/TiO2 are given as Fig. 4(a). For the GO, the absorption peak is at 235 nm. For the GO/TiO2 composites, the maximum absorptivity is at 240 nm, which is a red shift, and their intensity is higher than that of GO. It indicates the increased photocatalytic efficiency when the TiO2 load onto the surface of GO. In addition, they also have another absorption peak at 302 nm due to the photocatalytic activity of TiO2. Therefore, the composites are expected to improve the photocatalytic activity, which will be verified in the photocatalytic degradation of methylene orange dye.
UV-vis spectra of GO and GO/TiO2 composites (a), photocatalytic degradation of MO in sunlight and 48-W UV light irradiation by GO/TiO2 composites (b), photocatalytic degradation of GO, TiO2, and GO/TiO2 composites in 48-W UV light irradiation (c), influence of H2O2 on the degradation of MO with GO/TiO2 composites in sunlight (d).
The degradation ratio of GO/TiO2 for MO is studied, as shown in Fig. 4(b). First, the MO degradation efficiency of GO/TiO2 composites in sunlight and under a 48W ultraviolet light was determined. The degradation ratio of MO from ultraviolet irradiation is 85.73%, while that in sunlight is 40% [Fig. 4(b)], which is obviously lower than that in ultraviolet light because it is difficult to excite electron-hole pairs at a low energy in sunlight. Fig. 4(c) shows the MO degradation ratio of three samples in ultraviolet irradiation for 4 h. However, the GO and TiO2 display similar degradation ratios (36.8% and 38.5%, respectively), and GO/TiO2 composites display 85.4% degradation ratios after 4 h. For GO, the degradation ratio mainly depends on the adsorption of GO, and the degradation ratio remains unchanged when the adsorption reaches equilibrium. TiO2 nanomaterials eliminate the MO in solution via degradation reaction of active groups under ultraviolet light. Therefore, the GO and TiO2 display lower degradation ratios than GO/TiO2 composites. The degradation ratio of GO/TiO2 is the fastest within 1 h, resulting from the adsorption of GO via π–π interaction between GO and MO. The MO transfer from solution to the surface of GO/TiO2 occurs in the first 1 h. The adsorption reaction reached equilibrium after 1 h, so the degradation ratio increased linearly with time. In addition, GO/TiO2 has a micro-mesoporous structure, which further increases its specific surface area, and also has physical adsorption on MO, and it is multilayer adsorption. Finally, in the solution, the active group produced by embedding TiO2 on GO surface can be rapidly combined with MO, which can also promote the degradation of MO.
In order to further increase the degradation ratio of MO, 2 mL hydrogen peroxide (H2O2) was added into the solution to evaluate the influence of hydrogen peroxide on the photodegradation rate, which is shown in Fig. 4(d). The MO degradation ratio of GO/TiO2 increased with the irradiation time. Approximately 40% of MO was degraded by GO/TiO2 composites in the absence of H2O2 after 4 h. In comparison, 90% of MO was degraded when hydrogen peroxide was dripped into the solution. Hydrogen peroxide promotes the rapid formation of OH radical groups to degrade more MO dye. On the other hand, hydrogen peroxide can be used as an electron remover, which can effectively inhibit the simple combination of photogenerated electrons (e−) and photogenerated holes (h+) on the surface of the catalyst, and improve the reaction rate of photocatalytic degradation. This may be due to the rapid formation of OH active groups on the surface of GO/TiO2 by H2O2 to accelerate the degradation of MO.
Equations (1)–(7) and Fig. 5 show the pathway of electron transfer and mechanism of the degradation of MO dye by GO/TiO2 composites. First, MO is adsorbed onto the surface of the GO/TiO2 composites due to the π–π bond of graphene sheets. The GO/TiO2 composites generate photogenerated electrons (e−) and holes (h+) on their surface under the irradiation of ultraviolet light. The water in the solution and the absorbed OH− on the surface of composites are the capture agent of photogenerated holes (h+) to form the hydroxyl radical (•OH). The free oxygen absorbed on the surface of nanocomposites react with photogenerated electrons (e−) to obtain the H2O2, further forming •OH. The •OH is an active species and has a strong oxidation ability, and therefore can directly decompose the MO molecule. The GO promotes the contact between MO and TiO2. In addition, GO, as the recipient of TiO2 photogenerated electricity, effectively promotes the migration of photogenerated electrons and prevents the recombination of photogenerated electrons and holes. H2O2 as an electron remover effectively inhibits the simple combination of photogenerated electrons (e−) and photogenerated holes (h+) and promotes the increase in the number of hydroxyl radicals. So, the GO/TiO2 nanocomposites exhibit excellent photocatalysis when adding H2O2, which can effectively decompose the MO dye.
Conclusion
In this article, GO/TiO2 composites were prepared by the hydrothermal method, and the adsorption and degradation conditions of MO by GO/TiO2 were studied. The specific surface area of GO/TiO2 is about 152.5 m2/g, the pore volume is about 0.2199 cm3/g, and the pore size is 20–40 nm, which is a micro-mesoporous material. The adsorption and degradation rate of GO/TiO2 was 85.62% under ultraviolet lamp irradiation for 4 h. Compared with the traditional photocatalyst TiO2, the composite has a higher catalytic activity for MO under mild conditions. Experimental results demonstrated that the adsorption and degradation efficiency of GO/TiO2 is higher than that of TiO2. The H2O2 obviously improves the degradation efficiency of MO. The adsorption and degradation of GO/TiO2 dioxide consists of two parts: the first pathway is the adsorption of MO molecules by GO/TiO2 and the second is the chemical photodegradation of MO on GO surface.
Experimental section
Materials
MO was purchased from Sigma-Aldrich and was used without further purification. TiCl3 (20–35%) was purchased from NANO Co. H2O2 (30%) was obtained from Duksan Pure Chemical company. Graphite powder, KMnO4, and H2SO4 were purchased from Kanto Chemical Co., Daejung Chemicals & Metals Co., and Yakuri Pure Chemicals Co., respectively.
Preparation of GO/TiO2 nanocomposites
The synthesis of GO was conducted using the modified Hummers method with graphite powder as the raw materials according to the process described in the previous work [63, 64]. The synthetized GO was dispersed in 50 mL H2O by ultrasonic treatment under ambient conditions to yield a brown GO dispersion. The GO/TiO2 composites were fabricated by a hydrothermal reaction method. The concrete procedures were as follows. First, titanium tetrachloride (TiCl3) solution (25–30%, 4 mL) was added into a three-neck flask, and then NH3·H2O solution (37%, 5 mL) was added dropwise into the TiCl3 solution with vigorous agitation at ambient temperature to obtain a turbid solution. Subsequently, GO dispersion (100 mg/L, 100 mL) was added rapidly into the above solution to stir for 30 min. Then, the mixture was put into a hydrothermal reactor at 180 °C for 6 h. Finally, the product was filtrated and washed several times with deionized water and dried at 80 °C to obtain GO/TiO2 composites. The preparation process for GO/TiO2 nanocomposites was displayed in Fig. 6.
Characterization
The structure composition of samples was characterized by a Fourier transform-infrared (FTIR) spectrometer (FTS2000) with KBr pellets, scanning from 400 cm−1 to 4000 cm−1 at the resolution of 1.5 cm−1 for eight times. The Raman spectra of GO and GO/TiO2 composites were measured by using a RLX Renishaw 1000 Raman spectrometer with 514 nm of excitation wavelength. Thermalgravimetric analysis (TGA; Mettler Company, Jiangsu Science equipment Co., Ltd.) was conducted at a temperature range of 25–1000 °C and a heating rate of 15 °C/min. The crystalline phase of GO/TiO2 composites was tested by X-ray diffractometer (XRD; XRD-600, Japan Shimadzu Co., Ltd.) scanning from 10° to 90° in the conditions of voltage of 40 kV, current of 30 mA, and scanning rate of 8°/min, respectively. The surface area and porosity measurements were performed by N2 adsorption at 0–300 °C range with an automated volumetric apparatus (ASAP2020, Micromeritics Instrument Ltd., USA). The surface morphology and microstructures of the GO/TiO2 were investigated by using a scanning electron microscope (SEM; Apollo 300). The photocatalytic properties were tested by using an ultraviolet spectrophotometer UV-vis-L6S. The range of the wavelength test is 200–900 nm.
Photocatalytic degradation of MO
0.1 g sample was added into 100 mL of MO (20 mg/L) solution and the solution ultrasonic treated in the dark for 30 min. The solution was placed under sunlight or a 48W UV lamp with 240 nm wavelength with a 6-cm distance between the lamp tube and sample for different time periods. The solution was gathered every half hour and then centrifuged for 10 min to collect the supernatant used for measuring the absorbance by UV-vis spectrophotometry (721E Vis spectrophotometer) to detect the MO content. The degradation rate of MO was calculated by drawing a standard curve and formula.
where D is the degradation ratio of MO, C0 is the initial concentration of MO in the solution, and C1 presents the equilibrium concentration of MO after photocatalytic degradation.
References
S.D. Perera, R.G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal, and K.J. Balkus, Jr.: Hydrothermal synthesis of graphene–TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2, 949 (2012).
F. Guo, W. Shi, X. Lin, and G. Che: Hydrothermal synthesis of graphitic carbon nitride–BiVO4 composites with enhanced visible light photocatalytic activities and the mechanism study. J. Phys. Chem. Solids 75, 1217 (2014).
Y. Huo, R. Hou, X. Chen, H. Yin, Y. Gao, and H. Li: BiOBr visible-light photocatalytic films in a rotating disk reactor for the degradation of organics. J. Mater. Chem. A 3, 14801 (2015).
Q. Wang, S. Dong, D. Zhang, C. Yu, J. Lu, D. Wang, and J. Sun: Magnetically recyclable visible-light-responsive MoS2@Fe3O4 photocatalysts targeting efficient wastewater treatment. J. Mater. Sci. 53, 1135 (2018).
Z. Zhang, J. Zhang, S. Li, J. Liu, M. Dong, Y. Li, N. Lu, S. Lei, J. Tang, and J. Fan: Effect of graphene liquid crystal on dielectric properties of polydimethylsiloxane nanocomposites. Composites, Part B 176, 107338 (2019).
L. Wang, H. Hu, J. Xu, S. Zhu, A. Ding, and C. Deng: WO3 nanocubes: Hydrothermal synthesis, growth mechanism, and photocatalytic performance. J. Mater. Res. 34, 2955 (2019).
W. Wang, J. Fang, S. Shao, M. Lai, and C. Lu: Compact and uniform TiO2@g-C3N4 core–shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal., B 217, 57 (2017).
H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri, and J. Ye: Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 24, 229 (2012).
S. Liu, Y. Wang, L. Ma, and H. Zhang: Ni2P/ZnS(CdS) core/shell composites with their photocatalytic performance. J. Mater. Res. 33, 3580 (2018).
H. Wang, K. Zhu, L. Yan, C. Wei, Y. Zhang, C. Gong, J. Guo, J. Zhang, D. Zhang, and J. Zhang: Efficient and scalable high-quality graphene nanodot fabrication through confined lattice plane electrochemical exfoliation. Chem. Commun. 55, 5805 (2019).
Y. Wei, Y. Shi, Z. Jiang, X. Zhang, H. Chen, Y. Zhang, J. Zhang, and C. Gong: High performance and lightweight electromagnetic wave absorbers based on TiN/RGO flakes. J. Alloys Compd. 810, 151950 (2019).
Y. Guo, K. Ruan, X. Yang, T. Ma, J. Kong, N. Wu, J. Zhang, J. Gu, and Z. Guo: Constructing fully carbon-based fillers with hierarchical structure to fabricate highly thermally conductive polyimide nanocomposites. J. Mater. Chem. C 7, 7035 (2019).
W. Zhang, C. Wang, X. Liu, and J. Li: Enhanced photocatalytic activity in porphyrin-sensitized TiO2 nanorods. J. Mater. Res. 32, 2773 (2017).
X. Lang, X. Chen, and J. Zhao: Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 43, 473 (2014).
J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo, and D.W. Bahnemann: Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 114, 9919 (2014).
J. Zhang, J. Yi, and Y. Jiao: Preparation and application of water-soluble TiO2-ionic liquids hybrid nanomaterials. J. Inorg. Mater. 33, 577 (2018).
Y. Guo, X. Yang, K. Ruan, J. Kong, M. Dong, J. Zhang, J. Gu, and Z. Guo: Reduced graphene oxide heterostructured silver nanoparticles significantly enhanced thermal conductivities in hot-pressed electrospun polyimide nanocomposites. ACS Appl. Mater. Interfaces 11, 25465 (2019).
C. Liang, P. Song, A. Ma, X. Shi, H. Gu, L. Wang, H. Qiu, J. Kong, and J. Gu: Highly oriented three-dimensional structures of Fe3O4 decorated CNTs/reduced graphene oxide foam/epoxy nanocomposites against electromagnetic pollution. Compos. Sci. Technol. 181, 107683 (2019).
C. Liang, P. Song, H. Qiu, Y. Zhang, X. Ma, F. Qi, H. Gu, J. Kong, D. Cao, and J. Gu: Constructing interconnected spherical hollow conductive networks in silver platelets/reduced graphene oxide foam/epoxy nanocomposites for superior electromagnetic interference shielding effectiveness. Nanoscale 11, 22590 (2019).
J. Zhang, W. Zhang, L. Wei, L. Pu, J. Liu, H. Liu, Y. Li, J. Fan, T. Ding, and Z. Guo: Alternating multilayer structural epoxy composite coating for corrosion protection of steel. Macromol. Mater. Eng. 304, 1900374 (2019).
Z. Lyu, B. Liu, R. Wang, and L. Tian: Synergy of palladium species and hydrogenation for enhanced photocatalytic activity of {001} facets dominant TiO2 nanosheets. J. Mater. Res. 32, 2781 (2017).
L. Thirugnanam, S. Kaveri, M. Dutta, N.V. Jaya, and N. Fukata: Porous tubular rutile TiO2 nanofibers: Synthesis, characterization and photocatalytic properties. J. Nanosci. Nanotechnol. 14, 3034 (2014).
S. Banerjee, S.C. Pillai, P. Falaras, K.E. O’shea, J.A. Byrne, and D.D. Dionysiou: New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 5, 2543 (2014).
M. Pelaez, N.T. Nolan, S.C. Pillai, M.K. Seery, P. Falaras, A.G. Kontos, P.S. Dunlop, J.W. Hamilton, J.A. Byrne, and K. O’shea: A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 125, 331 (2012).
X. Wang, M. Utsumi, Y. Yang, D. Li, Y. Zhao, Z. Zhang, C. Feng, N. Sugiura, and J.J. Cheng: Degradation of microcystin-LR by highly efficient AgBr/Ag3PO4/TiO2 heterojunction photocatalyst under simulated solar light irradiation. J. Phys. Chem. Lett. 325, 1 (2015).
P. Song, C. Liang, L. Wang, H. Qiu, H. Gu, J. Kong, and J. Gu: Obviously improved electromagnetic interference shielding performances for epoxy composites via constructing honeycomb structural reduced graphene oxide. Compos. Sci. Technol. 181, 107698 (2019).
X. Yang, S. Fan, Y. Li, Y. Guo, Y. Li, K. Ruan, S. Zhang, J. Zhang, J. Kong, and J. Gu: Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Composites, Part A 128, 105670 (2020).
G. Liu, Y. Zhao, C. Sun, F. Li, G.Q. Lu, and H.M. Cheng: Synergistic effects of B/N doping on the visible-light photocatalytic activity of mesoporous TiO2. Angew. Chem., Int. Ed. 47, 4516 (2008).
D.H. Kim, H.S. Hong, S.J. Kim, J.S. Song, and K.S. Lee: Photocatalytic behaviors and structural characterization of nanocrystalline Fe-doped TiO2 synthesized by mechanical alloying. J. Alloys Compd. 375, 259 (2004).
L. Zhang, X. Li, Z. Chang, and D. Li: Preparation, characterization and photoactivity of hollow N, Co co-doped TiO2/SiO2 microspheres. Mater. Sci. Semicond. Process. 14, 52 (2011).
L.G. Devi and R. Kavitha: A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal., B 140, 559 (2013).
F. Huang, D. Chen, X.L. Zhang, R.A. Caruso, and Y.B. Cheng: Dual-function scattering layer of submicrometer-sized mesoporous TiO2 beads for high-efficiency dye-sensitized solar cells. Adv. Funct. Mater. 20, 1301 (2010).
S. Wooh, T. Kim, D. Song, Y. Lee, T.K. Lee, V.W. Bergmann, S.A. Weber, J. Bisquert, Y.S. Kang, and K. Char: Surface modification of TiO2 photoanodes with fluorinated self-assembled monolayers for highly efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7, 25741 (2015).
J. Zhang, S. Liu, C. Yan, X. Wang, L. Wang, Y. Yu, and S. Li: Abrasion properties of self-suspended hairy titanium dioxide nanomaterials. Appl. Nanosci. 7, 691 (2017).
Y. Xie, G. Ali, S.H. Yoo, and S.O. Cho: Sonication-assisted synthesis of CdS quantum-dot-sensitized TiO2 nanotube arrays with enhanced photoelectrochemical and photocatalytic activity. ACS Appl. Mater. Interfaces 2, 2910 (2010).
A.A. Ismail, I. Abdelfattah, A. Helal, S. Al-Sayari, L. Robben, and D. Bahnemann: Ease synthesis of mesoporous WO3–TiO2 nanocomposites with enhanced photocatalytic performance for photodegradation of herbicide imazapyr under visible light and UV illumination. J. Hazard. Mater. 307, 43 (2016).
J. Sheng, H. Tong, H. Xu, and C. Tang: Preparation and photocatalytic activity of SnO2@TiO2 core–shell composites modified by Ag. Catal. Surv. Asia 20, 167 (2016).
J. Zhang, Z. Zhang, Y. Jiao, H. Yang, Y. Li, J. Zhang, and P. Gao: The graphene/lanthanum oxide nanocomposites as electrode materials of supercapacitors. J. Power Sources 419, 99 (2019).
Y. Jiao, J. Zhang, S. Liu, Y. Liang, S. Li, H. Zhou, and J. Zhang: The graphene oxide ionic solvent-free nanofluids and their battery performances. Sci. Adv. Mater. 10, 1706 (2018).
J. Paredes, S. Villar-Rodil, A. Martínez-Alonso, and J. Tascon: Graphene oxide dispersions in organic solvents. Langmuir 24, 10560 (2008).
J. Zhang, P. Li, Z. Zhang, X. Wang, J. Tang, H. Liu, Q. Shao, T. Ding, A. Umar, and Z. Guo: Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 542, 159 (2019).
Y. Guo, G. Xu, X. Yang, K. Ruan, T. Ma, Q. Zhang, J. Gu, Y. Wu, H. Liu, and Z. Guo: Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 6, 3004 (2018).
Y. Li, T. Jing, G. Xu, J. Tian, M. Dong, Q. Shao, B. Wang, Z. Wang, Y. Zheng, and C. Yang: 3-D magnetic graphene oxide-magnetite poly(vinyl alcohol) nanocomposite substrates for immobilizing enzyme. Polymer 149, 13 (2018).
K. Sun, J. Dong, Z. Wang, Z. Wang, G. Fan, Q. Hou, L. An, M. Dong, R. Fan, and Z. Guo: Tunable negative permittivity in flexible graphene/PDMS metacomposites. J. Phys. Chem. C 123, 23635 (2019).
M. Idrees, S. Batool, J. Kong, Q. Zhuang, H. Liu, Q. Shao, N. Lu, Y. Feng, E.K. Wujcik, and Q. Gao: Polyborosilazane derived ceramics-nitrogen sulfur dual doped graphene nanocomposite anode for enhanced lithium ion batteries. Electrochim. Acta 296, 925 (2019).
V. Murugadoss, J. Lin, H. Liu, X. Mai, T. Ding, Z. Guo, and S. Angaiah: Optimizing graphene content in a NiSe/graphene nanohybrid counter electrode to enhance the photovoltaic performance of dye-sensitized solar cells. Nanoscale 11, 17579 (2019).
Y. He, Q. Chen, H. Liu, L. Zhang, D. Wu, C. Lu, W. OuYang, D. Jiang, M. Wu, and J. Zhang: Friction and wear of MoO3/graphene oxide modified glass fiber reinforced epoxy nanocomposites. Macromol. Mater. Eng. 304, 1900166 (2019).
L. Ma, Y. Zhu, P. Feng, G. Song, Y. Huang, H. Liu, J. Zhang, J. Fan, H. Hou, and Z. Guo: Reinforcing carbon fiber epoxy composites with triazine derivatives functionalized graphene oxide modified sizing agent. Composites, Part B 176, 107078 (2019).
B. Kumar, D.K. Verma, A.K. Singh, Kavita, N. Shukla, and R.B. Rastogi: Nanohybrid Cu@C: Synthesis, characterization and application in enhancement of lubricity. Compos. Interfaces, 1 (2019) https://doi.org/10.1080/09276440.2019.1697134.
C. Lou, T. Jing, J. Tian, Y. Zheng, J. Zhang, M. Dong, C. Wang, C. Hou, J. Fan, and Z. Guo: 3-Dimensional graphene/Cu/Fe3O4 composites: Immobilized laccase electrodes for detecting bisphenol A. J. Mater. Res. 34, 2964 (2019).
Y. He, Q. Chen, S. Yang, C. Lu, M. Feng, Y. Jiang, G. Cao, J. Zhang, and C. Liu: Micro-crack behavior of carbon fiber reinforced Fe3O4/graphene oxide modified epoxy composites for cryogenic application. Composites, Part A 108, 12 (2018).
M. Liu, Q. Meng, Z. Yang, X. Zhao, and T. Liu: Ultra-long-term cycling stability of an integrated carbon–sulfur membrane with dual shuttle-inhibiting layers of graphene “nets” and a porous carbon skin. Chem. Commun. 54, 5090 (2018).
B.S. Gonçalves, L.M. Silva, T.C. de Souza, V.G. de Castro, G.G. Silva, B.C. Silva, K. Krambrock, R.B. Soares, V.F. Lins, and M. Houmard: Solvent effect on the structure and photocatalytic behavior of TiO2-RGO nanocomposites. J. Mater. Res. 34, 3918 (2019).
X. Yan, X. Yuan, J. Wang, Q. Wang, C. Zhou, D. Wang, H. Tang, J. Pan, and X. Cheng: Construction of novel ternary dual Z-scheme Ag3VO4/C3N4/reduced TiO2 composite with excellent visible-light photodegradation activity. J. Mater. Res. 34, 2024 (2019).
V. Labunov, L. Tabulina, I. Komissarov, D. Grapov, E. Prudnikova, Y.P. Shaman, S. Basaev, and A. Pavlov: Features of the reduction of graphene from graphene oxide. Russ. J. Phys. Chem. A 91, 1088 (2017).
B. Cao, H. Liu, L. Yang, X. Li, H. Liu, P. Dong, X. Mai, C. Hou, N. Wang, and J. Zhang: Interfacial engineering for high-efficiency nanorod array-structured perovskite solar cells. ACS Appl. Mater. Interfaces. 11, 33770 (2019).
L. Yang, X. Wang, X. Mai, T. Wang, C. Wang, X. Li, V. Murugadoss, Q. Shao, S. Angaiah, and Z. Guo: Constructing efficient mixed-ion perovskite solar cells based on TiO2 nanorod array. J. Colloid Interface Sci. 534, 459 (2019).
L. Zhang, M. Qin, W. Yu, Q. Zhang, H. Xie, Z. Sun, Q. Shao, X. Guo, L. Hao, and Y. Zheng: Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 164, H1086 (2017).
L. Zhang, W. Yu, C. Han, J. Guo, Q. Zhang, H. Xie, Q. Shao, Z. Sun, and Z. Guo: Large scaled synthesis of heterostructured electrospun TiO2/SnO2 nanofibers with an enhanced photocatalytic activity. J. Electrochem. Soc. 164, H651 (2017).
L. Shindume, Z. Zhao, N. Wang, H. Liu, A. Umar, J. Zhang, T. Wu, and Z. Guo: Enhanced photocatalytic activity of B, N-codoped TiO2 by a new molten nitrate process. J. Nanosci. Nanotechnol. 19, 839 (2019).
J. Tian, Q. Shao, J. Zhao, D. Pan, M. Dong, C. Jia, T. Ding, T. Wu, and Z. Guo: Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO2/ZrO2 composites toward enhanced photocatalytic degradation of rhodamine B. J. Colloid Interface Sci. 541, 18 (2019).
L. Ma, N. Li, G. Wu, G. Song, X. Li, P. Han, G. Wang, and Y. Huang: Interfacial enhancement of carbon fiber composites by growing TiO2 nanowires onto amine-based functionalized carbon fiber surface in supercritical water. Appl. Surf. Sci. 433, 560 (2018).
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, and R.S. Ruoff: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558 (2007).
J. Zhang, Y. Liang, X. Wang, H. Zhou, S. Li, J. Zhang, Y. Feng, N. Lu, Q. Wang, and Z. Guo: Strengthened epoxy resin with hyperbranched polyamine-ester anchored graphene oxide via novel phase transfer approach. Adv. Compos. Hybrid Mater. 1, 300 (2018).
Acknowledgments
We gratefully acknowledge the support from the special fund of Shaanxi Provincial Education Department (16JK1612), the Key Laboratory of High-tech Research on Marine Functional Thin Film Materials in Zhenjiang (ZHZ2019008), the key research and development project of Shaanxi Province in 2017 (2017GY-180), and the Provincial College Students Innovation and Entrepreneurship Program (201819018).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, C., Gao, Y., Zhang, J. et al. GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange. Journal of Materials Research 35, 1307–1315 (2020). https://doi.org/10.1557/jmr.2020.41
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2020.41