Abstract
ZrO2:Eu3+ hollow spheres were successfully fabricated with the resin microspheres as the template. The sample characterizations were carried out by means of x-ray diffraction (XRD), scanning electron microscope (SEM), and photoluminescence spectra. XRD results revealed that Eu3+-doped samples were pure t-ZrO2 phase after being calcined at 873 K. SEM results exhibited that this Eu3+ doped ZrO2 was hollow spheres; the diameter and thickness of which were about 450 and 50 nm, respectively. Upon excitation at 394 nm, the orange-red emission bands at the wave length longer than 570 nm were from 5D0 → 7FJ (J = 1, 2) transitions. The asymmetry ratio of (5D0 → 7F2)/(5D0 → 7F1) intensity is about 1.61, 1.26, 1.42, 1.42, 1.40, and 1.38 for the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. These values suggest that the asymmetry ratio of Eu3+ ions is independent of the doping concentration. The optimal doping concentration of Eu3+ ions in ZrO2 is 1.5 mol%. According to Dexter’s theory, the critical distance between Eu3+ ions for energy transfer was determined to be 16 Å.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Rare earth (RE) ion-doped materials have drawn great attention due to their significant technological importance and are used as solid state lighting, medical labeling, imaging, radiation detection, and other functional compounds based on their optical, electronic, and chemical characteristics.1–6 The macroscopic properties of phosphors, such as the emission spectrum or the luminous efficiency, rely on their composition, crystal structure, and morphology.7–12 Extensive work has been devoted to the development of effective morphology-controlled methods to synthesize functional materials with complex three-dimensional (3D) hierarchical architectures, such as Fe2O3 flower-like microspheres,13 urchin-like WO3 and MnWO4 microstructures,14,15 cactus-like β-Ga2O3 microarchitectures,16 dandelion-like ZnO and CuO architectures,17,18 3D hierarchical MWO4 (M = Mn, Bi, Ba, and Pb), and MMoO4 (M = Fe, Pb, and Ba) superstructures,9,19–23 which have been fabricated from low dimensional nanobuilding units by bottom-up approaches. Since sphere morphology of phosphor is favorable for high brightness and high resolution, spherical structured particles have been interestingly studied in these years.16–18
The selection of host materials for RE ions is crucial to obtain large luminescence signals.14 Materials with low phonon energies present small nonradiative losses owing to the multiphonon relaxation, and consequently, the luminescence efficiency is enhanced. A suitable host must also present large optical band gap along with good solubility and stability. Zirconia is a suitable host for RE material because it offers a large transparency window from the short ultraviolet to the near infrared frequencies and has excellent mechanical properties and good chemical stability.15 Generally speaking, the luminescence properties of phosphors strongly depend on the physical properties, such as specific surface area, crystallinity, particle size, size distribution, and morphology, of their host materials. To understand the detailed factors controlling the luminescence and to find the better ways to enhance the photoluminescence (PL) property, for instance, a lot of efforts related to photoluminescence of nanometer zirconia powders based on different synthetic methods have been made.24–27 To the best of our knowledge, there are few reports related to Eu3+-doped ZrO2 hollow nanospheres, which were exploited for photoluminescence properties.
Herein, we provide a facile and effective route for shape-controlled synthesis of ZrO2:Eu3+ hollow spheres with the help of phenolic resin microspheres as template. The luminescence properties were studied in detail and PL spectra indicated that the ZrO2:Eu3+ particles presented excellent orange-red emission at 614 nm and excited at 394 nm. The effects of doped content and critical distance of Eu3+ ions in the ZrO2 have been systematically explored.
II. EXPERIMENTAL
A. Synthesis of hollow sphere ZrO2:Eu3+
All the reagents are of the analytical grade. The phenolic resin microspheres with diameter around 400 nm were synthesized according to literature.28–30 As shown in Scheme 1, a typical synthesis of the hollow sphere ZrO2:Eu3+ with 500 nm diameter was synthesized as following procedures. 6.7 mmol of ZrClO2·8H2O aqueous solution (20 mL) was mixed with a solution (20 mL) containing 0.78 mmol of Y(NO3)3·6H2O, 1.6 mmol of citric acid, and 0.2 g of the template, phenolic resin microspheres. Subsequently, different amounts of Eu(NO3)3·6H2O (0.4%, 0.7%, 1.0%, 1.5%, 2.0%, and 2.5 mol%) aqueous solution were added and continually stirred for 30 min, respectively. 0.03 g of polyvinylpyridine (PVP) and 0.2 g of urea were then added to the reaction solution and stirred for 5 h. The ZrO2/phenolic resin composite microsphere-doped Eu3+ was recovered by centrifugation and air-dried at 100 °C for 12 h. For the sintering, the powder was heated under air atmosphere to 600 °C with a heating rate of 1 °C min−1, then maintained at 600 °C for 2 h before measurements.
B. Characterization
The crystal structure of the samples was identified by x-ray diffractometer (Rigaku D/max-2400, Tokyo, Japan). Their diffraction patterns were obtained using Cu Kα radiation of wave length λ = 0.15418 nm. The morphology of the powder was recorded by a field-emission scanning electron microscope (SEM, S-4700; Hitachi, Tokyo, Japan). The room temperature PL, photoluminescence excitation (PLE) spectra, and decay curves of the products were measured by a fluorescence spectrophotometer (Hitachi F-4600, Tokyo, Japan) with a 450-W xenon lamp. In all experiments, both excitation and emission slits were 1.0 nm.
III. RESULTS AND DISCUSSION
A. Crystallization behavior and morphology
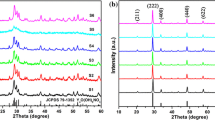
X-ray diffraction (XRD) analysis of ZrO2:Eu3+ products sintered at 873 K for 2 h was used to identify the sample phase and was shown in Fig. 1. The XRD pattern indicates that all the ZrO2:Eu3+ precursors underwent thermal transformation to ZrO2:Eu3+ samples and formed pure t-ZrO2 (JCPDS 50-1089) phase with good crystallization. No other phase was found because the template of the resin microspheres could be oxidized at high temperature.
The morphology of ZrO2:Eu3+ (1.5 mol%) powders prepared under the above mentioned conditions was studied by the SEM under different magnifications. Figure 2(a) shows that the templates, composed of phenolic resin microspheres, are regular spherical particles with uniform size about 400–500 nm. Figure 2(b) exhibited ZrO2-covered template spheres with slightly increased diameter of 500–600 nm. Seen from Figs. 2(c) and 2(d), after postheated at 873 K for 2 h, the as-synthesized ZrO2:Eu3+ was hollow sphere with the diameter and the thickness of about 450 nm and the 50 nm, respectively.
B. Luminescence properties of ZrO2:Eu3+
Due to the similarities in PLE and PL spectra of the samples with different Eu3+ concentrations, typical spectra of ZrO2 with 0.4 mol% Eu3+ content are shown in the Fig. 3. The PLE spectrum monitored at 614 nm presents a series of intraconfiguratioal 4f-4f transitions of Eu3+ as follows: 5D4(362 nm), 5G4(383 nm), and 5L6(394 nm) of Eu3+ ions, respectively.7 These transitions are followed by subsequent population decay to 5D0 luminescent excited state. With excitation at 394 nm, two main PL bands, associated with the 4f-4f internal orbital transitions of Eu3+ ions, are clearly resolved. They can be related to the radiative transitions from the 5D0 state to 7FJ (J = 1, 2) states of Eu3+ at 594 and 614 nm, respectively.
Figure 4 shows the PL spectra of ZrO2:Eu3+ synthesized at 873 K for 2 h with different doping concentrations of Eu3+ (0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%) and the comparison with pure ZrO2:Eu3+ powder. Upon excitation at 394 nm, the orange-red emission bands at the wave length longer than 570 nm are from 5D0 → 7FJ (J = 1, 2) transitions. The 5D0 → 7F1 band centered at 594 nm (orange) is a magnetic dipole transition and hardly varies with the crystal field strength around the Eu3+ ion. On the other hand, the hypersensitive transition 5D0 → 7F2 at 614 nm (red) is electric dipole allowed. Consequently, it depends on the local electric field, and hence, the local symmetry influences its strength. From these considerations, the (5D0 → 7F2)/(5D0 → 7F1) intensity ratio gives a measure of the degree of distortion from the inversion symmetry of Eu3+ ion local environment. The asymmetry ratio is about 1.61, 1.26, 1.42, 1.42, 1.40, and 1.38, which correspond to the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. These different values suggest that the asymmetry ratio of Eu3+ ions were independent of the doping concentration. In addition, it is evident that the emission intensity depends on the doping concentration. At low Eu3+ concentrations (χ < 1.5 mol%), the emission intensity is weak because there are no sufficient luminescence centers. However, under the condition of increasing Eu3+ concentration, the emission intensity increases and reaches the maximal value at 1.5 mol%. When the Eu3+ doping ratio is greater than 1.5 mol%, the luminescence intensity starts to reduce as concentration quenching occurs due to the decrease in Eu–Eu distance.20 Based on Dexter’s theory,21 the critical distance between Eu3+ ions for energy transfer can be calculated by the following relation:
where V is the volume of the unit cell, xc is the critical concentration of the doping ions, and Z is the number of host cation in the unit cell. For t-ZrO2, V = 66.7 Å3, Z = 2, and the critical concentration of Eu3+ in the ZrO2 host is 0.015. Therefore, the Rc of Eu3+ ions is determined to be 16 Å. What is more, the luminescence intensity of ZrO2:Eu3+ hollow sphere and pure ZrO2:Eu3+ powder was compared, which was shown in Fig. 4(b). Obviously, the luminescence intensity of ZrO2:Eu3+ hollow sphere was stronger than pure ZrO2:Eu3+ powder.
PL decay curves of ZrO2: Eu3+ with different concentrations of Eu3+ (0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%), shown in Fig. 5, were used to calculate the lifetime and investigate the luminescence dynamics of the samples. The samples were excited at 394 nm and monitored at 614 nm, and the curves were obtained with fluorescence spectrophotometer under Time Scan mode, and the lifetime values were calculated by the software of the spectrophotometer (Fluorescence Solutions for F-4600). It is found that the curves followed the single-exponential decay
where It is the intensity at time t, I0 is the intensity at t = 0, and τ is the decay lifetime. The fitted fluorescence lifetime values of ZrO2: Eu3+ are 3.43, 3.5, 3.42, 3.36, 3.45, and 3.35 ms corresponding to the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. The single-exponential decay curve reveals that these bands are not related to the defects of Eu host matrix, which is also confirmed by Podhorodecki.22
The color coordinates for the orange-red emission in the present experiment are calculated based on the corresponding PL spectra, and the results are shown in Fig. 6. The coordinates (x, y) of ZrO2:Eu3+ are (0.645, 0.354), (0.639, 0.361), (0.640, 0.360), (0.646, 0.353), (0.646, 0.353), and (0.647, 0.353), which correspond to the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. These results indicate that the as-obtained phosphors could show merits of orange-red emissions in the visible region when excited by a single wave length light, which might find potential applications in the fields such as light display systems and optoelectronic devices.23
IV. CONCLUSION
In summary, the controllable generation of ZrO2:Eu3+ hollow spherers has been successfully achieved with the resin microspheres as the template. The diameter and thickness of the samples were about 450 and 50 nm, respectively. Upon excitation at 394 nm, the orange-red emission bands at the wave length longer than 570 nm are from 5D0 → 7FJ (J = 1, 2) transitions. The asymmetry ratio is about 1.61, 1.26, 1.42, 1.42, 1.40, and 1.38, which correspond to the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. These different values suggest that the asymmetry ratio of Eu3+ ions was independent of the doping concentration. From the luminescence study, the optimum concentration of luminescence is found to be 1.5 mol%. The critical energy transfer distance of Eu3+ in t-ZrO2 host is calculated to be 16 Å. The single-exponential decay curves reveal that these bands do not relate to the defects of Eu host matrix. Because of the suitable excitation band, controllable morphology, good CIE chromaticity, and chemical stability, the luminescence materials may find potential applications in fields such as optoelectronic and nanoscale devices or biological technology.
References
K. Riwotzki, H. Meyssamy, and H. Schnablegger: Liquid-Phase Synthesis of Colloids and Redispersible Powders of Strongly Luminescing LaPO4−:Ce,Tb Nanocrystals., Angew. Angew. Chem. Int. Ed. 40, 573–576 (2001).
S. Sudhahar, M.K. Kumar, V. Jayaramakrishnan, R. Muralidharan, and R.M. Kumar: Effect of Sm+ Rare Earth Ion on the Structural, Thermal, Mechanical and Optical Properties of Potassium Hydrogen Phthalate Single Crystals. J. Mater. Sci. Technol. 30, 13–18 (2014).
W.X. Wang, Z.Y. Cheng, and P.P. Yang: Patterning of YVO4:Eu3+ Luminescent Films by Soft Lithography. Adv. Funct. Mater. 21, 456–463 (2011).
E.H. Penilla, Y. Kodera, and J.E. Garay: Blue–Green Emission in Terbium-Doped Alumina (Tb:Al2O3) Transparent Ceramics. Adv. Funct. Mater. 23, 6036–6043 (2013).
J. Joo, T. Yu, and Y.W. Kim: Multigram scale synthesis and characterization of monodisperse tetragonal zirconia nanocrystals. J. Am. Chem. Soc. 125, 6553–6557 (2003).
X.J. Liang, B.F. Liu, and N. Chen: Growth and Photoluminescence Properties of Tetrapod-Shaped ZnO Microcrystals-Whiskers and Microrods. J. Mater. Sci. Technol. 25, 427–432 (2009).
D.G. Liu and Z.F. Zhu: Photoluminescence properties of the Eu-doped alpha-Al2O3 microspheres. J. Alloys Compd. 583, 291–294 (2014).
M.M. Rahman, M.K.R. Khan, M.R. Islam, M.A. Halim, M. Shahjahan, M.A. Hakim, D.K. Saha, and J.U. Khan: Effect of Al Doping on Structural, Electrical, Optical and Photoluminescence Properties of Nano-Structural ZnO Thin Films. J. Mater. Sci. Technol. 28, 329–335 (2012).
D.G. Liu: Effects of Cr content and morphology on the luminescence properties of the Cr-doped alpha-Al2O3 powders. Ceram. Int. 39, 4765–4769 (2013).
D. Boyer and R. Mahiou: Powders and Coatings of LiYF4:Eu3+ Obtained via an Original Way Based on the Sol–Gel Process. Chem. Mater. 16, 2518–2521 (2004).
Z. Deng, X.D. Pi, J.J. Zhao, and D. Yang: Photoluminescence from Silicon Nanocrystals in Encapsulating Materials. J. Mater. Sci. Technol. 29, 221–224 (2013).
C.J. Jia, L.D. Sun, and L.P. You: Selective Synthesis of Monazite- and Zircon-type LaVO4 Nanocrystals. J. Phys. Chem. B 109, 3284–3290 (2005).
Q. Chen, Y.Q. Chang, C.J. Shao, J. Zhang, J. Chen, M.W. Wang, and Y. Long: Effect of Grain Size on Phase Transformation and Photoluminescence Property of the Nanocrystalline ZrO2 Powders Prepared by Sol-Gel Method. J. Mater. Sci. Technol. 30, 1103–1107 (2014).
L.X. Yu, H.W. Song, and S.Z. Lu: Luminescent Properties of LaPO4:Eu Nanoparticles and Nanowires. J. Phys. Chem. B 108, 16697–16702 (2004).
N. Rakov and G.S. Maciel: Enhancement of luminescence efficiency of f–f transitions from Tb3+ due to energy transfer from Ce3+ in Al2O3 crystalline ceramic powders prepared by low temperature direct combustion synthesis. Chem. Phys. Lett. 400, 553–557 (2004).
Q.H. Fan, P. Li, Z. Zheng, W.S. Wu, and C.L. Liu: Insights into sorption species of Eu(III) on γ-Al2O3 and bentonite under different pH: Studies at macro- and micro-scales. J. Radioanal. Nucl. Chem. 299, 1767–1775 (2014).
X.Y. Xu, Y.X. Liu, Z. Lv, J.Q. Song, M.Y. He, Q. Wang, L.J. Yan, and Z.F. Li: Thermal study in Eu3+-doped boehmite nanofibers and luminescence properties of the corresponding Eu3+:Al2O3. J. Therm. Anal. Calorim. 118, 1585–1592 (2014).
G.G. Li, C. Peng, and C.X. Li: Shape-controllable synthesis and morphology-dependent luminescence properties of GaOOH:Dy3+ and beta-Ga2O3:Dy3+. Inorg. Chem. 49, 1449–1457 (2010).
G. Jia, Y.J. Huang, and Y.H. Song: Controllable Synthesis and Luminescence Properties of La(OH)3 and La(OH)3:Tb3+Nanocrystals with Multiform Morphologies. Eur. J. Inorg. Chem. 2009, 3721–3726 (2009).
G. Blasse and B.C. Grabmaier: Luminescent Materials (Springer, Berlin, 1994).
G. Blasse: Energy Transfer in Oxidic Phosphors. Philips Res. Rep. 24, 131–143 (1969).
A. Podhorodecki, M. Banski, J. Misiewicz, and J. Serafińczuk: Influence of Annealing on Excitation of Terbium Luminescence in YAlO3 Films Deposited onto Porous Anodic Alumina Semiconductor Devices, Materials, and Processing. J. Electrochem. Soc. 157, H628 (2010).
N.V. Gaponenko, I.S. Molchan, O.V. Sergeev, and G.E. Thompson: Enhancement of Green Terbium-Related Photoluminescence from Highly Doped Microporous Alumina Xerogels in Mesoporous Anodic Aluminasensors and Displays: Principles, Materials, and Processing. J. Electrochem. Soc. 149, H49 (2002).
Q. Li, D. Ai, X. Dai, and J. Wang: Photoluminescence of nanometer zirconia powders. Powder Technol. 137, 34–40 (2003).
W. Du, Z. Zhu, X. Zhang, D. Wang, D. Liu, X. Qian, and J. Du: RE/ZrO2 (RE = Sm, Eu) composite oxide nano-materials: Synthesis and applications in photocatalysis. Mater. Res. Bull. 48, 3735–3742 (2013).
R. Marin, G. Sponchia, E. Zucchetta, P. Riello, G. De Portu, F. Enrichi, and A. Benedetti: Monitoring the t → m Martensitic Phase Transformation by Photoluminescence Emission in Eu3+-Doped Zirconia Powders. J. Am. Ceram. Soc. 96, 2628–2635 (2013).
X. Gao, D. He, H. Jiao, J. Chen, and X. Meng: Fabrication of SiO2@ZrO2@Y2O3:Eu3+ Core-Multi-Shell Structured Phosphor. J. Nanosci. Nanotechnol. 8, 6963–6968 (2011).
J. Liu, S. Qiao, H. Liu, J. Chen, A. Orpe, D. Zhao, and G. Lu: Extension of the Stöber Method to the Preparation of Monodisperse Resorcinol–Formaldehyde Resin Polymer and Carbon Spheres. Angew. Chem. 123, 6069–6073 (2011).
D. Chen, J. Liu, P. Wang, L. Zhang, J. Ren, F. Tang, and W. Wu: Fabrication of monodisperse zirconia-coated core–shell and hollow spheres in mixed solvents. Colloids Surf., A 302, 461–466 (2007).
F. Lin, W. Dong, C. Liu, Z. Liu, and M. Li: In situ source–template-interface reaction route to hollow ZrO2 microspheres with mesoporous shells. J. Colloid Interface Sci. 323, 365–371 (2008).
ACKNOWLEDGMENT
This work was financially supported by the National Natural Science Foundation of China under Grant Nos. 21404084 and 51532003, the foundation of State Key Laboratory for Modification of Chemical Fibers and Polymer Materials (Donghua University), the Fundamental Research Funds for the Central Universities under Grant No. B040307 and the funding from the State Key Laboratory of Solidification Processing in NWPU (Grant No. 107-QP-2014) and the 111 Project (B08040).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Co-first author
Rights and permissions
About this article
Cite this article
Zhang, M., Zuo, W., Zhu, M. et al. Synthesis and photoluminescence properties of Eu3+-doped ZrO2 hollow spheres. Journal of Materials Research 30, 3740–3745 (2015). https://doi.org/10.1557/jmr.2015.375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.375