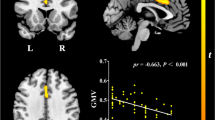
Abstract
Background
Cognitive reserve (CR) shows protective effects on cognitive function in older adult and in Alzheimer’s disease (AD). However, the brain mechanisms underlying the CR effect on the non-dementia AD spectrum (subjective cognitive decline (SCD) and mild cognitive impairment (MCI)) are unknown. The aim of this study was to investigate the potential moderate effect of CR on brain functional networks associated with cognitive performance.
Methods
We selected 200 participants, including 48 cognitively normal (CN) and 56 SCD, and 96 patients with MCI from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Seed-based locus coeruleus functional connectivity (LC FC) was conducted to detect early brain functional changes in the nondementia AD spectrum. CR was assessed via years of education and intelligence (IQ). The ANDI composite executive function scores (ADNI-EF) and ADNI composite memory scores (ANDI-MEM) at baseline and 24-month follow-up were used to assess cognitive performance.
Results
Compared to the CN group, the SCD group showed abnormal LC FC with the executive control network (dorsolateral prefrontal cortex, DLPFC), salience network, sensorimotor network, reward network, and hippocampus, while these alterations were inverted at the MCI stage. The LC-hippocampus FC was correlated with ADNI-MEM at baseline and follow-up, and these relationships were moderated by education. The LC-DLPFC FC was correlated with ADNI-EF at baseline, and this association was moderated by IQ.
Conclusion
Our results manifested that higher levels of CR would confer protective effects on SCD and MCI. Furthermore, IQ and education could moderate the relationship between LC FC and cognition through different pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive reserve (CR), is defined as the capacity to function quite well cognitively despite the presence of neuropathy, e.g., in Alzheimer’s disease (AD) (1, 2). CR is thought to be bestowed by more advanced mental capacities like IQ or long-term life experiences like education (3). According to the CR hypothesis, a person’s unique processing style for tasks acts as a buffer against brain dysfunction (3). Evidence from epidemiologic research suggesting that proxy measures of CR, such as IQ and years of formal education, are related to a lower risk of dementia reinforces the CR hypothesis (4, 5). However, the brain mechanisms underlying CR are not well understood.
Recently, an increasing number of neuroimaging studies have used tau and amyloid PET, structural MRI, task-related functional MRI (fMRI), and resting-state fMRI to investigate the neural substrates of CR in mild cognitive impairment (MCI) and AD (6–10). For example, Franzmeier et al. discovered that left frontal cortex global functional connectivity (FC) and frontal cortex connectivity with memory networks were critical in the CR-related protective effect of memory function in normal aging and MCI (9, 11). Using a graph theory analysis approach, Lee et al. found that the right middle-temporal pole was a pivotal neural substrate for CR in the AD spectrum, while the precentral gyrus was associated with CR in normal aging (8). However, whether and how CR affects cognitive function in the preclinical stage, particularly in cognitively normal older adults with subjective cognitive decline (SCD), has not yet been well explored.
The SCD represents older adults with subjective reports of cognitive changes but unimpaired performance on cognitive tests. SCD is considered to be at an increased risk of developing AD in the future before significant cognitive decline (12) and might even be the first symptomatic manifestation of AD (13). As previous MRI studies have shown circumscribed structural and functional brain changes in SCD (for review, see (14)), smaller hippocampal volume, thinner entorhinal cortex, and an abnormal default mode network (DMN) were frequently reported in individuals with SCD (15). The question here is whether the level of CR moderates the effects of early brain function changes on cognitive performance in SCD and MCI.
The locus coeruleus (LC) is considered to be the “ground zero” (the first region to develop neurofibrillary tangles) and serves a critical role in the pathogenesis of AD (especially the tau pathology) (16). In addition, the CR proxy of education has shown a significant moderating effect on tau pathology in AD patients (17). According to the noradrenergic theory of CR (18), the continuous upregulation of the noradrenergic system throughout the life-time can be a key component of CR. Importantly, LC produces the majority of the brain norepinephrine. Recently neuroimaging (19) and post-mortem studies (20) found that the LC structure (volume and density) was associated with cognitive function decline, and suggest the LC as a key mediator underpinning the cognitive reserve. However, it remains unclear how CR influences the LC functional networks in individuals with SCD and MCI, and how these alterations are related to cognitive performance.
The present study aims to fill this research gap by investigating the role of CR in the relationship between LC functional alterations and cognitive performance specifically in the non-dementia AD spectrum (including SCD and MCI). We used the resting-state functional MRI data to constructed LC-based whole-brain functional connectivity to assess the early functional network changes that underpin preclinical AD. Subsequently, we investigated the moderate effect of CR proxies (IQ or years of education) on the association between altered LC FC and cognition performance in the non-dementia AD spectrum. As previous neuropsychological results suggest that CR may be more connected with executive function and working memory (21, 22), we hypothesized that CR would moderate the association between altered LC FC and executive function performance in the non-dementia AD spectrum.
Methods
Participants
Data for this study were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) after a data request application was approved in August 2022. ADNI is a 63-site longitudinal study in the United States and Canada, directed by Principal Investigator Michael W. Weiner, MD, that began in 2003 as a public-private partnership. The primary goal of ADNI is to investigate whether the evolution of MCI and early AD can be tracked using serial MRI, PET, other biomarkers, and clinical and neuropsychological tests. The ADNI study was authorized by the institutional review boards of all participating institutions (http://www.adni-info.org/pdfs/adni_protocol_9_19_08.pdf)). Before the study began, all ADNI participants provided written informed consent. A comparable SCD group in the ADNI dataset was participants with significant memory concerns (SMC) (23). Thus, we used the SMC group as the SCD group. All ADNI participants’ inclusion and exclusion criteria can be found at http://www.adniinfo.org. The following criteria were used to identify subjects for this study: Caucasian, 3 Tesla MRI scanners with resting-state fMRI and 3D T1-weighted MRI data.
The diagnoses of SCD and MCI were based on the guidelines in the ADNI protocol. The following criteria were applied for inclusion of SCD participants: 1) a significant subjective memory concern expressed by the participant, study partner, or clinician; 2) a Cognitive Change Index score (CCI-S) greater than or equal to 16; 3) evidence of normal memory function demonstrated by performance above education-adjusted cutoffs on the Logical Memory II Subscale (above 9 for 16 years of education); and 4) a Clinical Dementia Rating (CDR) of 0. For MCI participants must have: 1) a memory complaint or a memory problem noted by their partner; 2) a specified education-adjusted cutoff score on Logical Memory (Mini Mental State Examination score between 24 and 30, a CDR score of 0.5, and a Memory Box score must be at least 0.5); 3) relatively preserving instrumental activities of daily living. Detailed inclusion and exclusion criteria can be found at https://adni.loni.usc.edu/wp-content/themes/freshnews-dev-v2/documents/clinical/ADNI-2_Protocol.pdf. 210 subjects met our criteria for inclusion, but 10 were removed due to inadequate scan signals on rs-fMRI images (N = 5) or excessive head motion (translations > 3 mm or rotation > 2°) (N = 5). The study was then performed on the remaining 200 subjects, which included 48 CN seniors, 56 SCD elders, and 96 MCI patients.
Neuropsychological assessment
A series of neuropsychological tests were conducted in the ADNI dataset. In the present study, we used the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) to assess general cognition. To improve statistical power, we used the ADNI global cognitive composite score of memory (ADNI-MEM) to measure memory performance and the composite score of executive function (ADNI-EF) to measure EF performance (24–26). The ADNI-EF is a standardized composite score based on a Clock Drawing Task (CDT), Animal and Vegetable Category Fluency Test (VFT), Trail-making Task (TMT), Digit Span Backwards (DS-B), and Digit-Symbol Substitution Task (DSST), while the ADNI-MEN is a standardized composite score based on the Alzheimer’s Disease Assessment Scale-cognitive (ADAS-Cog) subscale word list learning task, the Rey Auditory Verbal Learning Test (RAVLT), Logical Memory (LM), and the MMSE recall task. Changes in ADNI-MEM and ADNI-EF scores over 24 months of follow-up were used to measure changes in cognitive ability for each participant.
Cognitive reserve assessment
The most commonly used proxy of CR is years of education, and more education has been thought to be linked to a lower risk of dementia. In addition, reading ability and vocabulary tend to be more strongly associated with dementia risk than years of education (27). In the ADNI dataset, the American National Adult Reading Test (ANART) (28) was used to obtain the premorbid verbal intelligence quotient (IQ) for all participants at the baseline enrollment stage (29). Thus, we used both years of education and ANART as proxies for CR in the next data analysis.
MRI data acquisition
All rs-fMRIs were performed using an echo-planar imaging pulse sequence in a high-resolution 3 Tesla MRI scanner with an eight-channel head coil. The rs-fMRI parameters were as follows: repetition time = 3000 ms, echo time = 30 ms, flip angle = 80°, acquisition matrix = 64 × 64, field of view = 240 × 240 mm, thickness = 3.3 mm, gap = 0 mm, and the number of slices = 48. A total of 140 image volumes were acquired. In addition, 3D T1 MRI images were obtained using a magnetization-prepared gradient echo sequence with a spatial resolution of 1 × 1 × 1.2 mm3. Intracranial volume (ICV) was used as a covariate for the LC FC network analysis. A detailed list of MRI protocols can be found at http://adni.loni.usc.edu/methods/documents/mri-protocols/.
Rs-fMRI data preprocessing
The rs-fMRI data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and DPABI 6.0 (Data Processing & Analysis of Brain Imaging; http://rfmri.org/dpabi) implemented in MATLAB version 8.0 (The MathWorks, Inc., Natick, MA, USA) (30). Voxel-based morphometry (VBM) in SPM12 was used to segment the structural images and co-register with the functional images. The fMRI images underwent the following preprocessing: first five volumes discarded, slice timing, realigned, spatially normalized, resampled (3 × 3 × 3 mm3 cubic voxels), low-pass filtered (0.01–0.1Hz), and detrended. Subsequently, the 24 motion parameters, white matter signal, and cerebrospinal fluid (CSF) signals were removed from the data by linear regression. The global signal was not regressed given the controversy regarding its application to rs-fMRI data (31, 32). We also estimated the frame-wise displacement (FD), which depicts the misalignment of the head position with respect to the volume (33). Between the groups, there was no significant difference in FD (p > 0.05).
LC functional network construction
Using DPABI, a bilateral, LC-based, voxel-wise FC analysis was performed. For the construction of the LC FC network, the left and right LC were selected from Automated Anatomical Labeling Atlas 3 (AAL3) and used as the seed regions, respectively (34). The average time course of each LC region was correlated with the time course in all brain voxels using Pearson’s cross correlation, and the correlation coefficients were calculated with a Fisher’s Z-transformation to reach a normal distribution (35, 36).
Statistical analysis
First, a one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test was performed to compare the demographic information and neuropsychological tests among the three groups. Chi-square (χ2) tests were used for categorical data (gender, Aβ status, and APOE status). Statistical analysis was performed using IBM SPSS 24.0. The statistical significance level was set at 0.05.
Second, a voxel-wise, one-way analysis of covariance (ANCOVA) was used to obtain group differences in the LC FC network after controlling the effects of age, gender, education, Aβ status, APOE genotype, and ICV. Significance thresholds were set at P-values < 0.005 at the voxel level and P-values < 0.01 at the cluster level, with voxel sizes above 50, with the Gaussian random field (GRF) correction method for multiple comparisons. FC values for brain regions differing among groups were extracted, and a post hoc analysis was conducted by LSD test.
Third, partial correlation analysis was used to explore the potential associations between CR proxies, cognitive performance (ADNI-MEM and ADNI-EF), and LC FC alterations, controlling for sex, age, Aβ status, APOE status, and ICV in the non-dementia AD spectrum (pooled SMC and MCI groups). The correlation analysis also calculated in the SMC and MCI group separately. As the LC-hippocampus FC and LC-DLPFC FC were associated with cognitive performance, we just explored the altered LC FC in the hippocampus and DLPFC in the correlation analyses and the next moderation analyses.
Last, as a significant association between the CR, neuropsychological tests, and altered LC FC was found in the no-dementia AD spectrum group (see Results), a simple linear moderation model was conducted (PROCESS Marco version 2.16.3 in SPSS, Model 1) to address the moderation effect of CR on the association between LC FC and cognitive performance (37, 38). In this model, CR proxies (years of education, ANART) were entered as moderator (W), LC FC strengths (LC-hippocampus and LC-DLPFC) as independent variable (X), cognitive performance as dependent variable (Y), and age, gender, APOE status, Aβ status, and ICV were set as covariates, respectively. All models included a bootstrap sample of 5000 with a bias-corrected bootstrap confidence interval (CI). When a significant moderate effect was detected, the Johnson-Neyman’s region of significance approach (floodlight analysis) was conducted to detect significant interactions (39).
Results
Participants characteristics
Full demographic characteristics and clinical assessments, including APOE status, Aβ+ status, and cognitive performance for all participants, are illustrated in Table 1 and Table S1. In the present ADNI sample, there were no significant differences in age, gender, CR (years of education and verbal IQ), APOE status, and ICV among the three groups. The MCI group had a higher percentage of Aβ+ and poorer cognitive performance than the other two groups (all p < 0.05). The CN and SCD groups had no significant differences in Aβ+ ratio or cognitive performance.
Group differences in the LC functional network
For the left LC FC network, group differences were found in the left insula, superior frontal gyrus (SFG), temporal pole (TP), lateral orbital frontal cortex (lOFC), hippocampus, and right supplemental motor area (SMA) (Table 2 and Figure 1A). Post-hoc analysis revealed that the SCD group had significantly altered left LC FC in all seven brain regions when compared to the CN and MCI groups (Figure 2). While the MCI group showed significantly altered left LC FC in the insula, SFG, TP, and hippocampus compared to the CN group. No significant differences in SMA, caudate, and lOFC were found between the CN and MCI groups.
A, Left LC FC network; B, Right LC FC network. Abbreviations: DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; LC FC, locus coeruleus functional connectivity; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex; SFG, superior frontal gyrus; SMA, supplemental motor area; TP, temporal pole.
Abbreviations: CN, cognitively normal; DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; MCI, mild cognitive impairment; LC FC, locus coeruleus functional connectivity; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex; SCD, subjective cognitive decline; SFG, superior frontal gyrus; SMA, supplemental motor area; TP, temporal pole.
For the right LC FC network, group differences were found in the left dorsolateral prefrontal cortex (DLPFC) and bilateral medial orbital frontal cortex (mOFC) (Table 2 and Figure 1B). Post-hoc analysis revealed that when compared with the CN and MCI groups, the SCD group had higher right LC FC in the left DLPFC and lower right LC FC in the bilateral mOFC (Figure 2). In addition, the MCI group showed higher right LC FC in the left DLPFC than that in the CN group.
Taken together, the post hoc analyses revealed the U-shape and inverted U-shape trajectories curve of the LC FC alteration among the CN, SCD, and MCI groups (Figure 2).
The relationships between CR, cognition performance, and altered LC FC in the nondementia AD spectrum
For the non-dementia AD spectrum (pooled SCD and MCI groups, Table S2), partial correlation analyses showed that education was positively associated with EF performance at baseline (Figure 3A, r = 0.321, p = 1.84*10-4), whereas ANART scores were negatively associated with EF performance at 24 months follow-up (Figure 3C, r = −0.219, p = 0.012). There was no significant association between education and ANART scores (Figure 3E, r = −0.044, p = 0.595). Altered LC FC in the hippocampus was positively associated with MEM performance at baseline and follow-up (Figure 3F, baseline, r = 0.261, p = 0.004; Figure 3G, follow-up, r = 0.235, p = 0.009). Altered LC FC in the DLPFC was positively associated with EF performance at baseline (Figure 3H, r = 0.196, p = 0.025). No other significant associations were found between CR, cognition performance, and altered LC FC in the nondementia AD spectrum.
Abbreviations: ADNI-EF, ADNI composite executive function score; ADNI-MEN, ADNI composite memory score; ANART, American national adult reading test; CR, cognitive reserve; DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; FC, functional connectivity; LC, locus coeruleus; MCI, mild cognitive impairment; mOFC, medial orbitofrontal cortex; SCD, subjective cognitive decline.
Stratified by disease stage, for the SCD group (Table S3), education was not significantly associated with EF (Figure 3B, r = −0.039, p = 0.817), while ANART scores were negatively associated with EF performance in follow-up (Figure 3D, r = −0.337, p = 0.019). The altered LC FC in the DLPFC was positively associated with EF performance at baseline in the SCD group (r = 0.393, p = 0.015). For the MCI group (Table S4), education was associated with EF at baseline (Figure 3B, r = 0419, p = 1.10*10–4), and ANART was not significantly associated with EF at follow-up (Figure 3D, r = -0.160, p = 0.157). The altered LC FC in the hippocampus was positively associated with ANART scores in the MCI group (r = 0.316, p = 0.018). No other significant association was found in the MCI group.
The moderator role of CR in the associations between LC FC and cognitive performance
As illustrated in Table 3 and Figure 4, for the nondementia AD spectrum group, the interactive effect of LC-DLPFC FC and ANART scores on the EF composite scores at baseline was significant (t = 2.352, B = 0.399, SE = 0.16, p = 0.020). Floodlight analysis (JN approach) found that the moderate effect was significant when the ANART scores were higher than 14.67. The interactive effects of LC-Hippocampus FC and education on baseline and 24-month follow-up MEM performance across the non-dementia AD spectrum were significant (baseline, t = −2.026, B = 0.637, SE = 0.31, p = 0.048; follow-up, t = −2.044, B = −0.20, SE = 0.09, p = 0.042), with moderate effects significant when education was below 14.74 (baseline) and 16.36 (follow-up).
Abbreviations: ADNI-EF, ADNI composite executive function score; ADNI-MEN, ADNI composite memory score; CR, cognitive reserve; DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; FC, functional connectivity; IQ, intelligence quotient; LC, locus coeruleus.
Stratified by disease stage, for the SCD stage, the moderate effect of ANART on the association between LC-DLPFC FC and EF performance was also significant (t = 2.689, B = 0.429, SE = 0.16, p = 0.009), and the moderate effect was significant when the ARANT scores were above 20.91. For the MCI stage, this moderate effect was not significant. Subgroup analysis revealed no significant moderate effect of education on MEM performance in the SCD and MCI groups.
Discussion
The current study sought to identify the moderate role of CR in the relationships between early brain functional alteration and cognitive performance in older adults with SCD and MCI patients. First, we found that the trajectories of LC FC alteration among CN, SCD, and MCI were not linear. In particular, decreased and increased LC FC were found in the SCD group; however, these LC FC alterations were inverted in the MCI stage. Second, the LC-hippocampus FC values were associated with MEM performance, while the LC-DLPFC value was associated with EF performance in the non-dementia AD spectrum and the SCD stage only. Third, the CR proxy of education had a significant moderating effect on the association between LC-hippocampus FC and MEM performance at baseline and 24-month follow-up in the non-dementia AD spectrum. The CR proxy of IQ (ANART scores) showed a moderate effect on the relationships between LC-DLPFC FC and EF performance in the non-dementia AD spectrum and the SCD group only. These results suggest that higher levels of CR would confer protective effects on SCD and MCI. Furthermore, IQ and education could moderate the association between LC FC and cognition through different pathways.
The LC FC characteristic in the non-dementia AD spectrum
Our finding manifested that older individuals with SCD present early alteration in the LC FC network. Previous studies of LC in the AD spectrum, mainly using structural MRI, included LC signal intensity and LC-contrast ratios, and found significantly lower signal intensity and contrast in patients with AD but not in MCI (40). The LC-contrast ratios were found to be associated with general cognition performance (MMSE) in AD patients (41) and multiple cognitive domains (episodic memory, general verbal fluency, and information processing speed) in MCI patients (42). LC structure was negatively associated with the CSF amyloid levels in the AD spectrum, predominantly in the CN and MCI stages, suggesting a protective effect of the LC noradrenergic system in preclinical AD (40). Recently, interrupted functional connectivity of the LC has been investigated in individuals with neuropsychiatric disorders (43–46), as well as in healthy adults with a parental history of AD (47). As far as we know, the LC FC characteristics of SCD and MCI have not been explored.
Here, our results indicated a nonlinear curve (U-shape and inverted U-shape) of the trajectories of LC FC configuration change across the AD spectrum. Specifically, the SCD group showed significant alterations (both increased and decreased) in LC FC when compared with the CN group, but these alterations in the SCD group were inverted during the MCI stage. The results are consistent with studies evaluating the quadratic age effect on the LC FC change from childhood to older adults (aged between 8 and 83 years) (48). Herein, using LC-based FC analysis, both increased and decreased alterations were found in the SCD group. Decreased LC FCs were located in the hippocampus and reward network (caudate and OFC) (49, 50), while increased LC FCs were located in the ECN (DLPFC and SFG) (51), salience network (SN, TP, and insula) (51), and sensorimotor network (SMA) (52). Interestingly, the decreased regions of the LC network were mainly located in the early Braak tau stage of AD pathology, whereas the increased regions of the LC network were mainly located in the late Braak tau stage in the SCD group (17, 53). Thus, we speculated that the LC function presented a compensatory mechanism in the SCD stage while presenting a maladaptation mechanism in the MCI stage.
The relationships among CR, altered LC FC, and cognition performance
Furthermore, we found that in the non-dementia AD spectrum, altered LC-hippocampus FC was significantly correlated with MEM performance at baseline and 24-month follow-up, while altered LC-DLPFC FC was significantly correlated with EF performance at baseline. The hippocampus, a core brain area for learning and memory, is particularly vulnerable to damage in the early stages of AD and is dysfunctional in MCI (54). Previous studies have shown associations between LC integrity and memory performance in healthy older adults (55). Additionally, the hippocampus is one of the earliest regions to accumulate tau pathology in AD (53), and the noradrenergic neurotransmission from the LC to the hippocampus is known to facilitate spatial learning and memory (56). According to our findings, LC-hippocampus FC is not only associated with baseline but also 24-months follow-up memory performance within non-dementia AD spectrum. Therefore, we postulate that the disrupted LC-hippocampus connectivity may represent an early pathological pathway and server as a potential biomarker for detecting the memory stability in the non-dementia stage of AD.
On the other hand, the DLPFC, a critical hub for executive function performance in the ECN, is affected relatively late in the tau pathology of AD (17, 51). Age-related changes in the frontal cortex are mainly correlated with executive function, and the decline of EF influences the strategic, controlled processing at memory encoding and retrieval (57). Our results demonstrate an association between higher LC-DLPFC FC and better EF performance in the individuals with non-dementia AD and SCD. However, we did not observe and association between LC-DLPFC and EF performance at the 24-months follow-up. These findings suggest that the executive function may play a compensatory role during the SCD stage, while LC-DLPFC FC might not be associated with preclinical AD progression.
Previous longitudinal studies of aging and preclinical AD have found that CR was associated with cognitive performance but not with the rate of change in cognition (58) and that SCD with higher CR had a lower risk of conversion to MCI (59). In the present study, a significant relationship between CR proxies and EF performance was manifested in the non-dementia AD spectrum. Interestingly, subgroup analyses revealed that IQ was significantly associated with EF performance at the 24-month follow-up in the SCD stage, while education was significantly associated with EF performance at baseline in the MCI stage. Altered executive function is considered to be a common consequence of normal aging and AD (57). In the ADNI dataset, the composite score of EF performed as well or better than all other measures of EF in detecting changes over time and was the only measure significantly associated with the frontal cortex thickness (26). The findings of various CR proxy’s association with EF performance in different stages of the AD spectrum may point to a protective role of education at baseline as well as a predictive role of IQ in the future. As ADNI-MEM is a better predictor for the conversion from MCI to AD (24), the absence of a significant association between CR and MEM performance might indicate an indirect role for CR in memory performance in the non-dementia AD spectrum.
The moderate role of CR on LC FC associated cognition performance
The results of moderation analyses verified our hypothesis that CR proxies moderate the relationship between LC-ECN FC and executive function performance in the non-dementia AD spectrum. The results showed that the CR proxy of verbal IQ (ANART scores), but not education, showed a modest effect on the relationship between LC-DLPFC FC and EF performance in the AD spectrum. In addition, we found that the CR proxy of education had a significant moderating effect on the association between LC-hippocampus FC and MEM performance at baseline and 24-month follow-up in the non-dementia AD spectrum. These results were consistent with previous evidence that CR proxies differently moderate the negative effects of AD pathology on cognition (10). Previous postmortem and PET studies have manifested a moderating effect of education on the association between Aβ deposition and cognitive decline (60, 61). Another study using PET images found that IQ moderated the association between tau deposition and cognition but not Aβ status in a combined group of CN, MCI, and AD (62), and no significant modifying effect was found in the CN group only. Previous studies, including several functional connectivity analyses, reported a modulatory effect of CR proxies on memory performance related to the brain’s functional network in the MCI group (6, 9, 11). The current study demonstrated that CR plays a moderator role in both MEM and EF performance in the non-dementia AD spectrum. Subgroup analysis also found a similar moderate effect of IQ on LC FC associated EF performance in the SCD group. The current results in the SCD and MCI groups suggest that dysfunctional LC FC is associated with poor cognitive performance, which would be alleviated by different CR proxies. Higher levels of education and IQ confer protective effects on the non-dementia AD spectrum by moderating LC associated memory and executive function via various LC functional pathways.
Limitations
Nevertheless, several limitations need to be mentioned. First, only two CR proxies (education and IQ) were used in this study to explore the moderate effects of CR on cognition related to altered brain function. Future studies should explore the moderate effects of other CR proxies on brain function associated cognition, such as occupational complexity and lifetime cognition activity (1). Second, we did not explore the structural features of LC as the traditional structural MRI scan parameter of the ADNI dataset (41). Future studies using neuromelanin-sensitive MRI protocols could extend the knowledge of the moderate role of CR on LC structure features and cognition (41). Finally, since the current study was an exploratory one, we did not strictly account for the probability of increased type I error due to multiple comparisons in the correlation analyses. Further research is therefore required to support these findings, requiring a larger sample size and a stricter statistical significance criterion.
Conclusion
In summary, the present study found abnormal LC FC in the non-dementia AD spectrum. The LC-hippocampus FC was associated with memory performance and moderated by education level, whereas the LC-DLPFC FC was associated with executive function and moderated by IQ level in the non-dementia AD spectrum. These findings provide crucial support for the CR hypothesis and enrich the notion that higher CR is beneficial for cognitive performance via various LC functional pathways in the pre-dementia stage of AD.
References
Stern Y.: Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol, 2012;11(11), 1006–12, doi:https://doi.org/10.1016/s1474-4422(12)70191-6
Stern Y., Arenaza-Urquijo E. M., Bartrés-Faz D., Belleville S., Cantilon M., Chetelat G., et al.: Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement, 2020;16(9), 1305–1311, doi:https://doi.org/10.1016/j.jalz.2018.07.219
Stern Y.: Cognitive reserve. Neuropsychologia, 2009;47(10), 2015–2028, doi: https://doi.org/10.1016/j.neuropsychologia.2009.03.004
Esiri M. M. and Chance S. A.: Cognitive reserve, cortical plasticity and resistance to Alzheimer’s disease. Alzheimer’s Research & Therapy, 2012;4(2), 7, doi:https://doi.org/10.1186/alzrt105
Arenaza-Urquijo E. M., Wirth M. and Chételat G.: Cognitive reserve and lifestyle: moving towards preclinical Alzheimer’s disease. Front Aging Neurosci, 2015;7, 134, doi:https://doi.org/10.3389/fnagi.2015.00134
Franzmeier N., Göttler J., Grimmer T., Drzezga A., Áraque-Caballero M. A., Simon-Vermot L., et al.: Resting-State Connectivity of the Left Frontal Cortex to the Default Mode and Dorsal Attention Network Supports Reserve in Mild Cognitive Impairment. Frontiers in Aging Neuroscience, 2017;9, doi:https://doi.org/10.3389/fnagi.2017.00264
Chételat G.: Multimodal Neuroimaging in Alzheimer’s Disease: Early Diagnosis, Physiopathological Mechanisms, and Impact of Lifestyle. J Alzheimers Dis, 2018;64(s1), S199–s211, doi:https://doi.org/10.3233/jad-179920
Lee D. H., Lee P., Seo S. W., Roh J. H., Oh M., Oh J. S., et al.: Neural substrates of cognitive reserve in Alzheimer’s disease spectrum and normal aging. NeuroImage, 2019;186, 690–702, doi:https://doi.org/10.1016/j.neuroimage.2018.11.053
Franzmeier N., Duering M., Weiner M., Dichgans M. and Ewers M.: Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology, 2017;88(11), 1054–1061, doi:https://doi.org/10.1212/wnl.0000000000003711
Ko K., Yi D., Byun M. S., Lee J. H., Jeon S. Y., Kim W. J., et al.: Cognitive reserve proxies, Alzheimer pathologies, and cognition. Neurobiol Aging, 2022;110, 88–95, doi:https://doi.org/10.1016/j.neurobiolaging.2021.10.005
Franzmeier N., Buerger K., Teipel S., Stern Y., Dichgans M. and Ewers M.: Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol Aging, 2017;50, 152–162, doi:https://doi.org/10.1016/j.neurobiolaging.2016.11.013
Caselli R. J., Chen K., Locke D. E., Lee W., Roontiva A., Bandy D., et al.: Subjective cognitive decline: self and informant comparisons. Alzheimers Dement, 2014;10(1), 93–8, doi:https://doi.org/10.1016/j.jalz.2013.01.003
Jessen F., Amariglio R. E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G., et al.: A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement, 2014;10(6), 844–52, doi:https://doi.org/10.1016/j.jalz.2014.01.001
Wang X., Huang W., Su L., Xing Y., Jessen F., Sun Y., et al.: Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol Neurodegener, 2020;15(1), 55, doi:10.1186/s13024-020-00395-3
Parker A. F., Smart C. M., Scarapicchia V. and Gawryluk J. R.: Identification of Earlier Biomarkers for Alzheimer’s Disease: A Multimodal Neuroimaging Study of Individuals with Subjective Cognitive Decline. J Alzheimers Dis, 2020;77(3), 1067–1076, doi:https://doi.org/10.3233/jad-200299
Braak H., Thal D. R., Ghebremedhin E. and Del Tredici K.: Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol, 2011;70(11), 960–9, doi:10.1097/NEN.0b013e318232a379
Hoenig M. C., Bischof G. N., Hammes J., Faber J., Fliessbach K., van Eimeren T., et al.: Tau pathology and cognitive reserve in Alzheimer’s disease. Neurobiology of Aging, 2017;57, 1–7, doi:https://doi.org/10.1016/j.neurobiolaging.2017.05.004
Robertson I. H.: A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol Aging, 2013;34(1), 298–308, doi:https://doi.org/10.1016/j.neurobiolaging.2012.05.019
Plini E. R. G., O’Hanlon E., Boyle R., Sibilia F., Rikhye G., Kenney J., et al.: Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells, 2021;10(7), doi:https://doi.org/10.3390/cells10071829
Wilson R. S., Nag S., Boyle P. A., Hizel L. P., Yu L., Buchman A. S., et al.: Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology, 2013;80(13), 1202–8, doi:https://doi.org/10.1212/WNL.0b013e3182897103
Siedlecki K. L., Stern Y., Reuben A., Sacco R. L., Elkind M. S. and Wright C. B.: Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. J Int Neuropsychol Soc, 2009;15(4), 558–69, doi:10.1017/s1355617709090857
Lojo-Seoane C., Facal D., Guàrdia-Olmos J., Pereiro A. X. and Juncos-Rabadán O.: Effects of Cognitive Reserve on Cognitive Performance in a Follow-Up Study in Older Adults With Subjective Cognitive Complaints. The Role of Working Memory. Front Aging Neurosci, 2018;10, 189 doi:https://doi.org/10.3389/fnagi.2018.00189
Swinford C. G., Risacher S. L., Charil A., Schwarz A. J. and Saykin A. J.: Memory concerns in the early Alzheimer’s disease prodrome: Regional association with tau deposition. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 2018;10, 322–331, doi:https://doi.org/10.1016/j.dadm.2018.03.001
Crane P. K., Carle A., Gibbons L. E., Insel P., Mackin R. S., Gross A., et al.: Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging & Behavior, 2012;6(4), 502–516, doi:https://doi.org/10.1007/s11682-012-9186-z
Choi S.-E., Mukherjee S., Gibbons L. E., Sanders R. E., Jones R. N., Tommet D., et al.: Development and validation of language and visuospatial composite scores in ADNI. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2020;6(1), e12072, doi:https://doi.org/10.1002/trc2.12072
Gibbons L. E., Carle A. C., Mackin R. S., Harvey D., Mukherjee S., Insel P., et al.: A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging & Behavior, 2012;6(4), 517–527, doi:https://doi.org/10.1007/s11682-012-9176-1
Pettigrew C. and Soldan A.: Defining Cognitive Reserve and Implications for Cognitive Aging. Current Neurology and Neuroscience Reports, 2019;19(1), 1, doi:https://doi.org/10.1007/s11910-019-0917-z
Bright P., Jaldow E. and Kopelman M. D.: The National Adult Reading Test as a measure of premorbid intelligence: A comparison with estimates derived from demographic variables. Journal of the International Neuropsychological Society, 2002;8(6), 847–854,, doi:https://doi.org/10.1017/S1355617702860131
Franzmeier N., Buerger K., Teipel S., Stern Y., Dichgans M. and Ewers M.: Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiology of Aging, 2017;50, 152–162, doi:https://doi.org/10.1016/j.neurobiolaging.2016.11.013
Yan C. G., Wang X. D., Zuo X. N. and Zang Y. F.: DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics, 2016;14(3), 339–51, doi:https://doi.org/10.1007/s12021-016-9299-4
Chai X. J., Castañón A. N., Öngür D. and Whitfield-Gabrieli S.: Anticorrelations in resting state networks without global signal regression. Neuroimage, 2012;59(2), 1420–1428, doi: https://doi.org/10.1016/j.neuroimage.2011.08.048.
Murphy K., Birn R. M., Handwerker D. A., Jones T. B. and Bandettini P. A.: The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 2009;44(3), 893–905, doi: https://doi.org/10.1016/j.neuroimage.2008.09.036
Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L. and Petersen S. E.: Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage, 2013;76, 439–41, doi:https://doi.org/10.1016/j.neuroimage.2012.03.017
Rolls E. T., Huang C. C., Lin C. P., Feng J. and Joliot M.: Automated anatomical labelling atlas 3. Neuroimage, 2020;206, 116189, doi:https://doi.org/10.1016/j.neuroimage.2019.116189
Lowe M. J., Mock B. J. and Sorenson J. A.: Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage, 1998;7(2), 119–32, doi:https://doi.org/10.1006/nimg.1997.0315
Liu F., Wang Y., Li M., Wang W., Li R., Zhang Z., et al.: Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Human brain mapping, 2017;38(2), 957–973
Hayes A. F.: Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. The Guilford Press., New York, 2013
Gong L., Xu R., Lan L., Liu D., Shen J., Zhang B., et al.: The CD33 genotype associated cognitive performance was bidirectionally modulated by intrinsic functional connectivity in the Alzheimer’s disease spectrum. Biomedicine & Pharmacotherapy, 2019;115(7), doi: https://doi.org/10.1016/j.biopha.2019.108903
Bauer D. J. and Curran P. J.: Probing Interactions in Fixed and Multilevel Regression: Inferential and Graphical Techniques. Multivariate Behavioral Research, 2005;40(3), 373–400, doi:https://doi.org/10.1207/s15327906mbr4003_5
Betts M. J., Cardenas-Blanco A., Kanowski M., Spottke A., Teipel S. J., Kilimann I., et al.: Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aβ levels. Alzheimers Dement (Amst), 2019;11, 281–285, doi:https://doi.org/10.1016/j.dadm.2019.02.001
Hou R., Beardmore R., Holmes C., Osmond C. and Darekar A.: A case-control study of the locus coeruleus degeneration in Alzheimer’s disease. Eur Neuropsychopharmacol, 2021;43, 153–159, doi:https://doi.org/10.1016/j.euroneuro.2020.12.013
Elman J. A., Puckett O. K., Beck A., Fennema-Notestine C., Cross L. K., Dale A. M., et al.: MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimers Dement, 2021;17(6), 1017–1025, doi:https://doi.org/10.1002/alz.12261
Gong L., Shi M., Wang J., Xu R., Yu S., Liu D., et al.: The Abnormal Functional Connectivity in the Locus Coeruleus-Norepinephrine System Associated With Anxiety Symptom in Chronic Insomnia Disorder. Frontiers in Neuroscience, 2021;15, 678465, doi:https://doi.org/10.3389/fnins.2021.678465
Liu J., Tao J., Xia R., Li M., Huang M., Li S., et al.: Mind-Body Exercise Modulates Locus Coeruleus and Ventral Tegmental Area Functional Connectivity in Individuals With Mild Cognitive Impairment. Front Aging Neurosci, 2021;13, 646807, doi:https://doi.org/10.3389/fnagi.2021.646807
Huang Y., Yu S., Wilson G., Park J., Cheng M., Kong X., et al.: Altered Extended Locus Coeruleus and Ventral Tegmental Area Networks in Boys with Autism Spectrum Disorders: A Resting-State Functional Connectivity Study. Neuropsychiatr Dis Treat, 2021;17, 1207–1216, doi:https://doi.org/10.2147/ndt.S301106
McCall J. G., Siuda E. R., Bhatti D. L., Lawson L. A., McElligott Z. A., Stuber G. D., et al.: Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife, 2017;6, doi:https://doi.org/10.7554/eLife.18247
Del Cerro I., Villarreal M. F., Abulafia C., Duarte-Abritta B., Sánchez S. M., Castro M. N., et al.: Disrupted functional connectivity of the locus coeruleus in healthy adults with parental history of Alzheimer’s disease. J Psychiatr Res, 2020;123, 81–88, doi:https://doi.org/10.1016/j.jpsychires.2020.01.018
Song I., Neal J. and Lee T. H.: Age-Related Intrinsic Functional Connectivity Changes of Locus Coeruleus from Childhood to Older Adults. Brain Sci, 2021;11(11), doi:https://doi.org/10.3390/brainsci11111485
Belfi A. M. and Loui P.: Musical anhedonia and rewards of music listening: current advances and a proposed model. Ann N Y Acad Sci, 2020;1464(1), 99–114, doi:https://doi.org/10.1111/nyas.14241
Wang D., Belden A., Hanser S. B., Geddes M. R. and Loui P.: Resting-State Connectivity of Auditory and Reward Systems in Alzheimer’s Disease and Mild Cognitive Impairment. Front Hum Neurosci, 2020;14, 280, doi:https://doi.org/10.3389/fnhum.2020.00280
Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., et al.: Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 2007;27(9), 2349–56, doi:https://doi.org/10.1523/jneurosci.5587-06.2007
Vahdat S., Darainy M., Milner T. E. and Ostry D. J.: Functionally specific changes in resting-state sensorimotor networks after motor learning. Journal of Neuroscience, 2011;31(47), 16907–16915, doi: https://doi.org/10.1523/JNEUROSCI.2737-11.2011.
Schöll M., Lockhart Samuel N., Schonhaut Daniel R., O’Neil James P., Janabi M., Ossenkoppele R., et al.: PET Imaging of Tau Deposition in the Aging Human Brain. Neuron, 2016;89(5), 971–982, doi:https://doi.org/10.1016/j.neuron.2016.01.028
Mu Y. and Gage F. H.: Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener, 2011; 6, 85, doi:https://doi.org/10.1186/1750-1326-6-85
Dahl M. J., Mather M., Düzel S., Bodammer N. C., Lindenberger U., Kühn S., et al.: Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nature Human Behaviour, 2019;3(11), 1203–1214, doi:https://doi.org/10.1038/s41562-019-0715-2
Kempadoo K. A., Mosharov E. V., Choi S. J., Sulzer D. and Kandel E. R.: Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A, 2016;113(51), 14835–14840, doi:https://doi.org/10.1073/pnas.1616515114
Buckner R. L.: Memory and Executive Function in Aging and AD: Multiple Factors that Cause Decline and Reserve Factors that Compensate. Neuron, 2004;44(1), 195–208, doi:https://doi.org/10.1016/j.neuron.2004.09.006
Soldan A., Pettigrew C., Cai Q., Wang J., Wang M. C., Moghekar A., et al.: Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging, 2017;60, 164–172, doi:https://doi.org/10.1016/j.neurobiolaging.2017.09.002
Bessi V., Mazzeo S., Padiglioni S., Piccini C., Nacmias B., Sorbi S., et al.: From Subjective Cognitive Decline to Alzheimer’s Disease: The Predictive Role of Neuropsychological Assessment, Personality Traits, and Cognitive Reserve. A 7-Year Follow-Up Study. J Alzheimers Dis, 2018;63(4), 1523–1535, doi:https://doi.org/10.3233/jad-171180
Roe C. M., Mintun M. A., D’Angelo G., Xiong C., Grant E. A. and Morris J. C.: Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of neurology, 2008;65(11), 1467–1471, doi: https://doi.org/10.1001/archneur.65.11.1467.
Bennett D., Schneider J., Wilson R., Bienias J. and Arnold S.: Education modifies the association of amyloid but not tangles with cognitive function. Neurology, 2005;65(6), 953–955, doi: https://doi.org/10.1212/01.wnl.0000176286.17192.69
Rentz D. M., Mormino E. C., Papp K. V., Betensky R. A., Sperling R. A. and Johnson K. A.: Cognitive resilience in clinical and preclinical Alzheimer’s disease: the Association of Amyloid and Tau Burden on cognitive performance. Brain Imaging & Behavior, 2017;11(2), 383–390, doi:https://doi.org/10.1007/s11682-016-9640-4
Acknowledgements
The study was obtained by the ADNI investigators (http://www.adni-info.org/pdfs/adni_protocol_9_19_08.pdf).
Funding
Fundings: This study supported by the funding below: Chengdu Medical Research Project (2022161), Basic and clinical cooperative research Program of the Anhui Medical University-incubation project for the Third Affiliated Hospital (2022sfy001), Clinical Medical Research Transformation Project of Anhui Provincial Science and Technology Department (202204295107020024).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest: The authors declare no conflicts of interest.
Ethics declarations: The studies involving human participants were reviewed and approved by the Ethical approval was obtained by the ADNI investigators. The patients/participants provided their written informed consent to participate in this study.
Electronic supplementary material
42414_2023_268_MOESM1_ESM.docx
Higher cognitive reserve is beneficial for cognitive performance via various locus coeruleus functional pathways in the pre-dementia stage of Alzheimer’s disease
Rights and permissions
About this article
Cite this article
Gong, L., Chen, K., Zhang, H. et al. Higher Cognitive Reserve Is Beneficial for Cognitive Performance Via Various Locus Coeruleus Functional Pathways in the Pre-Dementia Stage of Alzheimer’s Disease. J Prev Alzheimers Dis 11, 484–494 (2024). https://doi.org/10.14283/jpad.2023.127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.127