Abstract
Background
Previous meta-analyses did not explore the immediate and long-term effect of non-invasive brain stimulation (NIBS) on different cognitive domains in Alzheimer’s disease (AD). The meta-analysis aimed to assess the therapy effect of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) on different cognitive domains in AD in randomized controlled trials (RCTs).
Methods
Studies published before December 2021 and exploring therapy effect of rTMS, tDCS on different cognitive domains in AD were searched in the following databases: PubMed and Web of Science. We used STATA 12.0 software to compute the standard mean difference (SMD) and a 95% confidence interval (CI).
Results
The present study included 16 articles (including 372 AD patients treated with rTMS and 310 treated with sham rTMS) for rTMS and 11 articles (including 152 AD patients treated with tDCS and 134 treated with sham tDCS) for tDCS. The present study showed better immediate and long-term general cognitive function increase effects in AD given rTMS, compared to those given sham rTMS with random effects models (immediate effect: SMD = 2.07, 95% CI = 0.37 to 3.77, I2 = 97.8%, p < 0.001; long-term effect: SMD = 5.04, 95% CI = 2.25 to 7.84, I2 = 97.8%, p < 0.001). The present study showed no significant immediate and long-term effects of rTMS on attention, executive, language and memory functions. In addition, the present study showed no significant difference in immediate or long-term effects of tDCS on general cognitive function, attention, language or memory functions between tDCS group and sham tDCS group.
Conclusions
RTMS was an effective treatment technique for general cognitive function in AD, whereas tDCS showed no significant therapy effect on cognitive function in AD. More large-scale studies were essential to explore the effect of NIBS on various cognitive function in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common type of dementia among the elderly (1). In addition, the prevalence of AD increases gradually with age (2). Up to date, the most commonly used therapy strategies for AD are approved pharmacological treatments, especially acetylcholinesterase inhibitors and Memantine (3). However, these pharmacological treatments act to control AD symptoms rather than alter the progression of the disease (4).
Recently, extensive non-pharmacological interventions have been used in AD therapy. Two safe, commonly used, non-invasive brain stimulation (NIBS) techniques, including transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), were widely used in AD therapy. TMS is a painless technique which can regulate the cortical function of the brain (5). Repetitive TMS (rTMS) includes trains of TMS pulses with various frequencies and intensities. Lin et al. (6) found that rTMS can significantly improve general cognitive function in patients with mild to moderate AD with a meta-analysis. TDCS has attracted interest because of its relative inexpensiveness and ease of administration (7). Cai et al. (8) reported that tDCS contributes to an improvement of general cognitive function in mild and moderate AD with a meta-analysis. The three meta-analyses (6–8) only focused on the effect of NIBS on general cognitive function, but not other different cognitive domains, such as attention function, executive function, language function, memory function and visuospatial function. Additionally, Chou et al (9) reported that rTMS can significantly improve memory and executive functions in mild cognitive impairment (MCI) and AD with a meta-analysis. Cruz Gonzalez et al. [10] reported that tDCS can result in improvement in various cognitive domains like memory and language in dementia and MCI with a meta-analysis. The two meta-analyses (9, 10) assessed the effect of NIBS on both MCI and AD. MCI is considered as a transitional stage between normal aging and early AD (11). The meta-analysis aimed to assess the therapy effect of rTMS and tDCS on different cognitive domains only in AD in randomized controlled trials (RCTs).
Methods
Search strategies
The meta-analysis was conducted to investigate the effect of NIBS on AD. Lihua Gu and Hui Xu independently performed the literature search. Studies published before December 2021 were searched in the following databases: PubMed and Web of Science. Search terms included: (‘transcranial magnetic stimulation’ OR ‘TMS’ OR ‘transcranial direct current stimulation’ OR ‘tDCS’) AND (‘Alzheimer’s disease’ OR ‘AD’ OR ‘dementia’). The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline (12) (Supplementary table 1).
Selection criteria
RCTs were included in the present study. Exclusion criteria included: (1) studies were excluded if they did not explore effect of NIBS on AD; (2) case reports, meta-analysis and reviews. Full texts were read by two independent investigators. If included studies did not provide sufficient information regarding pre- and post-treatment cognitive function in AD patients given NIBS (combined or not combined with conventional cognitive therapy) and sham stimulation (combined or not combined with conventional cognitive therapy).
Data collections
Two investigators (Lihua Gu and Fangyuan Qian) independently used an Excel file to extract data from finally included studies. The present study included the following data: authors, publication year, study location, research type, numbers of cases and controls, mean ages, gender, interventions, target area, intensity of stimulation, number of pulses, types of cognitive function, follow-up time and adverse effects. According to a previous study (13), neuropsychological examinations were divided into 6 cognitive domains: (1) general cognitive function, (2) attention function, (3) executive function, (4) language function, (5) memory function, (6) visuospatial function. General cognitive function was evaluated with scales including Mini Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog). Attention function was evaluated with scales including trail making test (TMT) part A and attentive matrices. Executive function was assessed with scales including Stroop color-word test, TMT part B and Go-no go test. Memory function was evaluated with scales including story recall, Rey auditory verbal learning test (RAVLT) and Rey-Osterrieth complex figure test (ROCFT) recall. Language function was evaluated with scales including picture-naming task, phonemic verbal fluency, semantic verbal fluency, Aachener aphasie test and Mississippi aphasia screening test (MAST). Visuospatial function was assessed with scales including Raven colored progressive matrices (RCPM), clock drawing test and ROCFT copy. In the present study, long-term effect refers to effect lasting a follow-up period after stimulation.
Meta-analysis
Mean values and standard deviation (SD) of increase or reduction rate of cognitive function associated scores were collected from studies. We used STATA 12.0 software to compute the standard mean difference (SMD) and a 95% confidence interval (CI). We used the Cochran Q test and I2 method to assess the heterogeneity between included studies. With high heterogeneity (I2 ≥ 50%, p value of Q test < 0.05), we applied a random effects model; with low heterogeneity (I2 < 50%, p value of Q test ≥ 0.05), we used a fixed effects model. Meta-regression analyses were applied to detect heterogeneity source. In addition, subgroup analysis (for different ethnicities, different frequencies of rTMS or different types of tDCS, stimulation over different brain regions) was conducted to explore the source of the heterogeneity. Sensitivity analysis was used to evaluate stability. Moreover, Begg’s test, Egger’s test and funnel plot were applied to assess publication bias. Quality appraisal was performed using the Cochrane Risk of Bias Tool. Data were analyzed using Review Manager 5.3.
Results
Studies information
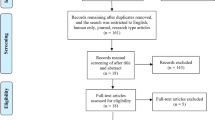
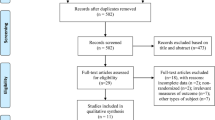
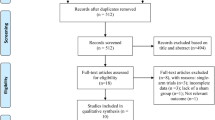
Tables 1 and 2 illustrated information about included studies. Figure 1 illustrated the gradual exclusion process. The present study included 16 articles (14–29) (including 372 AD patients treated with rTMS (combined or not combined with conventional cognitive therapy) and 310 treated with sham rTMS (combined or not combined with conventional cognitive therapy)) for rTMS and 11 articles (30–40) (including 152 AD patients treated with tDCS (combined or not combined with conventional cognitive therapy) and 134 treated with sham tDCS (combined or not combined with conventional cognitive therapy)) for tDCS.
Results of meta-analysis for rTMS
Effect of rTMS on general cognitive function in AD
N = 13 studies (14–16, 19–24, 26–29) (including 333 AD patients treated with rTMS (combined or not combined with conventional cognitive therapy) and 289 AD patients treated with sham rTMS (combined or not combined with conventional cognitive therapy)) explored the immediate effect of rTMS on general cognitive function in AD. Meta-analysis showed a significantly better immediate general cognitive function increase effect in AD given rTMS, compared to those given sham rTMS with a random effects model (SMD = 2.07, 95% CI = 0.37 to 3.77, I2 = 97.8%, p < 0.001, Figure 2. A). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE, intensity of stimulation and number of pulse were irresponsible for the heterogeneity between studies (Supplementary table 2). Subgroup analysis showed a significantly better immediate general cognitive function increase effect in AD given rTMS, compared to those given sham rTMS in Asian, whereas no significant difference in immediate effect of rTMS on general cognitive function between rTMS group and sham rTMS group in Caucasian (Table 3, Supplementary figure 1. A). Subgroup analysis showed a significantly better immediate general cognitive function increase effect in AD given high frequent (HF)-rTMS, compared to those given sham rTMS (Table 4, Supplementary figure 2. A). Subgroup analysis showed a significantly better immediate general cognitive function increase effect in AD given rTMS over the left dorsolateral prefrontal cortex (DLPFC), compared to those given sham rTMS, whereas no significant difference in immediate effect of rTMS on general cognitive function between rTMS over Broca and Wernicke group and sham rTMS group (Table 5, Supplementary figure 3. A). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. A). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.444; Egger’s test: p = 0.370; Supplementary figure 5. A).
N = 12 studies (14–16, 18, 20, 21, 23–26, 28, 29) (including 277 AD patients treated with rTMS (combined or not combined with conventional cognitive therapy) and 236 AD patients treated with sham rTMS (combined or not combined with conventional cognitive therapy)) explored the long-term effect of rTMS on general cognitive function in AD. Meta-analysis showed a significantly better long-term general cognitive function increase effect in AD given rTMS, compared to those given sham rTMS with a random effects model (SMD = 5.04, 95% CI = 2.25 to 7.84, I2 = 97.8%, p < 0.001, Figure 2. B). Meta-regression studies showed that intensity of stimulation was responsible for the heterogeneity between studies, whereas publication year, age, gender, educational level, baseline MMSE, number of pulse and follow-up duration were irresponsible for the heterogeneity between studies (Supplementary table 2). Subgroup analysis showed a significantly better long-term general cognitive function increase effect in AD given rTMS, compared to those given sham rTMS in Caucasian, whereas no significant difference in long-term effect of rTMS on general cognitive function between rTMS group and sham rTMS group in Asian (Table 3, Supplementary figure 1. B). Subgroup analysis showed a significantly better long-term general cognitive function increase effect in AD given HF-rTMS, compared to those given sham rTMS (Table 4, Supplementary figure 2. B). Subgroup analysis showed a significantly better long-term general cognitive function increase effect in AD given rTMS over the left DLPFC, compared to those given sham rTMS, whereas no significant difference in long-term effect of rTMS on general cognitive function between rTMS over bilateral DLPFC or Broca and Wernicke group and sham rTMS group (Table 5, Supplementary figure 3. B). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. B). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.428; Egger’s test: p = 0.574; Supplementary figure 5. B).
Effect of rTMS on attention function in AD
N = 3 studies (17, 19, 22) (including 50 AD patients treated with rTMS and 50 AD patients treated with sham rTMS) explored the immediate effect of rTMS on attention function in AD. Meta-analysis showed no significant difference in immediate effect of rTMS on attention function between rTMS group and sham rTMS group with a random effects model (SMD = 4.40, 95% CI = -0.53 to 9.34, I2 = 97.6%, p < 0.001, Figure 3. A). Meta-regression studies showed that publication year, age, gender, baseline MMSE, intensity of stimulation and number of pulse were irresponsible for the heterogeneity between studies (Supplementary table 2). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. C). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.296; Egger’s test: p = 0.266; Supplementary figure 5. C).
Forest plots regarding the immediate effect of rTMS on attention function (A), immediate effect of rTMS on executive function (B), immediate effect of rTMS on language function (C), long-term effect of rTMS on language function (D), immediate effect of rTMS on memory function (E) and long-term effect of rTMS on memory function (F)
Abbreviations: AD, Alzheimer’s disease; CI, confidence interval; rTMS, repetitive transcranial magnetic stimulation; SMD, standard mean difference.
Effect of rTMS on executive function in AD
N = 4 studies (14, 17, 22, 25) (including 38 AD patients treated with rTMS and 40 AD patients treated with sham rTMS) explored the immediate effect of rTMS on executive function in AD. Meta-analysis showed no significant difference in immediate effect of rTMS on executive function between rTMS group and sham rTMS group with a random effects model (SMD = 6.44, 95% CI = -5.39 to 18.27, I2 = 97.5%, p < 0.001, Figure 3. B). Meta-regression studies showed that number of pulse was responsible for the heterogeneity between studies, whereas publication year, age, gender, baseline MMSE and intensity of stimulation were irresponsible for the heterogeneity between studies (Supplementary table 2). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. D). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.734; Egger’s test: p = 0.798; Supplementary figure 5. D).
Effect of rTMS on language function in AD
N = 4 studies (14, 19, 23, 24) (including 73 AD patients treated with rTMS and 67 AD patients treated with sham rTMS) explored the immediate effect of rTMS on language function in AD. Meta-analysis showed no significant difference in immediate effect of rTMS on language function between rTMS group and sham rTMS group with a random effects model (SMD = -1.02, 95% CI = -3.03 to 0.99, I2 = 95.5%, p < 0.001, Figure 3. C). Meta-regression studies showed that publication year, age, gender, baseline MMSE, intensity of stimulation and number of pulse were irresponsible for the heterogeneity between studies (Supplementary table 2). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. E). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.734; Egger’s test: p = 0.795; Supplementary figure 5. E).
N = 3 studies (14, 23, 24) (including 47 AD patients treated with rTMS and 41 AD patients treated with sham rTMS) explored the long-term effect of rTMS on language function in AD. Meta-analysis showed no significant difference in long-term effect of rTMS on language function between rTMS group and sham rTMS group with a random effects model (SMD = 1.16, 95% CI = -2.45 to 4.78, I2 = 96.7%, p < 0.001, Figure 3. D). Meta-regression studies showed that publication year, age, baseline MMSE, intensity of stimulation, number of pulse and follow-up duration were irresponsible for the heterogeneity between studies (Supplementary table 2). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. F). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.602; Egger’s test: p = 0.657; Supplementary figure 5. F).
Effect of rTMS on memory function in AD
N = 6 studies (14, 17, 19, 22–24) (including 97 AD patients treated with rTMS and 91 AD patients treated with sham rTMS) explored the immediate effect of rTMS in AD on memory function in AD. Meta-analysis showed no significant difference in immediate effect of rTMS on memory function between rTMS group and sham rTMS group with a random effects model (SMD = -0.14, 95% CI = -1.86 to 1.57, I2 = 95.5%, p < 0.001, Figure 3. E). Metaregression studies showed that publication year, age, gender, educational level, baseline MMSE, intensity of stimulation and number of pulse were irresponsible for the heterogeneity between studies (Supplementary table 2). Subgroup analysis showed no significant difference in immediate effect of rTMS on memory function between rTMS group and sham rTMS group in Caucasian (Table 3, Supplementary figure 1. E). Subgroup analysis showed no significant difference in immediate effect of rTMS on memory function between rTMS over left DLPFC group and sham rTMS group (Table 5, Supplementary figure 3. D). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. G). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.851; Egger’s test: p = 0.911; Supplementary figure 5. G).
N = 3 studies (14, 23, 24) (including 47 AD patients treated with rTMS and 41 AD patients treated with sham rTMS) explored the long-term effect of rTMS on memory function in AD. Meta-analysis showed no significant difference in long-term effect of rTMS on memory function between rTMS group and sham rTMS group with a random effects model (SMD = 0.03, 95% CI = -2.13 to 2.20, I2 = 92.8%, p < 0.001, Figure 3. F). Meta-regression studies showed that publication year, age, educational level, baseline MMSE, intensity of stimulation and number of pulse were irresponsible for the heterogeneity between studies (Supplementary table 2). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 4. H). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.602; Egger’s test: p = 0.890; Supplementary figure 5. H).
Results of meta-analysis for tDCS
Effect of tDCS on general cognitive function in AD
N = 6 studies (31–34, 38, 39) (including 99 AD patients treated with tDCS and 84 AD patients treated with sham tDCS) explored the immediate effect of tDCS on general cognitive function in AD. Meta-analysis showed no significant difference in immediate effect of tDCS on general cognitive function between tDCS group and sham tDCS group with a random effects model (SMD = 1.96, 95% CI = -1.81 to 5.74, I2 = 97.1%, p < 0.001, Figure 4. A). Meta-regression studies showed that intensity of stimulation was responsible for the heterogeneity between studies, whereas publication year, age, gender, educational level and baseline MMSE were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in immediate effect of tDCS on general cognitive function between tDCS group and sham tDCS group in Caucasian (Table 3, Supplementary figure 6. A). Subgroup analysis showed no significant difference in immediate effect of AtDCS on general cognitive function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. A). Subgroup analysis showed no significant difference in immediate effect of tDCS on general cognitive function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. A). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. A). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.881; Egger’s test: p = 0.678; Supplementary figure 10. A).
N = 6 studies (31–35, 37) (including 86 AD patients treated with tDCS and 81 AD patients treated with sham tDCS) explored the long-term effect of tDCS on general cognitive function in AD. Meta-analysis showed no significant difference in long-term effect of tDCS on general cognitive function between tDCS group and sham tDCS group with a random effects model (SMD = 1.52, 95% CI = -1.08 to 4.13, I2 = 96.1%, p < 0.001, Figure 4. B). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE, intensity of stimulation and follow-up duration were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in long-term effect of tDCS on general cognitive function between tDCS group and sham tDCS group in Caucasian (Table 3, Supplementary figure 6. B). Subgroup analysis showed no significant difference in long-term effect of AtDCS on general cognitive function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. B). Subgroup analysis showed no significant difference in long-term effect of tDCS on general cognitive function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. B). Subgroup analysis showed no significant difference in long-term effect of tDCS on general cognitive function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. B). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. B).
Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.764; Egger’s test: p = 0.411; Supplementary figure 10. B).
Effect of tDCS on attention function in AD
N = 5 studies (32–34, 36, 37) (including 71 AD patients treated with tDCS and 55 AD patients treated with sham tDCS) explored the immediate effect of tDCS on attention function in AD. Meta-analysis showed no significant difference in immediate effect of tDCS on attention function between tDCS group and sham tDCS group with a fixed effects model (SMD = -0.22, 95% CI = -0.53 to 0.08, I2 = 0.0%, p = 0.473, Figure 5. A). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE and intensity of stimulation were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in immediate effect of tDCS on attention function between tDCS group and sham tDCS group in Caucasian (Table 3, Supplementary figure 6. C). Subgroup analysis showed no significant difference in immediate effect of AtDCS on attention function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. C). Subgroup analysis showed no significant difference in immediate effect of tDCS on attention function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. C). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. C). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.095; Egger’s test: p = 0.077; Supplementary figure 10. C).
Forest plots regarding the immediate effect of tDCS on attention function (A), long-term effect of tDCS on attention function (B), immediate effect of tDCS on language function (C), long-term effect of tDCS on language function (D), immediate effect of tDCS on memory function (E) and long-term effect of tDCS on memory function (F) in AD
Abbreviations: AD, Alzheimer’s disease; CI, confidence interval; SMD, standard mean difference; tDCS, transcranial direct current stimulation.
N = 6 studies (31–36) (including 80 AD patients treated with tDCS and 68 AD patients treated with sham tDCS) explored the long-term effect of tDCS on attention function in AD. Meta-analysis showed no significant difference in long-term effect of tDCS on attention function between tDCS group and sham tDCS group with a fixed effects model (SMD = -0.07, 95% CI = -0.39 to 0.24, I2 = 0.0%, p = 0.995, Figure 5. B). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE, intensity of stimulation and follow-up duration were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in long-term effect of AtDCS on attention function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. D). Subgroup analysis showed no significant difference in long-term effect of tDCS on attention function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. D). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. D). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.230; Egger’s test: p = 0.090; Supplementary figure 10. D).
Effect of tDCS on language function in AD
N = 6 studies (32–34, 36, 37, 40) (including 91 AD patients treated with tDCS and 75 AD patients treated with sham tDCS) explored the immediate effect of tDCS on language function in AD. Meta-analysis showed no significant difference in immediate effect of tDCS on language function between tDCS group and sham tDCS group with a fixed effects model (SMD = 0.15, 95% CI = -0.12 to 0.42, I2 = 0.0%, p = 0.531, Figure 5. C). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE and intensity of stimulation were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in immediate effect of tDCS on language function between tDCS group and sham tDCS group in Caucasian (Table 3, Supplementary figure 6. D). Subgroup analysis showed no significant difference in immediate effect of AtDCS on language function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. E). Subgroup analysis showed no significant difference in immediate effect of tDCS on language function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. E). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. E). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.068; Egger’s test: p = 0.514; Supplementary figure 10. E).
N = 4 studies (32–34, 36) (including 60 AD patients treated with tDCS and 48 AD patients treated with sham tDCS) explored the long-term effect of tDCS on language function in AD. Meta-analysis showed no significant difference in long-term effect of tDCS on language function between tDCS group and sham tDCS group with a fixed effects model (SMD = 0.18, 95% CI = -0.18 to 0.54, I2 = 0.0%, p = 0.744, Figure 5. D). Meta-regression studies showed that publication year, age, educational level, baseline MMSE and intensity of stimulation were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in long-term effect of AtDCS on language function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. F). Subgroup analysis showed no significant difference in long-term effect of tDCS on language function between tDCS over the left DLPFC group and sham tDCS group (Table 5, Supplementary figure 8. F). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. F). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.806; Egger’s test: p = 0.192; Supplementary figure 10. F).
Effect of tDCS on memory function in AD
N = 5 studies (30–32, 34, 37) (including 56 AD patients treated with tDCS and 51 AD patients treated with sham tDCS) explored the immediate effect of tDCS on memory function in AD. Meta-analysis showed no significant difference in immediate effect of tDCS on memory function between tDCS group and sham tDCS group with a random effects model (SMD = 0.33, 95% CI = -0.69 to 1.36, I2 = 81.3%, p < 0.001, Figure 5. E). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE and intensity of stimulation were irresponsible for the heterogeneity between studies (Supplementary table 3). Subgroup analysis showed no significant difference in immediate effect of tDCS on memory function between tDCS group and sham tDCS group in Caucasian (Table 3, Supplementary figure 6. G). Subgroup analysis showed no significant difference in immediate effect of AtDCS on memory function between AtDCS group and sham tDCS group (Table 4, Supplementary figure 7. G). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. G). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 0.573; Egger’s test: p = 0.065; Supplementary figure 10. G).
N = 4 studies (31, 32, 34, 35) (including 52 AD patients treated with tDCS and 52 AD patients treated with sham tDCS) explored the long-term effect of tDCS on memory function in AD. Meta-analysis showed no significant difference in long-term effect of tDCS on memory function between tDCS group and sham tDCS group with a fixed effects model (SMD = -0.28, 95% CI = -0.67 to 0.11, I2 = 9.3%, p = 0.346, Figure 5. F). Meta-regression studies showed that publication year, age, gender, educational level, baseline MMSE, intensity of stimulation and follow-up duration were irresponsible for the heterogeneity between studies (Supplementary table 3). Sensitivity analyses showed that the direction of effect showed no change when any one study was excluded (Supplementary figure 9. H). Additionally, Begg’s test, Egger’s test and funnel plot showed no significant risks of publication bias (Begg’s test: p = 1.000; Egger’s test: p = 0.208; Supplementary figure 10. H).
Risk of bias graph was showed in Supplementary figure 11. Details of the risk of bias summary was showed in Supplementary figure 12.
Discussion
The present study showed better immediate and long-term general cognitive function increase effects in AD given rTMS, compared to those given sham rTMS. The result regarding effect of rTMS on general cognitive function was corresponding to a recent meta-analysis (6) which demonstrated significantly improved general cognition in the rTMS group, compared with sham rTMS group. The present study reported that rTMS therapy showed both immediate and long-term effect. Previous in vivo and in vitro experimental studies supported that rTMS can raise long-term potentiation (LTP) (41). In various murine models of dementia, rTMS has been reported to rescue LTP deficits (42, 43). The study supported provide evidence for the persistent beneficial effects of rTMS on general cognitive function in AD patients.
The study showed a high heterogeneity between included studies evaluating effect of rTMS on general cognitive function in AD. The result was corresponding to the previous two studies (6, 9). Meta-regression analysis in the study showed that publication year and characteristics of participants (including age, gender, educational level and baseline MMSE) were not responsible for the heterogeneity between included studies, whereas intensity of stimulation might be a source of heterogeneity between studies. Subgroup analyses were conducted to determine which factor showed the greatest effects on cognitive outcome and explore sources of heterogeneity between studies. Subgroup analysis indicated that HF-rTMS showed better immediate and long-term general cognitive function increase effects in AD given HF-rTMS, compared to those given sham rTMS. HF-rTMS (> 1 Hz) works by promoting cortical excitability of the affected brain hemisphere, whereas low frequent (LF)-rTMS (≤ 1 Hz) works by inhibiting cortical excitability of unaffected brain hemisphere (44). Ahmed et al. (15) reported that the HF-rTMS group had more beneficial effects than the LF and sham groups in all general cognitive function related scales and at all time points after therapy. Another study (45) reported that LF-rTMS might worsen rather than improve cognitive function. HF-rTMS (> 1 Hz) seems to be confirmed in improving cognitive outcome. In addition, subgroup analysis showed significantly better immediate and long-term general cognitive function increase effect in AD given rTMS over the left DLPFC, compared to those given sham rTMS, whereas no significant difference in immediate or long-term effect of rTMS on general cognitive function between rTMS over Broca and Wernicke group and sham rTMS group. Previous studies supported that the frontal lobes are the “file clerk” of the episodic memory system (46). In addition, the left DLPFC is especially crucial for verbal working memory tasks (47) and is the most common target for rTMS therapy (48). The present meta-analysis supported the significant effect of rTMS over the left DLPFC on general cognitive function. In addition, heterogeneities might derive from participants’ heterogeneities and difference in detection methods for inflammatory biomarkers between studies.
The present study showed no significant immediate and long-term effects of rTMS on attention, executive, language and memory functions. The result was not consistent with a recent meta-analysis (9) exploring the effect of rTMS on cognitive enhancement in mild cognitive impairment and AD which reported that (1) HF-rTMS over the left DLPFC and LF-rTMS at the right DLPFC significantly improved memory function; (2) HF-rTMS targeting the right inferior frontal gyrus significantly enhanced executive function. The previous meta-analysis (9) focused on the effect of rTMS on mild cognitive impairment and AD, whereas our study focused on the effect of rTMS on AD. The different result might be due to the irreversible cognitive dysfunction in late stage of AD. The difference demonstrated that early intervention of rTMS on cognitive dysfunction at an early stage of AD is essential for AD therapy.
The present study showed no significant difference in immediate or long-term effects of tDCS on general cognitive function, attention, language or memory functions between tDCS group and sham tDCS group. Previous studies reported that tDCS modifies local cortical excitability to improve interhemispheric inhibition (49, 50). In addition, tDCS contributes to regional cerebral blood flow changes and puts off the aberrant neural synchronization in AD (7). A previous meta-analysis (8) reported that tDCS shows a significant effect on general cognitive function in mild and moderate AD. In addition, a recent meta-analysis (7) including 11 articles reported that tDCS significantly improves the score on cognition as compared to sham in AD. The previous meta-analyses (7, 8) computed results of comparison of post-treatment cognitive function, whereas our meta-analysis used increase or reduction rate of cognitive function. Thus, more studies were essential to explore the effect of tDCS on cognitive function in AD. In addition, the study showed a high heterogeneity between included studies. Meta-regression studies showed that intensity of stimulation might be a source of heterogeneity between studies.
There are some limitations in the study. Firstly, not sufficient numbers of studies were included to explore the therapy effect of rTMS or tDCS on visuospatial function in AD. Secondly, the amount of included studies was limited to exploring the therapy effect of LF-rTMS on general cognitive function in AD. Thirdly, the presence of heterogeneity between included studies was inevitable. The inconsistency may have influenced our results. To avoid this problem, we selected the most appropriate RCTs based on rigorous selection criteria.
Conclusions
In conclusion, the meta-analysis demonstrated that rTMS was an effective treatment technique for general cognitive function in AD, whereas tDCS showed no significant therapy effect on cognitive function in AD. More large-scale studies were essential to explore the effect of rTMS and tDCS on various cognitive function in AD.
References
Fargo KN, Aisen P, Albert M, Au R, Corrada MM, DeKosky S, Drachman D, Fillit H, Gitlin L, Haas M, Herrup K, Kawas C, Khachaturian AS, Khachaturian ZS, Klunk W, Knopman D, Kukull WA, Lamb B, Logsdon RG, Maruff P, Mesulam M, Mobley W, Mohs R, Morgan D, Nixon RA, Paul S, Petersen R, Plassman B, Potter W, Reiman E, Reisberg B, Sano M, Schindler R, Schneider LS, Snyder PJ, Sperling RA, Yaffe K, Bain LJ, Thies WH, Carrillo MC. 2014 Report on the Milestones for the US National Plan to Address Alzheimer’s Disease. Alzheimers Dement 2014;10, eS430–452; doi: https://doi.org/10.1016/j.jalz.2014.08.103.
Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050). Alzheimers Dement 2014;10, ee40–46; doi: https://doi.org/10.1016/j.jalz.2014.01.004.
Briggs R, Kennelly SP, O’Neill D. Drug treatments in Alzheimer’s disease. Clin Med (Lond) 2016;16, e247–253; doi: https://doi.org/10.7861/clinmedidne.16-3-247.
Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol 2012;73, e504–51; doi: https://doi.org/10.1111/j.1365-2125.2011.04134.x.
Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener 2015;4, e22; doi: https://doi.org/10.1186/s40035-015-0045-x.
Lin Y, Jiang WJ, Shan PY, Lu M, Wang T, Li RH, Zhang N, Ma L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J Neurol Sci 2019 398, 184–191; doi: https://doi.org/10.1016/j.jns.2019.01.038.
Saxena V, Pal A. Role of Transcranial Direct Current Stimulation in the Management of Alzheimer’s Disease: A Meta-analysis of Effects, Adherence and Adverse Effects. 2021;19, 589–599; doi: https://doi.org/10.9758/cpn.2021.19.4.589.
Cai M, Guo Z, Xing G, Peng H, Zhou L, Chen H, McClure MA, He L, Xiong L, He B, Du F, Mu Q. Transcranial Direct Current Stimulation Improves Cognitive Function in Mild to Moderate Alzheimer Disease: A Meta-Analysis. Alzheimer Dis Assoc Disord 2019;33, e170–178; doi: https://doi.org/10.1097/WAD.0000000000000304.
Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2020;86, 1–10; https://doi.org/10.1016/j.neurobiolaging.2019.08.020.
Cruz Gonzalez P, Fong KNK, Chung RCK, Ting KH, Law LLF, Brown T. Can Transcranial Direct-Current Stimulation Alone or Combined With Cognitive Training Be Used as a Clinical Intervention to Improve Cognitive Functioning in Persons With Mild Cognitive Impairment and Dementia? A Systematic Review and Meta-Analysis. Front Hum Neurosci 2018;12, e416; doi: https://doi.org/10.3389/fnhum.2018.00416.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56, e303–308; doi: https://doi.org/10.1001/archneur.56.3.303.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339, b2535; doi: https://doi.org/10.1371/journal.pmed.1000097.
Lezak MD. Neuropsychological assessment (3rd ed.). Journal of Neurology Neurosurgery & Psychiatry 2009;58, e655–664; doi: https://doi.org/10.1136/jnnp.58.6.655.
Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, Miniussi C. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry 2011. 82, 794–797; doi: https://doi.org/10.1136/jnnp.2009.197848.
Ahmed MA, Darwish ES, Khedr EM, El Serogy YM, Ali AM. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J Neurol 2012;259, e83–92; doi: https://doi.org/10.1007/s00415-011-6128-4.
Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm (Vienna) 2013;120, e813–819; doi: https://doi.org/10.1007/s00702-012-0902-z.
Eliasova I, Anderkova L, Marecek R, Rektorova I. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci 2014;346, e318–322; doi: https://doi.org/10.1016/j.jns.2014.08.036.
Rutherford G, Lithgow B, Moussavi Z. Short and Long-term Effects of rTMS Treatment on Alzheimer’s Disease at Different Stages: A Pilot Study. J Exp Neurosci 2015;9, e43–51; doi: https://doi.org/10.4137/JEN.S24004.
Wu Y, Xu W, Liu X, Xu Q, Tang L, Wu S. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: a randomized, double-blind, sham-controlled study. Shanghai Arch Psychiatry 2015;27, e280–288; doi: 10.11919/j.issn.100-0829.215107.
Lee J, Choi BH, Oh E, Sohn EH, Lee AY. Treatment of Alzheimer’s Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Neurol 2016;12, e57–64; doi: https://doi.org/10.3988/jcn.2016.12.1.57.
Zhao J, Li Z, Cong Y, Zhang J, Tan M, Zhang H, Geng N, Li M, Yu W, Shan P. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget 2017;8, e33864–33871; doi: https://doi.org/10.18632/oncotarget.13060.
Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C, Maiella M, Marra C, Cercignani M, Martorana A, Caltagirone C, Bozzali M. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018;169, e302–311; doi: https://doi.org/10.1016/j.neuroimage.2017.12.048.
Zhang F, Qin Y, Xie L, Zheng C, Huang X, Zhang M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm (Vienna) 2019;126, e1081–1094; doi: https://doi.org/10.1007/s00702-019-02022-y.
Bagattini C, Zanni M, Barocco F, Caffarra P, Brignani D, Miniussi C, Defanti CA. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul 2020;13, e1655–1664; doi: https://doi.org/10.1016/j.brs.2020.09.010.
Padala PR, Boozer EM, Lensing SY, Parkes CM, Hunter CR, Dennis RA, Caceda R, Padala KP. Neuromodulation for Apathy in Alzheimer’s Disease: A Double-Blind, Randomized, Sham-Controlled Pilot Study. J Alzheimers Dis 2020;77, e1483–1493; doi: https://doi.org/10.3233/JAD-200640.
Sabbagh M, Sadowsky C, Tousi B, Agronin ME, Alva G, Armon C, Bernick C, Keegan AP, Karantzoulis S, Baror E, Ploznik M, Pascual-Leone A. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement 2020;16, e641–650; doi: https://doi.org/10.1016/j.jalz.2019.08.197.
Jia Y, Xu L, Yang K, Zhang Y, Lv X, Zhu Z, Chen Z, Zhu Y, Wei L, Li X, Qian M, Shen Y, Hu W, Chen W. Precision Repetitive Transcranial Magnetic Stimulation Over the Left Parietal Cortex Improves Memory in Alzheimer’s Disease: A Randomized, Double-Blind, Sham-Controlled Study. Front Aging Neurosci 2021;13, e693611; doi: https://doi.org/10.3389/fnagi.2021.693611.
Li X, Qi G, Yu C, Lian G, Zheng H, Wu S, Yuan TF, Zhou D. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul 2021;14, e503–510; doi: https://doi.org/10.1016/j.brs.2021.01.012.
Zhou X, Wang Y, Lv S, Li Y, Jia S, Niu X, Peng D. Transcranial magnetic stimulation for sleep disorders in Alzheimer’s disease: A double-blind, randomized, and sham-controlled pilot study. Neurosci Lett 2021;766, e136337; doi: https://doi.org/10.1016/j.neulet.2021.136337.
Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Cogiamanian F, Barbieri S, Scarpini E, Priori A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 2008;71, e493–498; doi: https://doi.org/10.1212/01.wnl.0000317060.43722.a3.
Boggio PS, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Tadini L, Scarpini E, Fregni F, Priori A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul 2012;5, e223–230; doi: https://doi.org/10.1016/j.brs.2011.06.006.
Cotelli M, Manenti R, Brambilla M, Petesi M, Rosini S, Ferrari C, Zanetti O, Miniussi C. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front Aging Neurosci 2014;6, e38; doi: https://doi.org/10.3389/fnagi.2014.00038.
Khedr EM, Gamal NF, El-Fetoh NA, Khalifa H, Ahmed EM, Ali AM, Noaman M, El-Baki AA, Karim AA. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer’s disease. Front Aging Neurosci 2014;6, e275; doi: https://doi.org/10.3389/fnagi.2014.00275.
Suemoto CK, Apolinario D, Nakamura-Palacios EM, Lopes L, Leite RE, Sales MC, Nitrini R, Brucki SM, Morillo LS, Magaldi RM, Fregni F. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: a randomized, double-blind, sham-controlled trial. Brain Stimul 2014;7, e308–313; doi: https://doi.org/10.1016/j.brs.2013.10.003.
Bystad M, Grønli O, Rasmussen ID, Gundersen N, Nordvang L, Wang-Iversen H, Aslaksen PM. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: a randomized, placebo-controlled trial. Alzheimers Res Ther 2016;8, e13; doi: https://doi.org/10.1186/s13195-016-0180-3.
Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement (N Y) 2017;3, e247–253; doi: https://doi.org/10.1016/j.trci.2017.03.003.
Im JJ, Jeong H, Bikson M, Woods AJ, Unal G, Oh JK, Na S, Park JS, Knotkova H, Song IU, Chung YA. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul 2019;12, e1222–1228; doi: https://doi.org/10.1016/j.brs.2019.06.003.
Khedr EM, Salama RH, Abdel Hameed M, Abo Elfetoh N, Seif P. Therapeutic Role of Transcranial Direct Current Stimulation in Alzheimer Disease Patients: Double-Blind, Placebo-Controlled Clinical Trial. Neurorehabil Neural Repair 2019;33, e384–394; doi: https://doi.org/10.1177/1545968319840285.
Gangemi A, Colombo B. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: two randomized studies. 2021;33, 383–390; doi: https://doi.org/10.1007/s40520-020-01546-8.
Smirni D, Oliveri M, Misuraca E, Catania A, Vernuccio L, Picciolo V, Inzerillo F, Barbagallo M, Cipolotti L, Turriziani P. Verbal Fluency in Mild Alzheimer’s Disease: Transcranial Direct Current Stimulation over the Dorsolateral Prefrontal Cortex. J Alzheimers Dis 2021;81, e1273–1283; doi: https://doi.org/10.3233/JAD-210003.
Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res 2007;180, e583–593; doi: https://doi.org/10.1007/s00221-007-0991-3.
Tan T, Xie J, Liu T, Chen X, Zheng X, Tong Z, Tian X. Low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Aß(1–42)-mediated memory deficits in rats. Exp Gerontol 2013;48, e786–794; doi: https://doi.org/10.1016/j.exger.2013.05.001.
Zhen J, Qian Y, Weng X, Su W, Zhang J, Cai L, Dong L, An H, Su R, Wang J, Zheng Y, Wang X. Gamma rhythm low field magnetic stimulation alleviates neuropathologic changes and rescues memory and cognitive impairments in a mouse model of Alzheimer’s disease. Alzheimers Dement (N Y) 2017;3, e487–497; doi: https://doi.org/10.1016/j.trci.2017.07.002.
Sasaki N, Kakuda W, Abo M (2014) Bilateral high- and low-frequency rTMS in acute stroke patients with hemiparesis: A comparative study with unilateral high-frequency rTMS. Brain Injury 2014;28, 1682; doi: https://doi.org/10.3109/02699052.2014.947626.
Trojano L, Conson M, Maffei R, Grossi D. Categorical and coordinate spatial processing in the imagery domain investigated by rTMS. Neuropsychologia 2006;44, e1569–1574; doi: https://doi.org/10.1016/j.neuropsychologia.2006.01.017.
Budson AE, Price BH. Memory dysfunction. N Engl J Med 2005;352, e692–699; doi: https://doi.org/10.1056/NEJMra041071.
Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013;49, e1195–1205; doi: https://doi.org/10.1016/j.cortex.2012.05.022.
Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, Trinka E, Golaszewski S. Effect of transcranial brain stimulation for the treatment of Alzheimer disease: a review. Int J Alzheimers Dis 2012, 687909; doi: https://doi.org/10.1155/2012/687909.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt 3, 633–639; doi: https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x.
Peng Z, Zhou C, Xue S, Bai J, Yu S, Li X, Wang H, Tan Q. Mechanism of Repetitive Transcranial Magnetic Stimulation for Depression. Shanghai Arch Psychiatry 2018;30, e84–92; doi: https://doi.org/10.11919/j.issn.1002-0829.217047.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81901108). In addition, this study was supported by the Doctoral Fund for Entrepreneurship and Innovation of Jiangsu Province and the Nanjing Municipal Health Science and Technology Development Special Fund Project (No. YKK19161).
Author information
Authors and Affiliations
Corresponding author
Additional information
How to cite this article: L. Gu, H. Xu, F. Qian. Effects of Non-Invasive Brain Stimulation on Alzheimer’s Disease. J Prev Alz Dis 2022;3(9):410-424; https://doi.org/10.14283/jpad.2022.40
Disclosure statement
The authors have no potential conflicts of interest to disclose.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Gu, L., Xu, H. & Qian, F. Effects of Non-Invasive Brain Stimulation on Alzheimer’s Disease. J Prev Alzheimers Dis 9, 410–424 (2022). https://doi.org/10.14283/jpad.2022.40
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2022.40