Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation technique for Alzheimer’s disease (AD). rTMS, with high- or low-frequency, is thought to enhance or inhibit the cortical activities, respectively. This meta-analysis of randomized controlled trials (RCTs) was to summarize the efficacy of the rTMS on the cognition of AD patients and to identify its potential influential factors.
Methods
Literature from the Pubmed, Embase, Cochrane Library and Web of Science were searched and screened to identify eligible studies. Standardized mean difference (SMD) and 95% confidence interval were used to evaluate the therapeutic effects of rTMS. Subgroup analyses were performed to investigate the influential factors.
Results
Ten studies with 15 trials involving 240 patients were included. Compared with sham stimulation, rTMS could significantly improve cognition in AD (SMD, 0.42; 95% CI 0.18–0.67; P = 0.0006). Subgroup analysis suggested significant cognitive enhancement in participants receiving rTMS on multiple sites rather than on single site, and in patients receiving rTMS of more than 10 sessions, but not ≤ 10 sessions. Compared with rTMS as the single therapeutic method, rTMS with concurrent cognitive training seemed to produce greater improvement. Moreover, 20 Hz rTMS, seemed to be more effective than 10 Hz or 1 Hz rTMS. Furthermore, patients with higher education, or with mild-to-moderate AD were more likely to benefit from rTMS than patients with lower education, or with severe dementia, respectively.
Conclusions
Based on the current evidence, rTMS was an effective therapy for cognitive impairment in AD. Large RCTs are warranted to further validate the results of our subgroup analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a type of neurodegenerative disease characterized by various levels of cognitive impairment, constituting the most common cause of dementia among the elderly and one of the heaviest burdens on public health system [1,2,3]. There were about 5.4 million people suffering from AD worldwide in 2016, most of whom aged 60 years and older, and it was anticipated that the number of patients will double every 20 years by 2050 [4, 5]. People with AD may suffer from progressive memory loss, aphasia and declines in executive functions as well as psychiatric symptoms including hallucinations and apathy, which would seriously impact the life qualities of the patients and their caregivers [6,7,8].
Currently, there are two mainstream medications for AD, including cholinesterase inhibitors rivastigmine, donepezil and galantamine, and the glutamate antagonist memantine [3]. Nevertheless, these medications could only limitedly alleviate the symptoms and may be accompanied by adverse effects [9, 10]. Under this circumstances, it is imperative for us to develop alternative therapeutic method.
Transcranial magnetic stimulation (TMS) is a kind of noninvasive neuromodulation technique that could induce electrical current and change the cortical activities of the human brain via strong magnetic pulses delivering to the brain regions [11, 12]. Repetitive TMS (rTMS) involving trains of pulses in a certain repetitive form, could exert regulation effects on neural excitability as well as cortical function [13]. TMS could even modulate the activities of single neurons [14]. Previous studies have indicated that low-frequency rTMS could inhibit the stimulated cortex, whereas high-frequency rTMS was capable of facilitating the neural activities in the related areas [15]. Thus, rTMS has already been used in the treatment of dementia and cognitive impairment [16,17,18,19].
In spite of the accumulating evidence of positive outcomes brought by rTMS in the treatment of AD-related cognitive impairment, issues about the exact therapeutic effects and appropriate rTMS protocol have not reached a consensus and thus warrant further research. Obvious heterogeneities existed in the trial design, stimulation settings as well as the methodological qualities of the related clinical studies. To the best of our knowledge, three meta-analyses have been published to summarize the therapeutic efficacy of rTMS for cognitive outcomes in AD patients [20,21,22], among which only one study made meta-analysis rigorously based on randomized controlled trials (RCTs) [21]. However, only 5 RCTs were included in that study [21]. On the other hand, the inclusion of nonrandomized case series into the meta-analysis may increase the heterogeneity. Recently, several high-quality RCTs [23,24,25] have been published since the study of Chen et.al [21], which constitutes a proper opportunity for an updated meta-analysis.
Moreover, most clinical studies involving rTMS for AD only included patients with mild to moderate cognitive impairment [16, 19, 23,24,25,26,27,28,29], and there is a relative lack of the data about its therapeutic effects on severe dementia. Thus, an updated meta-analysis of RCTs was also imperative to summarize the effects of this therapy for patients with different levels of dementia and education. This meta-analysis, based on strict inclusion criteria, included the newly published RCTs, evaluated the pooled effects of rTMS and performed comprehensive subgroup analyses to investigate the proper stimulation patterns and identify the patients who are more likely to benefit from this procedure.
Methods
Search strategy
The systematic review was performed by two independent investigators following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30]. PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science were systematically searched until August 2019. The following search items were used: (‘Alzheimer’s disease’ OR ‘dementia’) AND (‘transcranial magnetic stimulation’ OR ‘noninvasive brain stimulation’ OR ‘TMS’ OR ‘rTMS’). Reference lists of the related studies were also reviewed to identify potential omitted literatures. Only studies written in English were eligible.
Study selection
Parallel design and crossover design trials were both accepted for the comprehensiveness of the included studies. The following inclusion criteria were used to identify eligible studies: (1) cognitive impairment attributed to AD; (2) patients were diagnosed with AD with specialized diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) or the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer disease and Related Disorders Association (NINCDS/ADRDA) criteria; (3) cognition outcomes of the patients were measured with specialized scales such as Mini-Mental State Examination (MMSE) and Alzheimer Disease Assessment Scale-cognitive subsection (ADAS-cog); (4) rTMS was the only intervention that differed between stimulation group and sham group; (5) mean cognition outcome scores changes and standard deviation (SD) were accessible, or absolute original scale scores have been given to calculate mean ± SD; (6) only original articles were included in the meta-analysis. The exclusion criteria included the following: (1) single-arm studies or studies without a sham group; (2) reviews, case report or case series, letters and conference abstracts; (3) insufficient data given in the study. Two independent investigators performed the literature search and selection, and any disagreements or uncertainties were resolved by negotiations based on the study selection criteria.
Quality assessment
The methodological quality of the included studies was evaluated by two authors independently with a modified checklist revised by Moher et al. [31] and the Cochrane Risk of Bias tool [32]. The checklist used the following criteria to evaluate the quality of each study: (1) randomization; (2) blinding procedure; (3) dropout number; (4) between-conditions statistical comparison; (5) point estimates and variability measures; (6) description of adverse events. If the study mentioned that patients were allocated randomly, the randomization was recorded as 1. With regard to the blinding procedure, 0 was used to indicate the non-blinded or non-described process, and single-blind and double-blind procedures were recorded as 1 and 2, respectively. Number of participants withdrew from the studies was recorded as the dropout number. When between-conditions comparison and point estimates and variability measures were given in the study, the related criterion was recorded as 1 in the corresponding items respectively. Regarding the adverse events, number of patients with adverse events and the type of these events were recorded.
Data extraction
The following information was extracted and summarized from each included studies by two authors independently: (1) general information: name of the first author, publication year, number of the patients and dropout numbers, study design, and blind procedure; (2) participants’ information: age, sex composition, disease duration, levels of education, levels of cognitive impairment (mild, moderate and severe) at baseline, scale scores at baseline; (3) main outcome measures of cognitive function, scores of assessment and adverse events; (4) stimulation protocol: stimulation sites, frequency, session numbers, pulses numbers per session, stimulation model (online vs offline) methods of sham stimulation. Level of cognitive impairment could be recorded as mild-to-moderate when MMSE scores ≥ 9 for illiterate patients, and scores ≥ 11 for educated individuals [33]. In this meta-analysis, MMSE or ADAS-cog was preferentially selected as the cognitive outcome measures if available in the studies, otherwise, the primary or the first-mentioned outcome measures were used.
Statistical analysis
The effect of rTMS on cognitive function in patients with AD was defined as the mean difference between the experimental groups and control groups for the changes in cognition measures relative to the baseline. Considering the variety of cognitive measures applied in the included studies, we used standardized mean difference (SMD) and 95% CI to summarize the pooled effects size of the eligible trials. Pre- and Post-stimulation scale scores of the groups were recorded. The first measurement taken after the stimulation was used for studies with “offline” design, and for “online” design studies, the cognitive performance measures based on the task during the stimulation was included. When studies did not present the net changes in the cognitive measures scores, the following formulas were used:
For the studies in which the original data were displayed as mean ± SEs, SD could be calculated using the following formula: SEs = SD/\(\surd n\) (n indicated the number of the participants).
Cochran Q statistic p value and I2 value were used to evaluate the heterogeneity of the included articles: a fixed-effect model was used when the I2 value < 50%; and when the I2 value ≥ 50%, a random model was applied to summarize the effect size. To further investigate the factors that might mediate the effects of rTMS on cognitive outcomes, the following 6 subgroup analyses were implemented: stimulation sites (single site vs. multiple sites), number of sessions (≤ 10 vs. > 10), severity of cognitive impairment (mild to moderate vs. severe), cognitive training (concurrent cognitive training vs. no concurrent cognitive training), rTMS frequency (20 Hz vs. 10 Hz vs. 1 Hz), as well as level of education (higher education [years of education ≥ 9] vs. lower education [years of education < 9]). The procedures of meta-analysis were performed using the Revman 5.3 statistical software (Cochrane Collaboration, 2014). Construction of funnel plots as well as Begg’s test and Egger’s test was also performed to evaluate the publication bias. In addition, a Trim and Fill procedure was implemented for the correction of publication bias. These procedures were finished with STATA 12.0 software (Stata Corp., College Station, TX).
Results
Search results
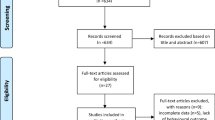
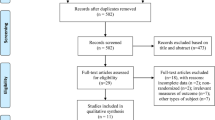
A total of 910 studies were yielded through the literature search, out of which 398 duplicate articles were excluded, followed by the removing of 494 studies through title and abstract scanning. Then the remaining 18 studies were evaluated for full-text reading. After excluding studies reporting single-arm trials (n = 3), with incomplete data (n = 3), without a sham stimulation group (n = 1) and reporting irrelevant outcomes (n = 1), a total of ten articles were included in our meta-analysis (Fig. 1).
Flow diagram based on PRISMA statement (www.prisma-statement.org)
Description of studies
A total of 15 trials involving 240 AD patients were reported in these 10 studies. Basic characteristics of the included studies are shown in Table 1. Seven articles reported parallel design trials [16, 17, 23, 25,26,27, 29], whereas the other 3 studies applied crossover patterns [19, 24, 28]. Most studies reported only one trial [16, 19, 24, 25, 27,28,29], whereas 3 articles reported 2 or more trials [17, 23, 26], as a result, 15 trials were included in our meta-analysis. All of these trials adopted an “offline stimulation” pattern. Nine studies only enrolled patients with mild-to-moderate AD [16, 19, 23,24,25,26,27,28,29], while 1 study also included patients with severe AD [17]. Single site rTMS was applied in 3 studies [16, 24, 27], while the other 7 studies applied multiple sites rTMS [17, 19, 23, 25, 26, 28, 29]. MMSE served as the cognitive measure in 6 studies [16, 17, 23,24,25,26], ADAS-cog was used in 3 studies [19, 27, 29], whereas the remaining 1 study used Trail making test [28]. With regard to the stimulation targets, in addition to the dorsolateral prefrontal cortex (DLPFC) serving as only the stimulation site in four studies [16, 17, 19, 27], other sites selected in the these articles included Broca and Wernicke area [26, 29], superior temporal gyrus (STG) [28], parietal somatosensory association cortex (pSAC) [26] and so on.
Quality assessment of the studies
As shown in Table 2 and Fig. 2, all of the included ten studies applied random allocation design, out of which double-blind design was adopted in seven studies [17, 19, 23, 24, 26, 27, 29]. Drop-out number of the patients was presented in five studies [19, 25,26,27, 29]. As for the adverse events, six studies presented the related information [19, 23, 25,26,27,28]. All of the reported adverse events were mild and tolerable, including mild extrapyramidal reactions, transient headache, fatigability and painful scalp sensation. Adverse events that occurred in the stimulation group and sham stimulation group were largely similar.
Effects of rTMS on AD
In total, 15 trials were selected to evaluate the pooled effects of rTMS on the cognitive function of patients with AD. The pooled results indicated that rTMS could significantly improve cognitive functions of the participants (SMD, 0.42; 95% CI 0.18–0.67; P = 0.0006) in the fixed-effects model analysis (P = 0.23, I2 = 20.0%) (Fig. 3).
Subgroup analyses
As shown in Table 3, we performed the following subgroup analyses to explore the effects of related factors on the clinical outcomes. The subgroup analysis of stimulation sites indicated that cognitive outcomes could be significantly improved by multiple sites stimulation (SMD, 0.47; 95% CI 0.14–0.79; P = 0.005), but not single-site stimulation (0.24; 95% CI − 0.45–0.92; P = 0.50, Fig.S1). The session numbers of rTMS in the studies ranged from 2 to 30, and the value of 10 was chosen as the cutoff point according to the distribution of the session numbers. In the subgroup analysis, it could be seen that cognitive scores were improved in patients receiving longer term treatment (0.59; 95% CI 0.28–0.90; P = 0.0002) instead of shorter term treatment (0.18; 95% CI − 0.20–0.56; P = 0.36, Fig.S2). In the subgroup analysis of cognitive training, the effect size of studies with and without cognitive training were 0.55 (95% CI 0.19–0.91) and 0.32 (95% CI − 0.00–0.65), respectively (Fig.S3). In addition, subgroup analysis of rTMS frequency indicated that 20 Hz rTMS (0.41; 95% CI 0.10–0.72; P = 0.01) were capable of producing greater enhancement than 10 Hz (0.68; − 0.03–1.38; P = 0.06) or 1 Hz rTMS (-0.04; 95% CI − 0.75–0.68; P = 0.92, Fig.S4).
As for the patient characteristics, the subgroup analyses indicated significant improved cognitive outcomes in patients with education of ≥ 9 years (0.64; 95% CI 0.04–1.24; P = 0.04), instead of < 9 years (0.15; 95% CI − 0.21–0.50; P = 0.42, Fig.S5). Besides, rTMS could significantly improve the outcome of participants with mild-to-moderate cognitive impairment (0.45; 95% CI 0.20–0.70; P = 0.0004) rather than patients with severe dementia (0.01; 95% CI − 0.95–0.97; P = 0.98, Fig.S6).
Publication bias and sensitivity analysis
As shown in Fig. 4, the funnel plot was visually symmetrical and the Begg’s test (P = 0.974) as well as the Egger’s test (P = 0.974) further validated the absence of the significant publication bias. Thus, no trimming was performed in the Trim and Fill test. In addition, no obvious change in the pooled results was detected when the comparisons were removed one by one, which indicated that our results were robust and reliable.
Discussion
The present updated meta-analysis was to further evaluate the efficacy of rTMS on cognitive outcomes of AD patients, to explore the proper stimulation patterns as well as the exact indications. Based on the current evidence, our meta-analysis further validated the significant therapeutic effects and the high level of safety and tolerability of this procedure. Our subgroup analyses suggested that rTMS protocols of > 10 sessions, multiple stimulation sites, 20 Hz, and with concurrent cognitive training were more effective than those of ≤ 10 sessions, single stimulation site, 10 Hz or 1 Hz, and without concurrent cognitive training, respectively.
As mentioned earlier, high- and low-frequency rTMS could facilitate and inhibit the stimulated areas, respectively [15]. In our meta-analysis, eight trials used 20 Hz rTMS, whereas 10 Hz and 1 Hz rTMS were applied in 5 and 2 trails, respectively. The study of Ahmed et al. [17] made comparisons of high- versus low- frequency rTMS in the long term, and concluded that high-frequency rTMS could serve as a useful adjuvant therapy in the treatment of AD. In addition, the meta-analysis of Liao et.al [22] also validated the significant effectiveness of high-frequency stimulation (> 1 Hz), rather than low-frequency stimulation (≤ 1 Hz). Our subgroup analyses showed that 20 Hz stimulation produced better cognitive outcome compared with 10 Hz or 1 Hz rTMS, and this result further validated the previous studies, and suggested the importance of selections of the appropriate frequency. However, only 2 trials from 1 study [17] applied 1 Hz rTMS, thus, further investigations are needed before the final conclusion of the best frequency.
With regard to the stimulation site, our subgroup analyses suggested that multiple sites rTMS was superior to single site rTMS (0.47 [0.14, 0.79] vs 0.24 [− 0.45, 0.92]), which was consistent with the previous study of Lin et.al [20]. In our meta-analysis, only 3 trials applied single site rTMS on left DLPFC or precuneus [16, 24, 27]. In addition, the most common choices of multiple sites rTMS included left and right DLPFC [19, 25, 26, 29]. The preferential selection of the prefrontal cortex may be due to the critical role played by this area in memory. As indicated by the hemispheric encoding retrieval asymmetry model, the left prefrontal cortex is critical for memory encoding, whereas the right side is involved in retrieval [8, 34, 35]. rTMS has been observed to be capable of inducing long-term potentiations or inhibitions on neuronal activity, thus producing after-effects [14, 36]. A previous neuroimaging study [37] suggested that rTMS even exerts remote effects on cortical or subcortical regions that have connections with the stimulated areas. However, conflicts still existed in the issues about the true effects of DLPFC stimulation as well as which side was better. The study of Liao et.al [22] summarized the clinical data of 94 patients with mild-to-moderate AD and suggested that, compared with left DLPFC rTMS, right or bilateral DLPFC stimulation seemed to be more effective. But the study of Ahmed et.al [17] found that the high frequency of rTMS on the left and then the right DLPDC was effective for cognitive outcome. The study of Cotelli, et.al [38] has validated the effects of rTMS over both the right and left DLPFC on the action naming instead of object naming in patients with mild AD. On the other hand, transcranial direct current stimulation (tDCS), another similar noninvasive neuromodulation technique, is also capable of altering the cortical activity. A recent tDCS study concluded that temporal cortex stimulation, rather than the left DLPFC tDCS, could improve cognitive function in AD [8]. Thus, further investigations are warranted to answer this question.
Our subgroup analysis of session numbers showed that rTMS of more than ten sessions was more effective than ≤ 10 sessions of stimulation (0.59 [0.28, 0.90] vs. 0.18 [− 0.20, 0.56]). Our result was in a larger extent in accordance with the previous meta-analysis [20], which has demonstrated that stimulation for ≥ 5 sessions produced a better effect than rTMS for ≤ 3 sessions. In our included studies, after-effects at a long stage could be witnessed after the application of multiple sessions of rTMS [16, 23, 25]. Thus, together with the previous studies, our results proved that a longer term treatment was more beneficial in a certain extent.
In the subgroup analyses, rTMS combined with cognitive training, instead of rTMS alone, yielded more significant clinical benefits. As for the necessities of combing with cognitive training, previous studies have reported conflict results. The study of Rabey et.al, [29], based on the randomized double-blind trials, provided evidence of the safety and efficacy of rTMS with concurrent cognitive training in the treatment of AD. However, another study found no significant effects in the studies with concurrent cognitive training, whereas rTMS without concurrent cognitive training showed significant effects [39]. Our study preliminarily suggested that rTMS with concurrent cognitive training could produce larger improvement. However, there was a possibility that this result was confounded with the session numbers. On the whole, rTMS combined with cognitive training was adopted in studies with larger session numbers and longer term treatment [19, 23, 25, 26, 29]. Thus, the true value of concurrent cognitive training in this condition needed to be evaluated in further investigations. Furthermore, there are also possibilities of interactions between all of the related variables including stimulation frequencies and cognitive training. However, evaluations of these interactions were hard to perform based on the available evidence. Thus, it is important to evaluate the potential interactions between these clinical factors and to identify the optimal combination of rTMS settings in the future studies.
With regard to the characteristics of the patients themselves, our study suggested that patients with a higher level of education exhibited better cognitive outcomes after receiving rTMS. Importantly, the study of tDCS presented a similar result [8]. As mentioned in the study of Cai et. al [8], one’s education may be a reflection of cognitive reserve (CR), which serves as an index of the discrepancy between the cognitive status and neuropathology [40]. Several previous studies have discussed the mechanism that response of noninvasive brain stimulation was better in subjects with higher levels of education [8, 41,42,43]. As for the severity of cognitive impairment in patients, Cotelli et al. [38], based on “online stimulation” trials, concluded that naming performance in patients with the advanced AD could also be improved after application of rTMS on the bilateral DLPFC. In most studies, the rTMS was applied to patients with mild-to-moderate AD. Our subgroup analysis showed that patients with severe AD may not benefit as much as patients with mild-to-moderate AD from this noninvasive brain stimulation therapy.
The present meta-analysis have included the recently published RCTs based on the rigorous inclusion criteria. Thus, our study enriched the previous literatures and presented high-level evidence in this field. In addition to a relatively lower level of heterogeneity, absence of significant publication bias as well as the higher sensitivity further validated the reliability of our results. Moreover, based on the existing evidence, we made the most comprehensive investigations about the influential factors through six subgroup analyses, and patients with severe dementia were also included, which would be helpful for finding out the best stimulation patterns and proper therapeutic indications, and thus facilitating this noninvasive therapy in the long term.
Several limitations need to be mentioned in our study. First, this meta-analysis included 10 studies with 15 trials, but the sample size was still small, thus further RCTs are needed in the future. Second, our study used the first measurements taken after the stimulation, and the long-term efficacy of rTMS for AD warrants to be summarized in the later studies. Third, only the cognitive outcomes were evaluated in our study, and the changes of behavioral and psychological symptoms need further research. Fourth, sufficient individual patient data were not available in the included studies, thus a correlation study is warranted to identify the potential relationship of particular clinical variables with the outcomes based on the individual patient data in the future. Finally, the sample size in some of our subgroup analyses was relatively small, thus, these results should be explained with caution and need to be validated in further clinical trials.
Conclusions
This meta-analysis provided further evidence of the efficacy of rTMS on cognition in AD. Subgroup analyses showed that rTMS of multiple stimulation sites, more than ten sessions, high-frequency, or with concurrent cognitive training was capable of producing greater improvement. In addition, significant improved cognitive outcomes were seen in patients with a higher level of education, or in patients with mild-to-moderate AD. However, considering the relative small sample sizes, the results in our subgroup analyses must be interpreted with caution. Large, double-blind RCTs are warranted in the future to investigate the appropriate stimulation patterns and to identify the patients who are more likely to benefit from this procedure.
Abbreviations
- AD:
-
Alzheimer’s disease
- rTMS:
-
Repetitive transcranial magnetic stimulation
- MMSE:
-
Mini-Mental State Examination
- ADAS-cog:
-
Alzheimer Disease Assessment Scale-cognitive subsection
- DLPFC:
-
Dorsolateral prefrontal cortex
- RCT:
-
Randomized controlled trial
References
Celsis P (2000) Age-related cognitive decline, mild cognitive impairment or preclinical Alzheimer's disease? Ann Med 32(1):6–14. https://doi.org/10.3109/07853890008995904
Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA (2012) The natural history of cognitive decline in Alzheimer's disease. Psychol Aging 27(4):1008–1017. https://doi.org/10.1037/a0029857
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer's disease. Lancet 388(10043):505–517. https://doi.org/10.1016/s0140-6736(15)01124-1
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9(1):63–75e62. https://doi.org/10.1016/j.jalz.2012.11.007
Alzheimer's A (2016) 2016 Alzheimer's disease facts and figures. Alzheimers Dement 12(4):459–509
Querfurth HW, LaFerla FM (2010) Alzheimer's disease. N Engl J Med 362(4):329–344. https://doi.org/10.1056/NEJMra0909142
Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch H, Popp J, Daamen M, Gorris D, Heneka MT, Boecker H, Biersack HJ, Maier W, Schild HH, Wagner M, Jessen F (2012) Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 79(13):1332–1339. https://doi.org/10.1212/WNL.0b013e31826c1a8d
Cai MJ, Guo ZW, Xing GQ, Peng HT, Zhou L, Chen HP, McClure MA, He L, Xiong LW, He B, Du F, Mu Q (2019) Transcranial direct current stimulation improves cognitive function in mild to moderate Alzheimer disease a meta-analysis. Alzheimer Dis Assoc Disord 33(2):170–178. https://doi.org/10.1097/wad.0000000000000304
Shafqat S (2008) Alzheimer disease therapeutics: perspectives from the developing world. J Alzheimers Dis 15(2):285–287
Bellanti F, Iannelli G, Blonda M, Tamborra R, Villani R, Romano A, Calcagnini S, Mazzoccoli G, Vinciguerra M, Gaetani S, Giudetti AM, Vendemiale G, Cassano T, Serviddio G (2017) Alterations of clock gene RNA expression in brain regions of a triple transgenic model of Alzheimer's disease. J Alzheimers Dis 59(2):615–631. https://doi.org/10.3233/jad-160942
Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1(8437):1106–1107. https://doi.org/10.1016/s0140-6736(85)92413-4
Ni Z, Chen R (2015) Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener 4:22. https://doi.org/10.1186/s40035-015-0045-x
Valero-Cabre A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA (2017) Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev 83:381–404. https://doi.org/10.1016/j.neubiorev.2017.10.006
Mueller JK, Grigsby EM, Prevosto V, Petraglia FW 3rd, Rao H, Deng ZD, Peterchev AV, Sommer MA, Egner T, Platt ML, Grill WM (2014) Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci 17(8):1130–1136. https://doi.org/10.1038/nn.3751
Gangitano M, Valero-Cabre A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A (2002) Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol 113(8):1249–1257. https://doi.org/10.1016/s1388-2457(02)00109-8
Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, Miniussi C (2011) Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry 82(7):794–797. https://doi.org/10.1136/jnnp.2009.197848
Ahmed MA, Darwish ES, Khedr EM, El Serogy YM, Ali AM (2012) Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer's dementia. J Neurol 259(1):83–92. https://doi.org/10.1007/s00415-011-6128-4
Padala PR, Padala KP, Lensing SY, Jackson AN, Hunter CR, Parkes CM, Dennis RA, Bopp MM, Caceda R, Mennemeier MS, Roberson PK, Sullivan DH (2018) Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: a double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 261:312–318. https://doi.org/10.1016/j.psychres.2017.12.063
Rutherford G, Lithgow B, Moussavi Z (2015) Short and long-term effects of rTMS treatment on Alzheimer's disease at different stages: a pilot study. J Exp Neurosci 2015(9):43–51. https://doi.org/10.4137/JEN.S24004
Lin Y, Jiang W-J, Shan P-Y, Lu M, Wang T, Li R-H, Zhang N, Ma L (2019) The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: a systematic review and meta-analysis. J Neurol Sci 398:184–191. https://doi.org/10.1016/j.jns.2019.01.038
Chen K, Dong X, Yan L, Huang L, Guan X, Dong C, Tao H, Wang T, Qin X, Wan Q (2018) Repetitive transcranial magnetic stimulation for the treatment of Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 13(10):e0205704. https://doi.org/10.1371/journal.pone.0205704
Liao X, Li G, Wang A, Liu T, Feng S, Guo Z, Tang Q, Jin Y, Xing G, McClure MA, Chen H, He B, Liu H, Mu Q (2015) Repetitive transcranial magnetic stimulation as an alternative therapy for cognitive impairment in Alzheimer's disease: a meta-analysis. J Alzheimers Dis 48(2):463–472. https://doi.org/10.3233/JAD-150346
Zhao J, Li Z, Cong Y, Zhang J, Tan M, Zhang H, Geng N, Li M, Yu W, Shan P (2017) Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer's disease patients. Oncotarget 8(20):33864–33871. https://doi.org/10.18632/oncotarget.13060
Koch G, Bonni S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C, Maiella M, Marra C, Cercignani M, Martorana A, Caltagirone C, Bozzali M (2018) Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. NeuroImage 169:302–311. https://doi.org/10.1016/j.neuroimage.2017.12.048
Zhang F, Qin Y, Xie L, Zheng C, Huang X, Zhang M (2019) High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm 126(8):1081–1094. https://doi.org/10.1007/s00702-019-02022-y
Lee J, Choi BH, Oh E, Sohn EH, Lee AY (2016) Treatment of Alzheimer's disease with repetitive transcranial magnetic stimulation combined with cognitive training: a prospective, randomized, double-blind, placebo-controlled study. J Clin Neurol 12(1):57–64. https://doi.org/10.3988/jcn.2016.12.1.57
Wu Y, Xu W, Liu X, Xu Q, Tang L, Wu S (2015) Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer's disease: a randomized, double-blind, sham-controlled study. Shanghai Arch Psychiatry 27(5):280–288. https://doi.org/10.11919/j.issn.1002-0829.215107
Eliasova I, Anderkova L, Marecek R, Rektorova I (2014) Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer's disease: a pilot study. J Neurol Sci 346(1–2):318–322. https://doi.org/10.1016/j.jns.2014.08.036
Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M (2013) Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer's disease: a randomized, double-blind study. J Neural Transm (Vienna) 120(5):813–819. https://doi.org/10.1007/s00702-012-0902-z
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 319(4):388–396. https://doi.org/10.1001/jama.2017.19163
Moher D, Schulz KF, Altman D, Group C (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 285(15):1987–1991. https://doi.org/10.1001/jama.285.15.1987
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:3189–3198. https://doi.org/10.1016/0022-3956(75)90026-6
Habib R, Nyberg L, Tulving E (2003) Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci 7(6):241–245
Turriziani P, Smirni D, Zappala G, Mangano GR, Oliveri M, Cipolotti L (2012) Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci 6:62. https://doi.org/10.3389/fnhum.2012.00062
Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G (2006) A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull 69(1):86–94. https://doi.org/10.1016/j.brainresbull.2005.11.003
Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J (2004) Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19(7):1950–1962. https://doi.org/10.1111/j.1460-9568.2004.03277.x
Cotelli M, Manenti R, Cappa SF, Zanetti O, Miniussi C (2008) Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol 15(12):1286–1292. https://doi.org/10.1111/j.1468-1331.2008.02202.x
Cheng CPW, Wong CSM, Lee KK, Chan APK, Yeung JWF, Chan WC (2018) Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry 33(1):e1–e13. https://doi.org/10.1002/gps.4726
Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8(3):448–460
Stern Y (2012) Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 11(11):1006–1012. https://doi.org/10.1016/s1474-4422(12)70191-6
Soldan A, Pettigrew C, Lu Y, Wang MC, Selnes O, Albert M, Brown T, Ratnanather JT, Younes L, Miller MI (2015) Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Hum Brain Mapp 36(7):2826–2841. https://doi.org/10.1002/hbm.22810
Bozzali M, Dowling C, Serra L, Spano B, Torso M, Marra C, Castelli D, Dowell NG, Koch G, Caltagirone C, Cercignani M (2015) The impact of cognitive reserve on brain functional connectivity in Alzheimer's disease. J Alzheimers Dis 44(1):243–250. https://doi.org/10.3233/jad-141824
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant no.8187052509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to report.
Ethical approval
All studies in this review have been approved by the local ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2019_9644_MOESM1_ESM.tif
Supplementary file1 Fig. S1 Forest plot for the standardized mean differences in subgroup of stimulation sites with 95% CI (TIF 4624 kb)
415_2019_9644_MOESM2_ESM.tif
Supplementary file2 Fig.S2 Forest plot for the standardized mean differences in subgroup of session numbers with 95% CI (TIF 4582 kb)
415_2019_9644_MOESM3_ESM.tif
Supplementary file3 Fig.S3 Forest plot for the standardized mean differences in subgroup of concurrent cognitive training with 95% CI (TIF 4653 kb)
415_2019_9644_MOESM4_ESM.tif
Supplementary file4 Fig.S4 Forest plot for the standardized mean differences in subgroup of stimulation frequency with 95% CI (TIF 5058 kb)
415_2019_9644_MOESM5_ESM.tif
Supplementary file5 Fig.S5 Forest plot for the standardized mean differences in subgroup of education levels with 95% CI (TIF 4320 kb)
415_2019_9644_MOESM6_ESM.tif
Supplementary file6 Fig.S6 Forest plot for the standardized mean differences in subgroup of cognitive impairment severity with 95% CI (TIF 4635 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Mao, Z., Ling, Z. et al. Repetitive transcranial magnetic stimulation for cognitive impairment in Alzheimer's disease: a meta-analysis of randomized controlled trials. J Neurol 267, 791–801 (2020). https://doi.org/10.1007/s00415-019-09644-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09644-y