Abstract
Laves phase plays a positive role in improving the strength of high-entropy alloys (HEAs); Nb and Ti elements have potential to promote Laves phase formation in some HEAs. For improving the strength of the face-centered cubic (FCC) CoCrFeMnNi HEA, a series of (CoCrFeMnNi)100−xNbx (atomic ratio: x = 0, 4, 8, 12, 16) and (CoCrFeMnNi)100−xTix (atomic ratio: x = 0, 2, 4, 6, 8, 12) HEAs were prepared by melting. The effects of Nb and Ti on the microstructure evolution and compressive properties of the CoCrFeMnNi HEAs were investigated. For (CoCrFeMnNi)100−xNbx HEAs, the second-phase (Laves and σ phase) volume fraction increased from 0 to 42%. The yield strength also increased gradually from 202 to 1010 MPa. However, the fracture strain decreased from 60% (no fracture) to 12% with increasing Nb content. For (CoCrFeMnNi)100−xTix HEAs, the yield strength increased from 202 to 1322 MPa. The Laves phase volume fraction also increased from 0 to 27%. However, the fracture strain decreased from 60% (no fracture) to 7.5% with increasing Ti content. Addition of Nb and Ti has a good effect on improving the strength of FCC CoCrFeMnNi HEA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past few years, high-entropy alloys (HEAs) have attracted considerable research attention because of their excellent mechanical properties and simple solid solution structure. Many materials scientists have conducted a lot of research in the fields such as forging [1, 2], heat treatment [3, 4, 5], alloying [6, 7, 8], casting [9, 10], and phase stability [11]. Since 2004, when they were first proposed independently by Yeh and Cantor [12, 13], HEAs have emerged as an important breakthrough in the traditional alloy design and have opened a new path for exploring new materials with excellent properties.
The traditional alloy design involves selecting the major component based on a specific property requirement and then conferring secondary properties by using alloying addition without sacrificing the primary property [13]. Several similar methods had been used in the field of HEAs. He et al. investigated the alloying effect of Al on the phase structure, strength, and ductility of the CoCrFeMnNi HEA system and found that the Al element had potential to directly change the phase structure and mechanical properties of CoCrFeMnNi HEA [14]. The crystal structure, microstructure, microhardness, and compression properties of CoCrFeMnNiVx (x = 0, 0.25, 0.5, 0.75, 1) HEAs were studied by Stepanov et al., who reported that the sigma phase volume fraction increased with increase in the V content; in CoCrFeMnNiV, they found that the sigma phase became the matrix phase [15]. In other studies, different elements (V/Nb/B/Mo/Si/Ti/Zr) were also added to AlCoCrFeNi HEA, and the alloying effect of these elements on the microstructure and mechanical properties was investigated [14, 15, 16, 17, 18, 19, 20, 21]. Table I summarizes some available literatures about the alloying effect on phase composition and mechanical properties of HEAs; the data indicate that some elements promote the formation of hard phase (body-centered cubic/Laves/δ phase) and improve the strength of alloys. In 2017 and 2018 [18, 19], Fang and his team designed two eutectic HEAs (CoCrFeNiTax and CoCrFeNiZrx HEAs) strengthened by Laves phase; these two HEAs have excellent mechanical properties, which again proved that some elements could promote the formation of Laves phase and improve the strength of alloys. Low enthalpy of mixing (ΔHmix) is helpful to form intermetallic in HEAs. Nb and Ti elements were added to FCC CoCrFeMnNi HEAs, which can decrease the ΔHmix of (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs. Thus, it is possible that the addition of Nb and Ti to (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs induces hard phase formation in CoCrFeMnNi matrix alloy. However, based on the existing reports in the literatures [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28], the effect of Nb and Ti addition on the structure evolution and mechanical properties of CoCrFeMnNi HEA has not been investigated thus far [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. The gap should be filled up.
In the present study, an HEA containing CoCrFeMnNi (all elements in equal atomic proportions) with a single face-centered cubic (FCC) phase solid solution was selected as the baseline alloy system. A systematic study on the alloying effect of Nb and Ti on the microstructure, phase evolution, and mechanical properties of the (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs was carried out. The correlations between the microstructure and the mechanical properties of these HEAs were determined. In addition, considering the combined effects of entropy of mixing (ΔSmix), enthalpy of mixing (ΔHmix), atomic radius difference (Δ), valence electron concentration (VEC), and the scald ratio of ΔSmix to ΔHmix (Ω), we tried to reveal the relevance between the phase translation and element properties.
Results and discussion
Phase composition
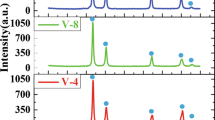
As shown in Fig. 1(a), the XRD patterns of (CoCrFeMnNi)100−xNbx (x = 0, 4, 8, 12, 16, hereafter in atomic ratio) HEAs indicate a phase selection from the FCC phase to the Laves phase and σ phase structure with increasing Nb content. The XRD patterns of (CoCrFeMnNi)100−xTix (x = 0, 2, 4, 6, 8, 12, hereafter in atomic ratio) HEAs also show a phase selection from the FCC phase to the Laves phase [see Fig. 1(b)]. This change enables the alloy system to attain a multiphase microstructure, in which both phases obtain the maximum benefit of the solid solution strengthening effect compared with only one phase [31]. A single FCC structure was observed when the Nb content was 0. When the Nb content was 4%, only a weak reflection peak corresponding to σ phase and Laves phase structure was found. When the Nb content was 4–16%, the alloys show a multiphase (FCC, Laves phase, and σ phase) structure, which was obviously induced by the addition of Nb element. As shown in Fig. 1(b), the phase of the alloys was identified as a single FCC solid solution when the Ti content was lower (x = 0, 2, 4, 6), at which point the alloy mainly was strengthened by the solid solution strengthening mechanism. The weak reflection peak corresponding to the Laves phase and Laves* phase appeared when the Ti content reached 8 at.%. Thus, this alloy obtained a multiphase (FCC, Laves phase, and Laves* phase) structure when the Ti content was 8–12 at.%, at which point the alloy mainly was strengthened by the solid solution strengthening mechanism and the second-phase strengthening mechanism. The Ti element had a considerable influence on the phase translation from the FCC to the Laves phase structure in the alloys.
Microstructure and mechanical property
Figure 2 shows the compressive mechanical behavior of (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs. The compressive yield strength of the (CoCrFeMnNi)100−xNbx HEAs increased from 202 to 1010 MPa; however, the fracture strain decreased from 60% (no fracture) to 12% with the addition of Nb from 0 to 16% [see Fig. 2(a)]. Figure 2(b) shows the stress–strain curve of the compression test for the (CoCrFeMnNi)100−xTix alloys. The yield strength of the alloys increased from 202 to 1322 MPa, whereas the fracture strain decreased from 60% (no fracture) to 7.5%, with the Ti content increased from 0 to 12 at.%. The results indicate that the addition of Nb and Ti has a positive effect on improving the strength of FCC HEAs.
Engineering stress–strain curves from compressive properties of HEAs. (a) (CoCrFeMnNi)100−xNbx (triangle, circular, quadrilateral, pentagon, and echelon represent the Nb-0, Nb-4, Nb-8, Nb-12, and Nb-16 HEAs, respectively). (b) (CoCrFeMnNi)100−xTix (triangle, circular, quadrilateral, pentagon, and echelon represent the Ti-0, Ti-4, Ti-6, Ti-8, and Ti-12 HEAs respectively).
Figure 3 shows the microstructures of the as-cast (CoCrFeMnNi)100−xNbx HEAs with different Nb contents, and the relationship between the yield strength and the second-phase (Laves and σ phase) volume fraction with different Nb contents in (CoCrFeMnNi)100−xNbx HEAs. Nb-0 alloys were identified by XRD (see Fig. 1) with a single FCC phase microstructure, in agreement with the previous study [14]. A small number of lath-shaped σ phase particles were generated when the Nb content was 4% [see Fig. 3(b)], at which point the alloy mainly consisted of the FCC phase. When the Nb content reached 8%, the Laves phase (eutectic structure, Nb-rich) appears and the volume increased gradually [see Figs 3(c) and 3(d)]. When the Nb content reached 16%, the alloy mainly consisted of the Laves phase [see Fig. 3(e)]. The number of Laves phases increases with increasing Nb content; this encourages growth in the phase boundary density, which creates additional obstacles for dislocation slipping, thus contributing to improving the strength of the alloy. In Fig. 3(c), the black areas indicate oxides; however, whether the oxygen was introduced through the raw material or during one of the subsequent processing steps is not known. However, because of their tiny volume fracture, they did not significantly influence mechanical properties, which is not surprising since they also have been previously found in these alloys [32, 33, 34, 35]. Figure 3(f) shows the relationship between the yield strength and the second-phase (Laves and σ phase) volume fraction with different Nb contents in (CoCrFeMnNi)100−xNbx HEAs. The second-phase volume fraction increased with increase in Nb content. The yield strength also increased as the second-phase volume fraction increased from 0 to 42%. This indicates that the increase in the second phase induced by adding Nb is the key factor for the improvement of the yield strength in this HEA system. In addition, the previous studies [16, 17, 20, 21, 23, 25, 29] also reported that Nb/Ti addition to CoCrFeNi/CoCrCuFeNi/AlCoCrFeNi HEAs, which also show the same result that Laves phase was generated in these HEAs. It proves that Nb and Ti elements have a positive effect on forming Laves phase, improving the strength of these HEAs.
Figures 4(a)–4(e) show the microstructure morphology of the as-cast (CoCrFeMnNi)100−xTix (x = 0, 4, 6, 8, 12) HEAs. As shown in Fig. 4(a), combined with the XRD pattern in Fig. 1(b), the Ti-0 alloy (Ti content of 0 at.%) presented a single FCC phase structure. Some Laves phases (Ti segregation particles) were observed in the Ti-4 alloy, as shown in Fig. 4(b). When the Ti content was 6%, a small number of Laves* phases were observed in the Ti-6 alloy. The volume fraction of the new Laves* phase increased with increase in the Ti content [see Figs. 4(c)–4(e)]. Figure 4(f) shows the yield strength and the second-phase (Laves phase and Laves* phase) volume fraction with different Ti contents in the (CoCrFeMnNi)100−xTix HEAs. The second-phase volume fraction increased from 0 to 27% as the Ti content increased. The yield strength also increased with the increase in the second-phase volume fraction, which indicates the second phase induced by the addition of Ti is the key factor for increasing the yield strength.
The FCC phase structure has better plasticity and low strength, whereas the Laves phase has higher strength and limited plasticity. The yield strength of the alloys can be estimated by the simple rule of mixtures [Eq. (1)] [36, 37, 38, 39].
where δfcc and δLaves denote the strength of the FCC and the Laves phase, respectively, and Vfcc and VLaves refer to the volume fraction of the FCC and the Laves phase, respectively. This implies that the strength and plasticity can be regulated based on practical application by changing the alloy element (Nb and Ti) content.
The elemental distribution is done by Energy Dispersive Spectrometer (EDS), and the compositions (at.%) of various phases in as-cast (CoCrFeMnNi)84Nb16 and (CoCrFeMnNi)92Ti8 HEAs are shown in Table II, which indicates the composition distribution in the (CoCrFeMnNi)84Nb16 HEA. In (CoCrFeMnNi)84Nb16 HEA, the Laves phase is shown to be an Nb-rich region, whereas the FCC phase is an Nb-poor region. The Laves phase and Laves* phase in the (CoCrFeMnNi)92Ti8 HEA are shown to be a Ti-rich region. The results prove that the Nb and Ti elements have a considerable influence on the phase selection from FCC to Laves phase structure in the alloys.
The atomic radius, entropy of mixing, and enthalpy of mixing have been proposed to predict the structural stability and phase formation in HEAs [38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57]. Considering from the electronic level, VEC or the itinerant electrons per atom ratio (e/a) is an important parameter for the atom arrangement. Guo et al. proposed this parameter for predicting phase formation and summed up the correlation of VEC and FCC/BCC phase formation. FCC phases have been found to be stable at higher VEC (≥8), whereas BCC phases are stable at lower VEC (<6.87) in HEAs [41, 42, 43]. Tsai et al. proposed that alloys having VEC values between 6.88 and 7.84 are prone to σ phase formation either in the as-cast state or during aging at suitable temperatures [43, 44]. They provided an important reference for the design of HEA. The value of VEC can be attained by Eq. (2) [41, 42, 43, 44].
where Ci is the atom percentage and (VEC)i is the VEC for the ith element.
Based on the correlation between VEC and the FCC/BCC phase, a function about the VEC and the content of Nb and Ti element was established. As shown in Fig. 5, VEC decreased with increasing Nb and Ti content because of the VEC values of the Nb and Ti were lower than the average VEC of the CoCrFeMnNi HEA. Contrary to previous reports [41, 42], no BCC phase formed in this alloy system; however, Laves phase and σ phase were generated in this alloy, which agrees with the criterion proposed by Tsai for σ phase formation [43, 44].
Enthalpy of mixing (ΔHmix), atomic radius difference (δ), entropy of mixing (ΔSmix), and the scald ratio of ΔSmix to ΔHmix (Ω) are important parameters for phase selection in HEAs based on the previous reports [41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57]. Guo suggested that solid solution is expected if all the three parameters satisfy the conditions of −22 ≤ ΔHmix ≤ 7 kJ/mol, 0 ≤ δ ≤ 8.5, and 11 ≤ ΔSmix ≤ 19.5 J/K mol [41]. Figure 6(a) shows the enthalpy of mixing (ΔHmix) of (CoCrFeMnNi)100−xNbx HEAs and (CoCrFeMnNi)100−xTix HEAs. The ΔHmix can be estimated by Eq. (3) [54].
where Ωij usually equals to 4 ΔHijmix and ΔHijmix is the enthalpy of mixing of the ith and jth component element [54, 55, 57].
It shows that the ΔHmix of both (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs decreases with increasing Nb/Ti content. The value of ΔHmix is less than zero, which indicates that the reaction is an exothermic reaction. In the process of alloy solidification, the temperature decreases, which is helpful to promote the reaction and form intermetallic. The lower the mixing enthalpy is, the higher the probability of the chemical reaction.
Figure 6(b) shows the atomic radius difference (δ) of (CoCrFeMnNi)100−xNbx HEAs and (CoCrFeMnNi)100−xTix HEAs. The atomic radius difference (δ) was calculated by Eq. (4) [14, 52, 53, 54, 55, 56, 57].
It shows that δ of both HEAs increases with increasing Nb/Ti content. The large lattice distortion energy, induced by Nb/Ti addition, can be released by the method of phase translation, so the increase of δ is helpful to promote phase translation in HEAs [14, 52, 53, 54, 55, 56].
Figure 7(a) shows the entropy of mixing (ΔSmix) of (CoCrFeMnNi)100−x(Nb/Ti)x HEAs. The ΔSmix was attained by Eq. (5) [48, 49, 50].
where n represents the number of component elements, ci represents the at.% of the ith component element, and R is the universal gas constant [8.314 k/(J mol)] [48, 49, 50]. It is shown that the value of ΔSmix decreases with increasing Nb/Ti content, which is helpful to promote solid solution formation based on the previous reports [48, 49, 50].
The scald ratio of ΔSmix to ΔHmix (Ω) was pointed out as an important parameter for prediction of solid solution formation by Yang et al. and Guo et al. [40, 41]. Figure 7(b) shows the scald ratio of ΔSmix to ΔHmix (Ω) of (CoCrFeMnNi)100−xNbx HEAs and (CoCrFeMnNi)100−xTix HEAs. The Ω was attained by Eq. (6) [40, 41].
where (Tm)i is the melting point of the ith constituent element, and it can be attained by Eq. (7) [40, 41].
Figure 7(b) shows that Ω of both HEAs increases with increasing Nb/Ti content, which plays a positive role in promoting solid solution formation based on the previous reports [40, 41].
When Nb element is added to the CoCrFeMnNi HEA, part of the Nb atoms were incorporated into the CoCrFeMnNi matrix and form FCC phase, whereas remnant Nb atoms will solid solute with other elements each other and form Laves phase finally. With increase in Nb content, part of FCC phase changes to Laves phase, and the Laves phase volume fraction increases with further increase in Nb.
Conclusions
In this study, the alloying effects of Nb and Ti elements on the microstructure evolution and compressive properties of CoCrFeMnNi HEAs were investigated. Based on the experimental and analytical results, the following conclusions were obtained:
-
(1)
Nb and Ti have the capacity to promote the phase selection from the FCC to the Laves phase.
-
(2)
The second-phase (Laves and σ phase) volume fraction of (CoCrFeMnNi)100−xNbx HEAs increases from 0 to 42% when Nb content is increased from 0 to 16 at.%. The second-phase (Laves phase and Laves* phase) volume fraction of (CoCrFeMnNi)100−xTix HEAs increases from 0 to 27% with increasing Ti content from 0 to 12 at.%.
-
(3)
The yield strength of (CoCrFeMnNi)100−xNbx HEA increases gradually from 202 to 1010 MPa with the addition of Nb at 0–16 at.%. The yield strength of (CoCrFeMnNi)100−xTix HEAs increases gradually from 202 to 1322 MPa with the addition of Ti at 0–12 at.%.
-
(4)
As a reinforced phase, the Laves phase is beneficial for improving the yield strength of HEAs. The increased volume fraction of Laves phase plays a positive role in improving the strength of CoCrFeMnNi HEA.
Experimental
A series of (CoCrFeMnNi)100−xNbx and (CoCrFeMnNi)100−xTix HEAs were synthesized by arc melting a mixture of pure metals (purity >99%) in a high-purity argon atmosphere. The alloys were melted seven times for composition homogeneity. The phase structure was identified by X-ray diffraction (XRD; Empyrean) with a step size of 0.026°. Microstructure was characterized by scanning electron microscopy (SEM) with the use of a Zeiss Supra 55 instrument (Carl Zeiss AG, Oberkochen, Germany) operated at 15 kV. Element distribution was measured with an electronic probe. For microstructure observation, the SEM samples were cut from the center of the ingot. These samples were sequentially ground and polished, followed by electropolishing in a mixture of 90% acetic acid and 10% perchloric acid at room temperature and supplied with the voltage of 27 V for 15 s. Samples with dimensions of Ø4 mm × 6 mm were prepared for compressive testing; at least three specimens were tested for each same composition sample to obtain reliable results. Compression tests were carried out on an AG-X plus 250 kN electronic universal material testing machine (Shimadzu, Kyoto, Japan) at a strain rate of 0.5 × 10−3 mm/min at room temperature.
References
S.J. Sun, Y.Z. Tian, H.R. Lin, X.G. Dong, Y.H. Wang, Z.J. Zhang, and Z.F. Zhang: Enhanced strength and ductility of bulk CoCrFeMnNi high entropy alloy having fully recrystallized ultrafine-grained structure. Mater. Des. 133, 122 (2017).
S.J. Sun, Y.Z. Tian, H.R. Lin, H.J. Yang, X.G. Dong, Y.H. Wang, and Z.F. Zhang: Transition of twinning behavior in CoCrFeMnNi high entropy alloy with grain refinement. Mater. Sci. Eng., A 712, 603 (2018).
Y. Ma, Q. Wang, C. Li, L. Santodonato, M. Feygenson, C. Dong, and P.K. Liaw: Chemical short-range orders and the induced structural transition in high-entropy alloys. Scr. Mater. 64, 144 (2018).
W.Q. Wu, S. Ni, Y. Liu, and M. Song: Effects of cold rolling and subsequent annealing on the microstructure of a HfNbTaTiZr high-entropy alloy. J. Mater. Res. 31, 3815 (2016).
Y. Zhang, Y. Liu, and T.X. Li: Microstructure and mechanical properties of a refractory HfNbTiVSi0.5 high-entropy alloy composite. Mater. Lett. 174, 82 (2016).
Y. Liu, Y. Zhang, H. Zhang, N.J. Wang, X. Chen, H.W. Zhang, and Y.X. Li: Microstructure and mechanical properties of refractory HfMo0.5NbTiV0.5Six high-entropy composites. J. Alloys Compd. 694, 869 (2017).
Y. Liu, M. Chen, Y.X. Li, and X. Chen: Microstructure and mechanical performance of AlxCoCrCuFeNi high-entropy alloys. Rare Met. Mater. Eng. 38, 602 (2009).
X.W. Liu, L. Liu, G. Liu, X.X. Wu, D.H. Lu, J.Q. Yao, W.M. Jiang, Z.T. Fan, and W.B. Zhang: The role of carbon in grain refinement of cast CrFeCoNi high-entropy alloys. Metall. Mater. Trans. A 49, 2151 (2018).
Y.P. Lu, Y. Dong, S. Guo, L. Jiang, H.J. Kang, T.M. Wang, B. Wen, Z.J. Wang, J.C. Jie, Z.Q. Cao, H.H. Ruan, and T.J. Li: A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 4, 6200 (2014).
Y.P. Lu, X.Z. Gao, L. Jiang, Z.N. Chen, T.M. Wang, J.C. Jie, H.J. Kang, Y.B. Zhang, S. Guo, H.H. Ruan, Y.H. Zhao, Z.Q. Cao, and T.J. Li: Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 124, 143 (2017).
J.Q. Yao, X.W. Liu, N. Gao, Q.H. Jiang, N. Li, G. Liu, W.B. Zhang, and Z.T. Fan: Phase stability of a ductile single-phase BCC Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy alloy. Intermetallics 98, 79 (2018).
J.W. Yeh, S.K. Chen, S.J. Lin, J.Y. Gan, T.S. Chin, T.T. Shun, C.H. Tsau, and S.Y. Chang: Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 6, 299 (2004).
B. Cantor, I.T.H. Chang, P. Knight, and A.J.B. Vincent: Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng., A 213, 375 (2004).
J.Y. He, W.H. Liu, H. Wang, Y. Wu, X.J. Liu, T.G. Nieh, and Z.P. Lu: Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 62, 105 (2014).
N.D. Stepanov, D.G. Shaysultanov, G.A. Salishchev, M.A. Tikhonovsky, E.E. Oleynik, A.S. Tortika, and O.N. Senkov: Effect of V content on microstructure and mechanical properties of the CoCrFeMnNiVx high entropy alloys. J. Alloys Compd. 628, 170 (2015).
W.H. Liu, J.Y. He, H.L. Huang, H. Wang, Z.P. Lu, and C.T. Liu: Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 60, 1 (2015).
X.F. Wang, Y. Zhang, Y. Qiao, and G.L. Chen: Novel microstructure and properties of multicomponent CoCrCuFeNiTix, alloys. Intermetallics 15, 357 (2007).
W.Y. Huo, H. Zhou, F. Fang, Z.H. Xie, and J.Q. Jiang: Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Mater. Des. 134, 226 (2017).
W.Y. Huo, H. Zhou, F. Fang, X.F. Zhou, Z.H. Xie, and J.Q. Jiang: Microstructure and properties of novel CoCrFeNiTax eutectic high-entropy alloys. J. Alloys Compd. 735, 897 (2018).
T.T. Shun, L.Y. Chang, and M.H. Shiu: Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng., A 556, 170 (2012).
W.H. Liu, Z.P. Lu, J.Y. He, J.H. Luan, Z.J. Wang, B. Liu, Y. Liu, M.W. Chen, and C.T. Liu: Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 116, 332 (2016).
H. Jiang, L. Jiang, D.X. Qiao, Y.P. Lu, T.M. Wang, Z.Q. Cao, and T.J. Li: Effect of niobium addition on microstructure and properties of the CoCrFeNbxNi high entropy alloys. J. Mater. Sci. Technol. 33, 712 (2016).
F. He, Z. Wang, P. Cheng, Q. Wang, J.J. Li, Y.Y. Dang, J.C. Wang, and C.T. Liu: Designing eutectic high entropy alloys of CoCrFeNiNbx. J. Alloys Compd. 656, 284 (2016).
Y. Dong, K.Y. Zhou, Y.P. Lu, X.X. Gao, T.M. Wang, and T.J. Li: Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 57, 67 (2015).
S.G. Ma and Y. Zhang: Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng., A 532, 480 (2012).
Q.S. Chen, Y.P. Lu, Y. Dong, T.M. Wang, and T.J. Li: Effect of minor B addition on microstructure and properties of AlCoCrFeNi multi-component alloy. Trans. Nonferrous Met. Soc. China 25, 2958 (2015).
J.M. Zhu, H.F. Zhang, H.M. Fu, A.M. Wang, H. Li, and Z.Q. Hu: Microstructures and compressive properties of multicomponent AlCoCrCuFeNiMox alloys. J. Alloys Compd. 497, 52 (2010).
J.M. Zhu, H.M. Fu, H.F. Zhang, A.M. Wang, H. Li, and Z.Q. Hu: Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater. Sci. Eng., A 527, 7210 (2010).
Y. Yu, J. Wang, J.S. Li, H.C. Kou, and W.M. Liu: Characterization of BCC phases in AlCoCrFeNiTix high entropy alloys. Mater. Lett. 138, 78 (2015).
J. Chen, P. Niu, Y. Liu, Y.Y. Lu, X.H. Wang, Y.L. Peng, and J.N. Liu: Effect of Zr content on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. Mater. Des. 94, 39 (2016).
Z.M. Li, K.G. Pradeep, and Y. Deng: Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 534, 227 (2016).
F. Otto, A. Dlouhý, K.G. Pradeep, M. Kuběnová, D. Raabe, G. Eggeler, and E.P. George: Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 112, 40 (2016).
A. Gali and E.P. George: Tensile properties of high- and medium-entropy alloys. Intermetallics 39, 74 (2013).
F. Otto, N.L. Hanold, and E.P. George: Microstructural evolution after thermo mechanical processing in an equiatomic, single-phase CoCrFeMnNi high entropy alloy with special focus on twin boundaries. Intermetallics 54, 39 (2014).
X. Chen, Y.W. Sui, J.Q. Qi, Y.Z. He, F.X. Wei, Q.K. Meng, and Z. Sun: Microstructure of Al1.3CrFeNi eutectic high entropy alloy and oxidation behavior at 1000 °C. J. Mater. Res. 11, 32 (2017).
Y.J. Zhou, Y. Zhang, Y.L. Wang, and G.L. Chen: Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 90, 253 (2007).
S.W. Tsai: Theory of Composites Design (Think Composites Press, San Francisco, 1992).
Y. Zhang, Y. Zhou, J. Lin, G. Chen, and P.K. Liaw: Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 10, 534 (2008).
T. Egami and Y. Waseda: Atomic size effect on the formability of metallic glasses. J. Non-Cryst. Solids 64, 113 (1984).
X. Yang and Y. Zhang: Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 132, 233 (2012).
S. Guo and C.T. Liu: Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci.: Mater. Int. 21, 433 (2011).
S. Guo, C. Ng, J. Lu, and C.T. Liu: Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 109, 645 (2011).
M.H. Tsai, K.Y. Tsai, C.W. Tsai, C. Lee, C.C. Juan, and J.W. Ye: Criterion for sigma phase formation in Cr- and V-containing high-entropy alloys. Mater. Res. Lett. 1, 207 (2013).
M.H. Tsai, K.C. Chang, J.H. Li, R.C. Tsai, and A.H. Cheng: A second criterion for sigma phase formation in high-entropy alloys. Mater. Res. Lett. 4, 1 (2016).
M.C. Troparevsky, J.R. Morris, P.R.C. Kent, A.R. Lupini, and G.M. Stocks: Criteria for predicting the formation of single-phase high-entropy alloys. Phys. Rev. X 5, 1 (2015).
N. Yurchenko, N. Stepanov, and G. Salishchev: Laves-phase formation criterion for high-entropy alloys. Met. Sci. J. 33, 17 (2016).
O.N. Senkov and D.B. Miracle: A new thermodynamic parameter to predict formation of solid solution or intermetallic phases in high entropy alloys. J. Alloys Compd. 658, 603 (2016).
B.S. Murty, J.W. Yeh, and S. Ranganathan: High Entropy Alloys (Elsevier, London, 2014).
Y. Zhang, T.T. Zuo, Z. Tang, M.C. Gao, K.A. Dahmen, P.K. Liaw, and Z.P. Lu: Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 61, 1 (2011).
S. Guo, Q. Hu, C. Ng, and C.T. Liu: More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 41, 96 (2013).
Y. Zhang, X. Yang, and P.K. Liaw: Alloy design and properties optimization of high entropy alloys. JOM 64, 830 (2012).
R.R. Chen, G. Qin, H.T. Zheng, L. Wang, Y.Q. Su, Y.L. Chiu, H.S. Ding, J.J. Guo, and H.Z. Fu: Composition design of high entropy alloys using the valence electron concentration to balance strength and ductility. Acta Mater. 144, 129 (2018).
G. Qin, W.T. Xue, C.L. Fan, R.R. Chen, L. Wang, Y.Q. Su, H.S. Ding, and J.J. Guo: Effect of Co content on phase formation and mechanical properties of (AlCoCrFeNi)100−xCox high-entropy alloys. Mater. Sci. Eng., A 710, 200 (2018).
G. Qin, S. Wang, R.R. Chen, X. Gong, L. Wang, Y.Q. Su, J.J. Guo, and H.Z. Fu: Microstructures and mechanical properties of Nb-alloyed CoCrCuFeNi high-entropy alloys. J. Mater. Sci. Technol. 34, 365 (2018).
R. Feng, M.C. Gao, C. Lee, M. Mathes, T.T. Zuo, S.Y. Chen, J.A. Hawk, Y. Zhang, and P.K. Liaw: Design of light-weight high-entropy alloys. Entropy 18, 333 (2016).
Y. Zhang, Z.P. Lu, S.G. Ma, P.K. Liaw, Z. Tang, Y.Q. Cheng, and M.C. Gao: Guidelines in predicting phase formation of high-entropy alloys. MRS Commun. 42, 57 (2014).
B. Chanda and J. Das: Composition dependence on the evolution of nanoeutectic in CoCrFeNiNbx (0.45 ≤ x ≤ 0.65) high entropy alloys. Adv. Eng. Mater. 20, 1700908 (2018).
Acknowledgment
This work was supported by the Fund of State Key Laboratory of Advanced Welding and Joining and National Natural Science Foundation of China (No. 51741404).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, G., Li, Z., Chen, R. et al. CoCrFeMnNi high-entropy alloys reinforced with Laves phase by adding Nb and Ti elements. Journal of Materials Research 34, 1011–1020 (2019). https://doi.org/10.1557/jmr.2018.468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2018.468