Abstract
Apolipoprotein E (APOE) ε4 allele is the best established genetic risk factor for sporadic Alzheimer’s disease (AD). However, there is a need to understand the effects of this genotype on the brain by simultaneously assessing intrinsic brain network and cerebral spinal fluid (CSF) biomarkers changes in healthy older ε4 carriers. Thirteen cognitively intact, elderly APOE ε4 carriers and 22 ε3 homozygotes were included in the present study. Eigenvector centrality mapping (ECM) was used to identify brain network hub organization based on resting-state functional MRI (rsfMRI). We evaluated comprehensive cognitive ability and tested levels of Aβ1–42, total-tau (t-tau) and phosphorylated-tau (p-tau181) in CSF. Comparisons of ECM between two groups were conducted, followed by correlations analyses between EC values with significant group differences and cognitive ability/CSF biomarkers. APOE ε4 carriers showed significantly decreased EC values in left medial temporal lobe (MTL), left lingual gyrus (LG) and increased EC values in left middle frontal gyrus (MFG) as compared to non-carriers. Correlation analysis demonstrated that left LG EC value correlated with Rey Auditory Verbal Learning Test total learning (RAVLT, r = 0.57, p < 0.05) and t-tau level (r = −0.57, p < 0.05), while left MFG EC values correlated with log-transformed Trail-Making Test B (TMT-B, r = −0.67, p < 0.05) in APOE ε4 carriers. This study suggests the APOE ε4 allele contributes to disruption of brain connectedness in certain functional nodes, which may result from neuronal death caused by toxicity of neurofibrillary tangles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia and is clinically characterized by memory loss and cognitive decline. The essential neuropathologic alteration of AD is extracellular β-amyloid deposits and intraneuronal neurofibrillary tangles (Braak and Braak 1997). Multiple risk factors contribute to pathogenesis of AD. The apolipoprotein E (apoE, protein; APOE, gene) allele is the strongest genetic risk factor for sporadic AD - which account for 40 % of cases (Corder et al. 2004; Rocchi et al. 2003; Strittmatter et al. 1993) - and epidemiologic studies have shown that APOE ε4 carriers are prone to develop AD (Corder et al. 1993; Reinvang et al. 2013). Presently, the specific role of APOE ε4 allele in the pathogenesis of AD is complex and remains unclear. Studies in animal models have demonstrated that the APOE ε4 allele might confer increased risk for AD via promoting tau hyperphosphorylation or reduction of beta-amyloid clearance (Jiang et al. 2008; Teter et al. 2002). The well-established association between APOE ε4 allele and AD suggests that assessing brain changes in healthy APOE ε4 carriers may enrich our understanding of the pathogenesis of this disease.
Resting-state functional magnetic resonance imaging (rsfMRI) is widely used to detect APOE ε4 allele-related effects on brain. Currently, most published connectivity pattern studies deal with two fundamental approaches: seed-based correlation analysis and independent component analysis (ICA) (Cole et al. 2010). By setting seed region in bilateral precuneus (PCu), Sheline and colleagues noted that ε4 carriers have decreased functional connectivity in MTL and dorsal anterior cingulate regions (Sheline et al. 2010). Using a posterior cingulate seed, Machulda et al. reported posterior default mode network (DMN) of ε4 carriers have decreased connectivity (Machulda et al. 2011); Based on ICA, increased hippocampal DMN synchronization has been observed in elderly ε4 carriers (Westlye et al. 2011). To date, most studies evaluating the effects of APOE genotype have needed to select pre-defined brain regions or subnetwork (e.g., number of independent component) to conduct networks analysis, which may result in a selection bias. Moreover, none of the previous studies had simultaneously assessed brain functional network and AD-related pathology (e.g., CSF β-amyloid1–42 and tau levels), which can help for understanding pathophysiologic substrates of APOE related effects.
Graph theory-based network analysis has increasingly been used as a theoretical framework to describe brain network characteristics. More specifically, centrality, a kind of graph theory-based network measures estimating the centrality or functional importance, is receiving great interest from functional neuroimaging (Koschützki et al. 2005). Unlike seed-based correlation analysis or ICA, centrality measure consider the brain as one large network rather than dividing it into several subnetwork, and it do not rely on a priori knowledge. There is a variety of metrics for network centrality, each emphasizing different aspects of connectivity. Presently, centrality measures mainly consist of: 1) degree centrality, counting the number of edges directly attaching to the node, 2) closeness centrality, computing the average distance from a node to all other nodes, and differentiates between both central nodes and end nodes 3) betweenness centrality, computing the fraction of shortest paths that go through a node, 4) leverage centrality, considering the extent of connectivity of a node relative to the connectivity of its neighbors (Joyce et al. 2010) and 5) eigenvector centrality (EC), the sum of centralities of the node’s direct neighbors (Zuo et al. 2012; Binnewijzend et al. 2014; Lohmann et al. 2010; Wink et al. 2012).
Among these centrality measures, EC has gained the most attention due to Google’s well-known “PageRank” algorithm, which employs a variant of EC (Langville and Meyer 2011). The recursive definition of EC is computing a node’s centrality by adding the centralities of its neighbors, makes it able to identify different layers in the network hierarchy (Newman 2008; Binnewijzend et al. 2014). More importantly, when used as a metric index for intrinsic brain network, EC has proven to be easily computable from the connectivity matrix (e.g., much faster to compute than betweenness centrality and closeness centrality), and robust against global physiological effects (Lohmann et al. 2010). As with its success in the context of internet search engines, eigenvector centrality mapping (ECM) has proven valuable in analyzing various neurodegenerative diseases, such as AD and depressed Parkinson’s disease (Binnewijzend et al. 2014; Lou et al. 2015). These findings suggest that the specific pattern of brain interconnectedness disruption could reflect the functional consequences of pathologic damages in the associated disease.
Our goal is to explore APOE ε4-related effects on the brain network of healthy older APOE ε4 carriers and well-matched ε3 homozygotes using the model-free, data-driven approach, fECM (Wink et al. 2012). We also investigate whether changes of hub organization may relate with cognitive ability and AD pathology.
Materials and methods
Alzheimer’s disease Neuroimaging Initiative
Data set used in this study was obtained from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians in developing new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
Study participants
This study was approved by the Institutional Review Boards of all of the participating institutions, and informed written consent was obtained from all participants at each site. At the time of analysis, individuals carrying at least one APOE ε4 allele (genotype ε4/ε4 and ε4/ε3) were classified as APOE ε4 carriers, while individuals with genotype ε3/ε3 were classified as non-carriers (controls). Individuals with ε2 allele were excluded due to its possible protective effects (Suri et al. 2013). Using the ADNI GO and ADNI 2 databases, 40 right-handed cognitively intact healthy participants, comprised of 14 APOE ε4 carriers and 26 non-carriers, who had undergone structural scans, rsfMRI scans, and neuropsychological evaluation, were identified. Study data were downloaded from the ADNI publically available database prior to June 15, 2015. For the ADNI, to be classified as control normal (CN), the subject had an MMSE between 24 and 30 (inclusive), a clinical dementia rating (CDR) score of 0. The subjects were also required to meet distinctive cutoffs for Wechsler Memory Scale-Logical Memory (WMS-LM) delay score (in detail: ≥ 9 for subjects with 16 or more years of education; ≥ 5 for subjects with 8–15 years of education; and ≥3 for 0–7 years of education). Additionally, no signs of depression (geriatric depression scale, GDS score < 5) or dementia were present. All subjects were screened and excluded for a history of obvious head trauma, other neurological or major psychiatric disorder and alcohol/drug abuse. We excluded those individuals who are subjective memory complaint defined by ADNI database. After careful screening, one participant was excluded due to depression; one participant was excluded due to big calcification in the occipital lobe; one participant was excluded for excessive head motion (details later); two subjects were excluded due to damaged structural image. Table 1 presents the demographic data for the remaining 35 subjects.
Neuropsychological assessment and APOE genotyping
All neuropsychological assessment used in the current study were downloaded from the ADNI clinical data repository on the Laboratory of Neuro Imaging (LONI) site. Specifically, we evaluated participant performance on the Mini-Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT; including the total learning and delayed recall score), Weschler’s Logical Memory Scale (including Immediate and Delayed), Clock Drawing Test (CDT), Trail-Making Test A&B, Boston Naming Test (BNT), Semantic Verbal Fluency (SVF) and stroke/vascular incident history (Modified Hachinski Ischemic Scale, HIS). Table 1 presents the details of the neuropsychological performances of the subjects.
APOE genotyping for all participants were performed as previously described (Saykin et al. 2010). In brief, APOE genotyping for all subjects was performed using DNA extracted from peripheral blood cells. The cells were collected in 1 EDTA plastic tubes (10 ml) and sent by overnight delivery, at room temperature, to the University of Pennsylvania AD Biofluid Bank Laboratory.
CSF samples and quantification of β-amyloid and tau
CSF was downloaded from the LONI site. Levels of Aβ1–42, total tau (t-tau) and phosphorylated tau (p-tau181) were measured from CSF samples, which were obtained using the standardized ADNI protocol as previously described (Shaw et al. 2011). Meanwhile, participants with CSF levels outside 3 SDs above or below the mean were excluded. It should be noted that not all subjects in present study have CSF sample because lumbar puncture is an invasive procedure and not obligatory for healthy subjects. Thus, the final samples for CSF analyses included 12 out of 13 APOE ε4 carriers and 18 out of 22 non-carriers.
Data acquisition
Both the structural scans and rsfMRI scans were downloaded from the ADNI database. All participants were scanned using a 3.0-Tesla Philips MRI scanner. Structural images were acquired using a 3D MPRAGE T1-weighted sequence with the following parameters: repetition time (TR) =2300 ms; echo time (TE) = 2.98 ms; inversion time (TI) =900 ms; 170 sagittal slices; within plane FOV = 256 × 240 mm2; voxel size = 1.1 × 1.1 × 1.2 mm3; flip angle = 9°; bandwidth = 240 Hz/pix. The rsfMRI images were obtained using an echo-planar imaging sequence with the following parameters: 140 time points; TR = 3000 ms; TE = 30 ms; flip angle = 80°; number of slices = 48; slice thickness = 3.3 mm; spatial resolution = 3.31 × 3.31 × 3.31 mm3; matrix = 64 × 64.
Imaging preprocessing
Data preprocessing was performed using the Data Processing Assistant for Resting-state fMRI, DPASF (http://www.rfmri.org/DPARSF) (Yan and Zang 2010), which is based on the Statistical Parametric Mapping software (SPM8) package (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Trust Center for Neuroimaging, University College London, United Kingdom) and Resting-State fMRI Data Analysis Toolkit, REST (http://www.resting-fmri.sourceforge.net/) (Song et al. 2011). The first 10 image volumes of rsfMRI scans were discarded for the signal equilibrium and subject’s adaptation to the scanning noise. The remaining 130 images were corrected for timing differences between each slice and head motion (six- parameter rigid body). Data set with more than 2.0 mm maximum displacement in any of the x, y, or z directions or 2.0° of any angular motion was discarded. Subsequently, based on through rigid-body transformation, the T1-weighted image was co-registered to the mean rsfMRI image, and spatially normalized to the Montreal Neurological Institute (MNI) stereotactic space, then re-sampled into of 3 mm × 3 mm × 3 mm cubic voxels. The functional images were spatially smoothed with a Gaussian kernel of 6 × 6 × 6 mm3 full width at half maximum to decrease spatial noise. Finally, linear trends and temporally filter (0.01 Hz < f < 0.08 Hz) were performed. To remove any residual effects of motion and other non-neuronal factors, six head motion parameters, WM signal, cerebrospinal fluid signal were used as nuisance variables in the functional connectivity analysis. The output of these preprocessing steps was a 4D residual functional volume in native functional space, for each participant. These 4D native data was registered to the MNI152 space with 2 mm resolution based on the affine transformation. Given the disputation of removing the global signal in the pre-processing of rsfMRI data, we omitted regress the global signal out. Given that two groups may display differences in the occurrence of micromotions’ artifact, the framewise displacement (FD) values were computed for each subject.
It should be noted that the cerebellum was excluded from the present analysis due to insufficient scanning range of the MRI (incomplete cerebellum). This may not dramatically influence our results as the cerebellum is the area known to be relatively unaffected by AD-related pathological changes (Klunk et al. 2004; Li et al. 2015a, b).
ECM
ECM of the rsfMRI time series in MNI-space were performed using fast ECM (fECM) software (https://www.github.com/amwink/bias/tree/master/matlab/fastECM) (Wink et al. 2012), which yielded a voxel-wise measure of relevance to the functional brain network. ECM required the computation of the voxel-wise connectivity matrix to calculate its eigenvector. The fECM software is faster and computationally more efficient because it computes matrix-vector products without having to compute or store the connectivity matrix.
As the comparisons of networks with different size are problematic, it is necessary to restrict graph computations beforehand (van Wijk et al. 2010). Therefore, a group mask was first generated to include all voxels presented across all subjects in our study (in MNI 152 standard space). All subjects’ masks were multiplied to produce the group mask and the subjects’ network analyses were then constrained within the group mask at voxel level (Fig. 1). As ECM does not require thresholding or binarizing of the connectivity matrix, all individuals’ network had the same topology and size (Adriaanse et al. 2016).
Statistical analyses
Statistical analyses were performed using SPSS statistical software (version 19; SPSS, Inc., Chicago, IL). Descriptive statistics for variables were expressed as means ± SD. The TMT scores were log-transformed due to positively skewed distribution. Group differences were assessed using Student’s t-test and Chi-square tests. Two sample t-tests were used for sociodemographic continuous variables, including age, education, neuropsychological assessments and mean FD value. The Chi-square test was used for categorical variables, including sex and family history. Statistical significance was set at p < 0.05.
Given that EC follows a Wishart distribution (non-Gaussian distribution), nonparametric permutation tests (5000 permutations) were performed to detect statistically significant differences of ECM between APOE ε4 carriers and ε3 homozygotes (p < 0.01, corrected with a single voxel height of p < 0.05 and a cluster size >24 using the AFNI AlphaSim program (Beckmann and Smith 2003; Cox 1996)). The nonparametric permutation tests were performed by SnPM13 (http://warwick.ac.uk/snpm) (Nichols and Holmes 2002).
Results
Demographics, behavioral and micromotions data
An overview of demographic variables, neuropsychological performance and micromotions data is presented in Table 1. There were no significant differences in age, gender, or education between APOE ε4 carriers and non-carriers. Moreover, the gender ratio and family history of dementia among first-degree relatives were equally distributed between groups. Other than the higher semantic verbal fluency (SVF) observed in APOE ε4 carriers versus non-carriers (p < 0.05), no significant differences in other neuropsychological assessments were observed between groups. No significant differences were detected in CSF biomarkers between groups nor were any significant differences in mean FD value observed.
EC difference
The mean EC maps of APOE ε3 homozygotes and ε4 carriers are shown in Fig. 2, where warm colors and cool colors indicate high and low EC values, respectively. Visual inspection demonstrated that spatially separated brain areas exhibited high EC in two groups, such as the posterior cingulate cortex (PCC), adjacent PCu, occipital regions of the lingual gyrus (LG), calcarine and temporal regions of the superior temporal gyrus. Of note, the PCC/PCu had the highest EC values in both groups. Notably, mean EC value was higher in controls when compared to ε4 carriers. With regard to laterality, we observed that left hemisphere exhibited a generally higher EC value than the right in both two groups.
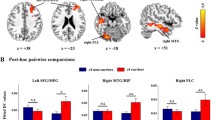
The ECM between-group analysis is shown in Fig. 3. MNI-coordinates and number of voxels of all clusters are shown in Table 2. APOE ε4 carriers demonstrated significantly decreased EC values in left MTL region (including left hippocampal/parahippocampal gyrus, HP/PHG) and left LG as compared to non-carriers; meanwhile, APOE ε4 carriers showed significantly increased EC values in left MFG.
a Group-wise ECM differences (APOE ε4 carriers VS. ε3 homozygotes). Cool areas represent clusters of EC value decreases in ε4 carriers when compared to ε3 homozygotes; Warm areas represent cluster of EC value increased inε4 carriers when compared to ε3 homozygotes (p < 0.01 and cluster size >24, corrected by Alphasim p < 0.05); b Box graph displays mean EC value for MTL (Red), LG (Pink) and MFG (Blue) in each group from left to right. Abbreviation: MTL, medial temporal lobe; LG, lingual gyrus; MFG, middle frontal gyrus
Correlation of EC with cognition and CSF biomarkers
Averaged EC values from each cluster with significant group differences were extracted. A one-sample KS test demonstrated these EC values were fitted with Gaussian distribution. Pearson’s correlation analyses were performed to examine the possible relationship between the EC values and cognitive ability/CSF biomarkers.
Across groups, no statistically significant correlation of mean EC values with behavioral data was observed. In ε4 carriers, mean EC in left LG was negatively related with t-tau level (r = −0.57, p < 0.05), mean EC value in LG was positively related with RAVLT total learning score (r = 0.57, p < 0.05). Following correction with HIS, sex, age, and family, correlation of mean EC in LG with t-tau level was still alive (r = −0.71, p < 0.05). Meanwhile, left MFG EC values was correlated with log-transformed TMT-B (r = −0.67, p < 0.05) in APOE ε4 carriers. The scatter diagram map is displayed in Fig. 4.
Figure 4 shows scatter plot association between a) mean EC value in left LG region and RAVLT total learning performance in ε4 carriers (r = 0.55, p < 0.05); b) mean EC value in left MFG and log-transformed TMT-B in ε4 carriers (r = −0.67, p < 0.05); c) mean EC value in left LG and t-tau level in ε4 carriers (r = −0.59, p < 0.05). Abbreviation: LG, lingual gyrus; MFG, middle frontal gyrus
Discussion
By assessing brain interconnectedness and CSF biomarkers simultaneously, the present study aimed to explore the effects of APOE ε4 on the brains of cognitively intact elderly populations. The main findings include: 1) ε4 carriers had significantly decreased functional connectivity in left MTL and left LG regions, and increased functional connectivity in left MFG as compared to the non-carriers. 2) Among ε4 carriers, there was significant correlation of EC in left LG with RAVLT, and with t-tau level.
Papez circuit, mainly including MTL, cingulate cortices and mammillary body, is a core pathway of the limbic system involved in the formation and consolidation of the episodic memory (Lech and Suchan 2013; Squire and Zola-Morgan 1991). The present study’s findings of significantly decreased brain interconnectedness in left MTL region among ε4 carriers are generally consistent with early neuropathologic outcome (Sparks 1997). Similarly, extensive structural studies of non-demented subjects have demonstrated that APOE ε4 allele is linked to smaller HP volume (Pievani et al. 2011), accelerated HP volume atrophy (Jak et al. 2007) and disruption of white matter integrity in HP or its projection tract (Salminen et al. 2013; Smith et al. 2010). Meanwhile, our results are consistent with most rsfMRI studies documenting the association between the ε4 allele and decreased functional connectivity or task-related reduced deactivation in MTL regions (Luo et al. 2016; Machulda et al. 2011; Persson et al. 2008; Sheline et al. 2010). Our results thus suggest memory-related functional hubs are disrupted in APOE ε4 carriers.
Using the same ADNI database, one previous study constructed structural cortical networks of normal elderly subjects using average gray matter volumes of each AAL area, and described communication hub regions involved in LG (Yao et al. 2010). LG is susceptible to AD-related neuropathology, and multiple neuroimaging investigations have reported MCI or AD patients have hyperactivity (Zhang et al. 2012), reduced grey matter density and disrupted white matter integrity in LG relative to controls (Braak et al. 2006; Zhuang et al. 2010). Moreover, one recent meta-analysis (including 36 task-based fMRI studies) reported that AD patients demonstrate hypoactivation in regions within the visual network relative to controls (Li et al. 2015a, b). On that basis, in AD high-risk subjects, our data further revealed a possible brain interconnectedness disruption in LG. Additionally, subsequent correlation analysis demonstrated that mean LG EC value was associated with RAVLT, which is widely used and generally reflects verbal memory function (Ricci et al. 2012). It is known that LG is involved in processing vision or dreaming rather than typically a primary target site for memory-related research (Bogousslavsky et al. 1987). However, LG anatomically possesses robust structural connections with PHG, which plays a key role in verbal memory (Cipolotti and Bird 2006; Mendoza and Foundas 2007). According to fMRI studies involving memory encoding, LG activation has also frequently been reported (Cho et al. 2012; Sulpizio et al. 2013). Thus, we hypothesize that brain interconnectedness of LG may contribute to verbal memory by means of regulating encoding strategies (e.g., using visual imagery or integrating visual tokens of the items into an imagistic representation (Huijbers et al. 2011; Leshikar et al. 2012)).
Another notable observation in our study is that APOE ε4 carriers had significantly increased brain interconnectedness in left MTG. Similarly, some recently meta-analyses of task-based fMRI studies have consistently reported the elderly, MCI individuals and AD patients recruit more activity than controls in prefrontal regions across memory encoding and retrieval tasks (Schwindt and Black 2009; Li et al. 2015a, b). Notably, our results are supported by prior rsfMRI studies using a similar cohort, which also reported increased functional connectivity of prefrontal cortex in aging APOE ε4 carriers relative to non-carriers (Machulda et al. 2011; Sheline et al. 2010; Song et al. 2015). Subsequent correlation analysis demonstrated that mean EC value in left MTG was significantly related with TMT-B in ε4 carriers, which generally reflecting executive function (Richeson et al. 2003; Reitan 1958). However, no differences in general cognitive ability or TMT-B between groups were found. Taken together, we hypothesize that APOE ε4 carriers may counteract possible cognitive declines by invoking additional network hub to strengthen brain interconnectedness; furthermore, frontal-executive cognitive processes predominantly mediate these compensatory mechanism (Han and Bondi 2008).
As for the observation that APOE ε4 carriers had significantly higher SVF scores than non-carriers, this result is consistent with the work of Alexander and colleagues (Alexander et al. 2007). Herein, relatively higher education level and younger age of APOE ε4 may account for this result. The antagonistic pleiotropy of ε4 allele (the carriers experience positive effects during their youth, despite being associated with a terminal illness in the elderly population), may also support our observation (Mondadori et al. 2007). It should be noted that subjects received various behavioral tests, however, only RAVLT and log-transformed TMT-B performance were significantly related with regional EC value with group differences. Two reasons may account for these findings: 1) All the subjects in our study are cognitively intact, and a relative narrow range of behavioral data may weaken the statistical power; 2) Small size of sample weakens the statistical power. Thus, further studies with larger sample sizes are urgently needed.
Previous neuropathologic studies have demonstrated that the left hemisphere could be more susceptible to AD-related neurodegeneration than the right (Janke et al. 2001). It is interesting to note that the differences of brain interconnectedness between groups including MTL, LG and MFG are all located in the left hemisphere. One longitudinal study reported, in ε4 carriers, HP volume decreased in the left side is faster than the right side (Jak et al. 2007). Furthermore, Donix and Burggren also observed that healthy middle aged or elderly ε4 carriers have greater volumetric declines over time in the left entorhinal cortex when compared with the right side (Donix et al. 2013). When considered in conjunction with results from animal studies that have documented the link between APOE ε4 and increased amyloid plaque production and tau tangle phosphorylation (Kim et al. 2009), we hypothesize that APOE ε4 allele may exacerbate lateral spatial pattern of AD-related neuropathology and contribute to left-sided disruption or compensation of brain interconnectedness.
Apart from neuroimaging biomarkers that could be associated with proposed clinical cascade of AD, CSF biomarkers including Aβ1–42, t-tau and p-tau181, are also effective candidates as they are intimately related to amyloid plaques, neuronal death and accumulation of tangles respectively (Blennow and Hampel 2003; Braak and Braak 1991). Early studies examining CSF have demonstrated that elevated concentrations of t-tau, p-tau181 and decreased concentrations of Aβ1–42 in AD or MCI patients relative to controls (for review, see (Babic et al. 2014)). Recently, some studies have reported that healthy ε4 carriers also have significantly decreased Aβ1–42 and elevated tau levels as compared to non-carriers (Randall et al. 2013; Sunderland et al. 2004). Although ε4 carriers in the present study have increased trend of t-tau/p-tau181 levels relative to non-carriers, no statistically significant differences were detected. The reason for discrepancies may be attributed to differences of in the subject populations. In the work of Sunderland (Sunderland et al. 2004), 113 out of 142 subjects had a first-degree relative with AD, which is a much higher rate than observed in our cohort (14 out of 35 subjects). It is known that family history risk of AD may reflect yet unknown genetic and non-genetic risks for AD (Donix et al. 2012).
As an exploratory analysis, we further examined the relationship between mean EC in regions with significant differences between groups and CSF biomarker. We found only mean LG EC was significantly related with t-tau level among ε4 carriers. After controlling HIS, sex, age, and family, correlation of mean EC in LG with t-tau level was still alive. These results are supported by neuropathological study of AD documenting selective distribution of tau aggregation initially starting in LG (Braak et al. 2006). We hypothesize that brain interconnectedness abnormalities of LG in ε4 carriers probably result from subtle elevated neurofibrillary tangles, whose toxicity could cause neuronal death. However, it should be noted that increased t-tau concentration merely reflects degree of neuronal degeneration and is not specific to AD (Randall et al. 2013). The APOE ε4-related effects on brain should not be interpreted in relation to AD alone. Unexpectedly, some recent studies have reported that APOE ε2 and ε4 allele exerts similar effect on brain structure and function (for review, see (Suri et al. 2013)). Therefore, it is possible that decreased brain interconnectedness in ε4 carriers simply is a reflection of a general change linked to susceptibility to future other neurological disease, but not specifically to AD.
Limitation
It is important to note that there exist several limitations with this exploratory study. This cross-sectional study is lacking clinical follow-up to make any possible inference between current findings and AD, and thus, longitudinal studies are needed to determine whether decreased brain interconnectedness within MTL and LG region is associated with a higher risk of developing AD-related pathological changes. Moreover, the sample we used was small with a relatively narrow APOE genotype and weak statistical power. Finally, not all subjects in present study have CSF sample because lumbar puncture is an invasive procedure; although CSF material is available of 92.3 % among APOE ε4 carriers, only 81 % non-carriers have it. These may have influenced statistical tests sensitivity. Future studies with larger sample sizes and wider APOE ε2 carriers are urgently required.
Conclusion
To the best of our knowledge, this is the first study using ECM to analyze APOE ε4-related effects on whole-brain functional connectivity in healthy elderly populations. We initially document that presence of APOE ε4 allele is linked to decreased brain interconnectedness in brain regions susceptible to AD-related pathology, including MTL and LG. Moreover, we observed a possible compensatory effect for ε4 allele in MFG. Subsequent correlation analyses suggest that functional changes of LG in APOE ε4 carriers may result from neuronal death caused by toxicity of neurofibrillary changes. Finally, we demonstrate that ECM is sensitive to APOE genotypic differences several years before the occurrence of dementia symptoms.
References
Adriaanse, S. M., Wink, A. M., Tijms, B. M., Ossenkoppele, R., Verfaillie, S. C., Lammertsma, A. A., Boellaard, R., Scheltens, P., van Berckel, B. N., & Barkhof, F. (2016). The Association of Glucose Metabolism and Eigenvector Centrality in Alzheimer’s disease. Brain Connectivity, 6, 1–8.
Alexander, D. M., Williams, L. M., Gatt, J. M., Dobson-Stone, C., Kuan, S. A., Todd, E. G., Schofield, P. R., Cooper, N. J., & Gordon, E. (2007). The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biological Psychology, 75, 229–238.
Babic, M., Svob, S. D., Muck-Seler, D., Pivac, N., Stanic, G., Hof, P. R., & Simic, G. (2014). Update on the core and developing cerebrospinal fluid biomarkers for Alzheimer disease. Croatian Medical Journal, 55, 347–365.
Beckmann, C. F., & Smith, S. M. (2003). Probabilistic ICA for FMRI-noise and inference. In Fourth Int. Symp. On Independent Component Analysis and Blind Signal Separation. ISBN (Vol. 695185812).
Binnewijzend, M. A., Adriaanse, S. M., Van der Flier, W. M., Teunissen, C. E., de Munck, J. C., Stam, C. J., Scheltens, P., van Berckel, B. N., Barkhof, F., & Wink, A. M. (2014). Brain network alterations in Alzheimer’s disease measured by eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Human Brain Mapping, 35, 2383–2393.
Blennow, K., & Hampel, H. (2003). CSF markers for incipient Alzheimer’s disease. Lancet Neurology, 2, 605–613.
Bogousslavsky, J., Miklossy, J., Deruaz, J. P., Assal, G., & Regli, F. (1987). Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. Journal of Neurology, Neurosurgery, and Psychiatry, 50, 607–614.
Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82, 239–259.
Braak, H., & Braak, E. (1997). Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiology of Aging, 18, S85–S88.
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H., & Del, T. K. (2006). Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica, 112, 389–404.
Cho, S., Metcalfe, A. W., Young, C. B., Ryali, S., Geary, D. C., & Menon, V. (2012). Hippocampal-prefrontal engagement and dynamic causal interactions in the maturation of children’s fact retrieval. Journal of Cognitive Neuroscience, 24, 1849–1866.
Cipolotti, L., & Bird, C. M. (2006). Amnesia and the hippocampus. Current Opinion in Neurology, 19, 593–598.
Cole, D. M., Smith, S. M., & Beckmann, C. F. (2010). Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience, 4, 8.
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L., & Pericak-Vance, M. A. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science, 261, 921–923.
Corder, E. H., Ghebremedhin, E., Taylor, M. G., Thal, D. R., Ohm, T. G., & Braak, H. (2004). The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Annals of the New York Academy of Sciences, 1019, 24–28.
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173.
Donix, M., Small, G. W., & Bookheimer, S. Y. (2012). Family history and APOE-4 genetic risk in Alzheimer’s disease. Neuropsychology Review, 22, 298–309.
Donix, M., Burggren, A. C., Scharf, M., Marschner, K., Suthana, N. A., Siddarth, P., Krupa, A. K., Jones, M., Martin-Harris, L., Ercoli, L. M., Miller, K. J., Werner, A., von Kummer, R., Sauer, C., Small, G. W., Holthoff, V. A., & Bookheimer, S. Y. (2013). APOE associated hemispheric asymmetry of entorhinal cortical thickness in aging and Alzheimer’s disease. Psychiatry Research, 214, 212–220.
Han, S. D., & Bondi, M. W. (2008). Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement, 4, 251–254.
Huijbers, W., Pennartz, C. M., Rubin, D. C., & Daselaar, S. M. (2011). Imagery and retrieval of auditory and visual information: neural correlates of successful and unsuccessful performance. Neuropsychologia, 49, 1730–1740.
Jak, A. J., Houston, W. S., Nagel, B. J., Corey-Bloom, J., & Bondi, M. W. (2007). Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dementia and Geriatric Cognitive Disorders, 23, 382–389.
Janke, A. L., de Zubicaray, G., Rose, S. E., Griffin, M., Chalk, J. B., & Galloway, G. J. (2001). 4D deformation modeling of cortical disease progression in Alzheimer’s dementia. Magnetic Resonance in Medicine, 46, 661–666.
Jiang, Q., Lee, C. Y., Mandrekar, S., Wilkinson, B., Cramer, P., Zelcer, N., Mann, K., Lamb, B., Willson, T. M., Collins, J. L., Richardson, J. C., Smith, J. D., Comery, T. A., Riddell, D., Holtzman, D. M., Tontonoz, P., & Landreth, G. E. (2008). ApoE promotes the proteolytic degradation of Abeta. Neuron, 58, 681–693.
Joyce, K. E., Laurienti, P. J., Burdette, J. H., & Hayasaka, S. (2010). A new measure of centrality for brain networks. PloS One, 5, e12200.
Kim, J., Basak, J. M., & Holtzman, D. M. (2009). The role of apolipoprotein E in Alzheimer’s disease. Neuron, 63, 287–303.
Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D. P., Bergstrom, M., Savitcheva, I., Huang, G. F., Estrada, S., Ausen, B., Debnath, M. L., Barletta, J., Price, J. C., Sandell, J., Lopresti, B. J., Wall, A., Koivisto, P., Antoni, G., Mathis, C. A., & Langstrom, B. (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Annals of Neurology, 55, 306–319.
Koschützki, D., Lehmann, K. A., Peeters, L., Richter, S., Tenfelde-Podehl, D., Zlotowski, O. (2005). Centrality indices. In Network analysis (pp. 16–61). Berlin Heidelberg: Springer.
Langville, A. N., & Meyer, C. D. (2011). Google’s PageRank and beyond: The science of search engine rankings. Princeton: Princeton University Press.
Lech, R. K., & Suchan, B. (2013). The medial temporal lobe: memory and beyond. Behavioural Brain Research, 254, 45–49.
Leshikar, E. D., Duarte, A., & Hertzog, C. (2012). Task-selective memory effects for successfully implemented encoding strategies. PloS One, 7, e38160.
Li, H. J., Hou, X. H., Liu, H. H., Yue, C. L., He, Y., & Zuo, X. N. (2015a). Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: a meta-analysis of 75 fMRI studies. Human Brain Mapping, 36, 1217–1232.
Li, H. J., Hou, X. H., Liu, H. H., Yue, C. L., Lu, G. M., & Zuo, X. N. (2015b). Putting age-related task activation into large-scale brain networks: a meta-analysis of 114 fMRI studies on healthy aging. Neuroscience and Biobehavioral Reviews, 57, 156–174.
Lohmann, G., Margulies, D. S., Horstmann, A., Pleger, B., Lepsien, J., Goldhahn, D., Schloegl, H., Stumvoll, M., Villringer, A., & Turner, R. (2010). Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PloS One, 5, e10232.
Lou, Y., Huang, P., Li, D., Cen, Z., Wang, B., Gao, J., Xuan, M., Yu, H., Zhang, M., & Luo, W. (2015). Altered brain network centrality in depressed Parkinson’s disease patients. Movement Disorders, 30, 1777–1784.
Luo, X., Qiu, T., Xu, X., Huang, P., Gu, Q., Shen, Z., Yu, X., Jia, Y., Guan, X., Song, R., & Zhang, M. (2016). Decreased inter-hemispheric functional connectivity in cognitively intact elderly APOE varepsilon4 carriers: a preliminary study. Journal of Alzheimers Disease, 50, 1137–1148.
Machulda, M. M., Jones, D. T., Vemuri, P., McDade, E., Avula, R., Przybelski, S., Boeve, B. F., Knopman, D. S., Petersen, R. C., & Jack, C. J. (2011). Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Archives of Neurology, 68, 1131–1136.
Mendoza, J., & Foundas, A. (2007). Clinical neuroanatomy: A neurobehavioral approach. Berlin: Springer Science & Business Media.
Mondadori, C. R., de Quervain, D. J., Buchmann, A., Mustovic, H., Wollmer, M. A., Schmidt, C. F., Boesiger, P., Hock, C., Nitsch, R. M., Papassotiropoulos, A., & Henke, K. (2007). Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cerebral Cortex, 17, 1934–1947.
Newman, M. E. (2008). The mathematics of networks. The NEW Palgrave Encyclopedia of Economics, 2, 1–12.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15, 1–25.
Persson, J., Lind, J., Larsson, A., Ingvar, M., Sleegers, K., Van Broeckhoven, C., Adolfsson, R., Nilsson, L. G., & Nyberg, L. (2008). Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia, 46, 1679–1687.
Pievani, M., Galluzzi, S., Thompson, P. M., Rasser, P. E., Bonetti, M., & Frisoni, G. B. (2011). APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer’s disease. NeuroImage, 55, 909–919.
Randall, C., Mosconi, L., de Leon, M., & Glodzik, L. (2013). Cerebrospinal fluid biomarkers of Alzheimer’s disease in healthy elderly. Frontiers in Bioscience (Landmark Ed), 18, 1150–1173.
Reinvang, I., Espeseth, T., & Westlye, L. T. (2013). APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neuroscience and Biobehavioral Reviews, 37, 1322–1335.
Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276.
Ricci, M., Graef, S., Blundo, C., & Miller, L. A. (2012). Using the Rey auditory verbal learning test (RAVLT) to differentiate Alzheimer’s dementia and behavioural variant fronto-temporal dementia. The Clinical Neuropsychologist, 26, 926–941.
Richeson, J. A., Baird, A. A., Gordon, H. L., Heatherton, T. F., Wyland, C. L., Trawalter, S., & Shelton, J. N. (2003). An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience, 6, 1323–1328.
Rocchi, A., Pellegrini, S., Siciliano, G., & Murri, L. (2003). Causative and susceptibility genes for Alzheimer’s disease: a review. Brain Research Bulletin, 61, 1–24.
Salminen, L. E., Schofield, P. R., Lane, E. M., Heaps, J. M., Pierce, K. D., Cabeen, R., Laidlaw, D. H., Akbudak, E., Conturo, T. E., Correia, S., & Paul, R. H. (2013). Neuronal fiber bundle lengths in healthy adult carriers of the ApoE4 allele: a quantitative tractography DTI study. Brain Imaging and Behavior, 7, 274–281.
Saykin, A. J., Shen, L., Foroud, T. M., Potkin, S. G., Swaminathan, S., Kim, S., Risacher, S. L., Nho, K., Huentelman, M. J., Craig, D. W., Thompson, P. M., Stein, J. L., Moore, J. H., Farrer, L. A., Green, R. C., Bertram, L., Jack, C. J., & Weiner, M. W. (2010). Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement, 6, 265–273.
Schwindt, G. C., & Black, S. E. (2009). Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. NeuroImage, 45, 181–190.
Shaw, L. M., Vanderstichele, H., Knapik-Czajka, M., Figurski, M., Coart, E., Blennow, K., Soares, H., Simon, A. J., Lewczuk, P., Dean, R. A., Siemers, E., Potter, W., Lee, V. M., & Trojanowski, J. Q. (2011). Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathologica, 121, 597–609.
Sheline, Y. I., Morris, J. C., Snyder, A. Z., Price, J. L., Yan, Z., D’Angelo, G., Liu, C., Dixit, S., Benzinger, T., Fagan, A., Goate, A., & Mintun, M. A. (2010). APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. Journal of Neuroscience, 30, 17035–17040.
Smith, C. D., Chebrolu, H., Andersen, A. H., Powell, D. A., Lovell, M. A., Xiong, S., & Gold, B. T. (2010). White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiology of Aging, 31, 1122–1131.
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., He, Y., Yan, C. G., & Zang, Y. F. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One, 6, e25031.
Song, H., Long, H., Zuo, X., Yu, C., Liu, B., Wang, Z., Wang, Q., Wang, F., Han, Y., & Jia, J. (2015). APOE effects on default mode network in Chinese cognitive normal elderly: relationship with clinical cognitive performance. PloS One, 10, e133179.
Sparks, D. L. (1997). Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Annals of the New York Academy of Sciences, 826, 128–146.
Squire, L. R., & Zola-Morgan, S. (1991). The medial temporal lobe memory system. Science, 253, 1380–1386.
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., & Roses, A. D. (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90, 1977–1981.
Sulpizio, V., Committeri, G., Lambrey, S., Berthoz, A., & Galati, G. (2013). Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behavioural Brain Research, 242, 62–75.
Sunderland, T., Mirza, N., Putnam, K. T., Linker, G., Bhupali, D., Durham, R., Soares, H., Kimmel, L., Friedman, D., Bergeson, J., Csako, G., Levy, J. A., Bartko, J. J., & Cohen, R. M. (2004). Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biological Psychiatry, 56, 670–676.
Suri, S., Heise, V., Trachtenberg, A. J., & Mackay, C. E. (2013). The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neuroscience and Biobehavioral Reviews, 37, 2878–2886.
Teter, B., Raber, J., Nathan, B., & Crutcher, K. A. (2002). The presence of apoE4, not the absence of apoE3, contributes to AD pathology. Journal of Alzheimers Disease, 4, 155–163.
van Wijk, B. C., Stam, C. J., & Daffertshofer, A. (2010). Comparing brain networks of different size and connectivity density using graph theory. PloS One, 5, e13701.
Westlye, E. T., Lundervold, A., Rootwelt, H., Lundervold, A. J., & Westlye, L. T. (2011). Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE epsilon4 carriers: relationships with memory performance. Journal of Neuroscience, 31, 7775–7783.
Wink, A. M., de Munck, J. C., van der Werf, Y. D., van den Heuvel, O. A., & Barkhof, F. (2012). Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connectivity, 2, 265–274.
Yan, C., & Zang, Y. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13.
Yao, Z., Zhang, Y., Lin, L., Zhou, Y., Xu, C., & Jiang, T. (2010). Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Computational Biology, 6, e1001006.
Zhang, Z., Liu, Y., Jiang, T., Zhou, B., An, N., Dai, H., Wang, P., Niu, Y., Wang, L., & Zhang, X. (2012). Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by Regional Homogeneity. NeuroImage, 59, 1429–1440.
Zhuang, L., Wen, W., Zhu, W., Trollor, J., Kochan, N., Crawford, J., Reppermund, S., Brodaty, H., & Sachdev, P. (2010). White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. NeuroImage, 53, 16–25.
Zuo, X. N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., & Milham, M. P. (2012). Network centrality in the human functional connectome. Cerebral Cortex, 22, 1862–1875.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; Bio Clinica, Inc.; Biogen; Bristol-Myers Squibb Company; Cere Spir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; Euro Immun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; MesoScale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was funded by the 12th Five-year Plan for National Science and Technology Supporting Program of China (Grant No. 2012BAI10B04), Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ14H180001 and Grant No. Y16H090026).
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all participants and/or authorized representatives and the study partners before any protocol-specific procedures were carried out in the ADNI study.
Additional information
Xiao Luo and Tiantian Qiu contributed equally to this paper.
Data used in preparation of this article were obtained from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database (www.adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Rights and permissions
About this article
Cite this article
Luo, X., Qiu, T., Jia, Y. et al. Intrinsic functional connectivity alterations in cognitively intact elderly APOE ε4 carriers measured by eigenvector centrality mapping are related to cognition and CSF biomarkers: a preliminary study. Brain Imaging and Behavior 11, 1290–1301 (2017). https://doi.org/10.1007/s11682-016-9600-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9600-z