Abstract
Background
To develop a novel nomogram for predicting 2-year and 5-year disease-free survival (DFS) and overall survival (OS) in patients with cT1-clear cell renal cell carcinoma (ccRCC) undergoing partial nephrectomy (PN).
Methods
A retrospective study was conducted across five urological centers, including 940 patients who underwent PN for cT1N0M0-ccRCC. Four centers were randomly selected to constitute the training group, while the remaining center served as the testing group. We employed the LASSO and multivariate Cox regression to develop new nomograms. The 1,000 bootstrap-corrected c-index, net reclassification improvement (NRI) and receiver operating characteristic curve were employed to compare the predictive abilities of new nomograms with the widely used UUIS and SSIGN models. Finally, the novel nomograms underwent external validation.
Results
The training group included 714 patients, while the testing group consisted of 226 patients. The bootstrap-corrected c-indexes for the DFS and OS model were 0.870 and 0.902, respectively. In the training cohort, the AUC for the DFS and OS models at 2 years and 5 years were 0.953, 0.902, 0.988, and 0.911, respectively. These values were also assessed in the testing cohort. The predictive capabilities of the new nomograms surpassed those of the UUIS and SSIGN models (NRI > 0). Decision curve analysis demonstrated that the novel nomograms provide greater net benefits compared to the UUIS and SSIGN models.
Conclusions
Our novel nomograms demonstrated strong predictive ability for forecasting oncological outcomes in cT1-ccRCC patients after PN. These user-friendly nomograms are simple and convenient for clinical application, providing tangible clinical benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Renal cell carcinoma (RCC) is the most common solid lesion in the kidney, with clear cell RCC (ccRCC) being its most prevalent subtype, making up about 70–80% of all cases.1 Localized RCC represents 65% of all RCC diagnosed.2 Partial nephrectomy (PN) is recommended by most global urological guidelines for T1-ccRCC, because it provides comparable tumor control while better preserving renal function compared with radical nephrectomy (RN).1,3,4 Although various models have been developed to predict the oncological outcomes of RCC, these models have included all stages of RCC and have not adequately addressed the issue of multicollinearity among variables.5,6,7,8,9,10,11,12 Moreover, most of these nomograms were created more than a decade ago and failed to incorporate crucial prognostic factors, such as sarcomatoid differentiation.5,6,7 Futhermore, while most existing models primarily predict cancer-specific survival (CSS) or overall survival (OS),5,6,7,8,9,10,11,12 few have been developed to specifically predict DFS. As the factors influencing disease-free survival (DFS) and OS in cT1-ccRCC patients differ significantly, it is difficult for a single model to predict both outcomes accurately. Although the emphasis of most prognostic models has been on OS/CSS, accurately predicting DFS is equally important. For surgeons, accurate DFS predictions are essential for follow-up and treatment planning. For patients, disease progression often necessitates the initiation of further therapy, significantly impacting their prognosis and financial situation, which is a primary concern after surgery.
Additionally, the clinical applicability of the latest model for predicting postsurgical oncological outcomes of RCC is limited by the lack of decision curve analysis (DCA) and validation.12 Currently, no study has developed a nomogram specifically designed to predict the oncological outcomes of cT1-ccRCC following PN. This study was designed to develop a new nomogram to predict 2-year and 5-year DFS and OS for cT1-ccRCC patients undergoing PN.
Materials and Methods
Ethics
Written informed consent was obtained preoperatively from all patients. The study was conducted in accordance with the Declaration of Helsinki. All surgical procedures were performed by experienced surgeons. The study was approved by the Institutional Review Board (No: 202201007) and reported in line with the STROCSS criteria.13
Patient Selection
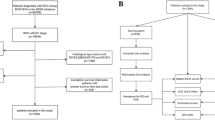
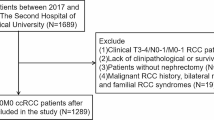
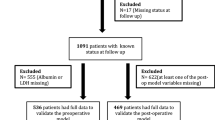
We identified 940 Asian patients who underwent PN for cT1N0M0-ccRCC without positive surgical margins and neoadjuvant therapy between 2010 and 2019 at five urological centers. Four centers were randomly selected to constitute the training group, while the remaining center served as the testing group. All participants were of Asian race.
Patient Features
The clinical features included gender, age at surgery, smoking status, lumbago, hematuresis, laterality, Eastern Cooperative Oncology Group (ECOG) performance status, age-adjusted Charlson Comorbidity Index (aCCI),14 baseline cardiovascular disease (defined using the myocardial infarction, congestive heart failure, and peripheral vascular disease components of the Charlson score), body mass index (BMI), preoperative estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), and surgical approach (open versus laparoscopic). Enhanced computed tomography (CT) was used to assess the radiographic features. Radiographic features were rereviewed by a genitourinary radiologist and included cT-stage, hemorrhage, necrosis, calcification, and cystic formation. The pathologic features were rereviewed by one genitourinary pathologist, included tumor size, pT-stage, WHO/ISUP grade, sarcomatoid differentiation and coagulative tumor necrosis.15,16
The clinical tumor (cT) stage and pathological tumor (pT) stage was determined according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th edition).17
Outcomes
The primary outcomes were disease progression and all-cause mortality. Disease progression was defined as local ipsilateral recurrence, contralateral recurrence, or distant metastasis. Follow-up duration was calculated from the date of treatment to the date of the primary outcome or the last follow-up.
Statistical Analysis
The patient features were summarized as frequencies and percentages for categorical variables and as medians with interquartile ranges (IQRs) for continuous variables that were not normally distributed. To compare patient features between the training and testing cohort, the Wilcoxon rank-sum test was used for nonnormally distributed variables, whereas Fisher exact test and the chi-square test were used for categorical variables.
To mitigate the impact of multicollinearity among variables, patient features within the training cohort underwent least absolute selection and shrinkage operator (LASSO) regression with tenfold cross-validation. This process was designed to identify relevant variables affecting disease-free survival (DFS) and overall survival (OS). Subsequently, multivariate Cox proportional hazards regression was employed to validate the impact of the selected features on outcomes and to construct a nomogram for forecasting 2-year and 5-year outcomes. The results of the Cox regression were summarized with hazard ratios (HR) and 95% confidence intervals (CIs). Following 1,000 rounds of internal verification, the predictive performance of the nomogram was assessed using calibration plots. The 1,000 times bootstrap-corrected concordance indexes (c-indexes) and time-dependent receiver operator characteristic (timeROC) curves for both the training and testing cohorts quantified the discrimination accuracy of the new nomograms versus University of California at Los Angeles Integrated Staging System (UUIS) and Stage, Size, Grade and Necrosis (SSIGN) score.5,18 The area under the curve (AUC) and net reclassification improvement (NRI) were used to compare the predictive abilities of the new nomograms with the UUIS and SSIGN models. Ultimately, decision curve analysis (DCA) was employed to assess the net benefit conferred by the models.
Statistical analyses were performed using R version 4.3.2. All statistical tests were two-sided with significance set at p < 0.05.
Results
Baseline and Features of Patients
The overall cohort included 940 patients, divided into a training group (n = 714) and a testing group (n = 226). Except for radiographic evidence of hemorrhage (8.0% vs. 14.1%), all patient features were balanced between the training and testing cohorts. The median follow-up time for the 940 patients was 52 months (IQR 24–75). During this period, 60 patients experienced disease progression and 39 died (Table 1).
Risk Factors for Modeling
After employing LASSO regression with tenfold cross-validation and multivariate Cox regression, pT stage (pT3: HR = 3.663, 95% CI 1.128–11.896, P = 0.03), grade (grade 3: HR = 2.687, 95% CI 1.019–7.083, P = 0.046; grade 4: HR = 3.568, 95% CI 1.073–11.858, P = 0.038), tumor size (HR= 1.41, 95% CI 1.006–1.977, P = 0.046), coagulative tumor necrosis (HR = 3.416, 95% CI 1.549–7.536, P = 0.002), and sarcomatoid differentiation (HR = 3.881, 95% CI 1.486–10.136, P = 0.006) were identified as relevant variables for DFS (Supplementary Tables 1–2, Supplementary Fig. 1). Meanwhile, tumor size (HR = 1.654, 95% CI 1.271–2.153, P < 0.001), sarcomatoid differentiation (HR= 2.941, 95% CI 0.832–10.387, P = 0.094), and aCCI (HR = 2.723, 95% CI 2.049–3.619, P < 0.001) were identified as relevant variables for OS (Supplementary Tables 3–4, Supplementary Fig. 1).
Development and Assessment of New Nomograms
After 1,000 times internal verification with Bootstrap, the resulting multivariable models for DFS and OS had bootstrap-corrected c-indices of 0.87 and 0.902 in the training cohort, and 0.864 and 0.894 in the testing cohort, respectively. Meanwhile, the bootstrap-corrected c-indices for the SSIGN and UUIS models were 0.751, 0.681 for DFS and 0.634, 0.666 for OS in the training cohort. In the testing cohort, the c-indices were 0.771 and 0.631 for DFS, and 0.721 and 0.713 for OS, respectively.
For ease of clinical use, the two newly established models were transformed into visual nomograms to predict 2-year and 5-year outcomes (Fig. 1). A higher total score on the nomograms indicates a greater probability of disease progression or all-cause mortality. Calibration plots for the nomograms predicting DFS and OS are shown in Fig. 2. The predicted risks for 2-year DFS, 5-year DFS, 2-year OS, and 5-year OS demonstrated good agreement between the model predictions and actual outcomes.
Calibration of the nomogram models. A Calibration of 2 years DFS prediction; B calibration of 5 years DFS prediction; C calibration of 2 years OS prediction; D calibration of 5 years OS prediction. Plot of observed incidence versus the predicted probability according to the nomograms. DFS disease-free survival; OS overall survival
In the training cohort, the AUC for the DFS prediction model is 0.953 (95% CI 90.35–100.31) at 2 years and 0.902 (95% CI 84.53–95.84) at 5 years. The AUC for the OS prediction model is 0.988 (95% CI 97.78–99.89) at 2 years and 0.911 (95% CI 83.39–98.84) at 5 years. In the testing cohort, the AUC for the DFS prediction model is 0.917 (95% CI 82.84–100.6) at 2 years and 0.878 (95% CI 76.26–99.32) at 5 years. The AUC for the OS prediction model is 0.945 (95% CI 88.4–100.68) at 2 years and 0.894 (95% CI 79.99–98.77) at 5 years (Table 2; Figs. 3–4).
Receiver operating characteristic curve for nomograms, SSIGN, and UUIS models predicting A DFS at 2 years, B DFS at 5 years, C OS at 2 years, D OS at 5 years in the training cohort. SSIGN Stage, Size, Grade and Necrosis score; UUIS University of California at Los Angeles Integrated Staging System; AUC area under the receiver operating characteristic curve; DFS disease-free survival; OS overall survival
Receiver operating characteristic curve for nomograms, SSIGN and UUIS models predicting A DFS at 2 years, B DFS at 5 years, C OS at 2 years, D OS at 5 years in the testing cohort. SSIGN Stage, Size, Grade and Necrosis score; UUIS University of California at Los Angeles Integrated Staging System; AUC area under the receiver operating characteristic curve; DFS disease-free survival; OS overall survival
DCA revealed that the established prediction models provided a net benefit for the 2-year DFS, 5-year DFS, 2-year OS, and 5-year OS at threshold probabilities below 57.5%, 75%, 50%, and 73%, respectively (Fig. 5).
Decision curve showing the benefit of the nomograms, SSIGN, and UUIS models compared with treating all cases or none as at risk of A DFS at 2 years, B DFS at 5 years, C OS at 2 years, D OS at 5 years. SSIGN Stage, Size, Grade and Necrosis score; UUIS University of California at Los Angeles Integrated Staging System; DFS disease-free survival; OS overall survival
Improvement of New Nomograms Compared with SSIGN and UUIS Models
Furthermore, we evaluated the enhanced predictive accuracy of the two novel nomograms compared to the SSIGN and UUIS models using NRI. DFS and OS risks were categorized at cutoff points of 0.2 and 0.6. The results indicated that both the predictive and discriminatory performance of our novel nomograms surpassed that of the SSIGN and UUIS models (NRI > 0). Additionally, a higher AUC indicated that our new models exhibited superior predictive capability compared to the SSIGN and UUIS models (Table 2). Finally, the DCA curve demonstrated that the net benefit of the new models surpassed that of the SSIGN and UUIS models (Fig. 5).
Discussion
With PN demonstrating comparable tumor control to RN, its adoption in RCC management has been progressively increasing.19,20 A tool that accurately predicts oncologic events while remaining user-friendly in clinical practice is crucial for both patients and clinicians.
To contribute to the field, our study developed two novel nomograms to predict DFS and OS in patients diagnosed with cT1-ccRCC who underwent PN. Given the favorable oncologic outcomes observed in cT1-ccRCC, with few patients experiencing tumor recurrence or death within the first year, we chose 2- and 5-year time points to forecast both short-term and long-term oncologic results following PN.
Our study revealed that the risk of DFS in patients is primarily related to the following factors: pT stage, nuclear grade, tumor size, coagulative tumor necrosis, and sarcomatoid differentiation. Whereas our DFS prediction model might appear similar to the SSIGN model, notable distinctions exist between the two.5 First, the SSIGN model utilized the fifth edition of the AJCC Cancer Staging Manual for tumor staging, which poses challenges for contemporary physicians and affects the model's predictive efficacy. Then, while the SSIGN model categorizes tumor size based on whether it exceeds 5 cm, our model treats tumor size as a continuous variable. We quantified its risk using a nomogram, which improved the accuracy of our model. Finally, our model incorporated sarcomatoid differentiation, a crucial factor influencing DFS.21 Indeed, our results demonstrated that the predictive efficacy of our new model was significantly superior to that of the SSIGN model.
Regarding our OS prediction model, we identified three factors associated with OS through LASSO regression: tumor size, aCCI, and sarcomatoid differentiation. However, the results of the multivariate Cox regression indicated that sarcomatoid differentiation was not an independent risk factor for OS (P = 0.094). Interestingly, excluding sarcomatoid differentiation from the model reduced the model's c-index and AUC, indicating a decline in predictive efficacy. Similar situations have been observed in many predictive models,22,23,24 demonstrating that the factors included in the final predictive model are not necessarily significant in multifactor regression.
After constructing our new models, we validated their predictive capability using bootstrap-corrected c-index and ROC analysis. The DCA results further confirmed the net benefit of our model, especially at lower threshold probabilities. Finally, the results of external validation reinforced the accuracy of our predicted results.
The UUIS and SSIGN score are widely used tools for assessing the oncological prognosis of ccRCC.5,18 Despite some models undergoing external validation, the outcomes of such validations display variability.25,26 For example, the UUIS model has undergone multiple external validations; however, the outcomes have been inconsistent.27,28 In Parker et al.'s study, the c-index of the SSIGN model in the external cohort was 0.82, whereas the c-index for external validation in Correa et al.'s study was only 0.688 (95% CI 0.686–0.689).9,28 Our study compared the predictive accuracy and net benefit of our novel models against the SSIGN and UUIS models using the c-index, AUC, NRI, and DCA. The results indicate that the precision and net benefits of the new models significantly surpass those of the SSIGN and UUIS models.
Recently, Karakiewicz's research team introduced novel predictive models tailored to ccRCC, papillary RCC (papRCC), and chromophobe RCC (chrRCC). For ccRCC, their latest model achieved a c-index of 0.83 for progression-free survival (PFS) and 0.86 for CSS.12 Although the c-index confirms the model's accuracy, it includes 13 predictive factors, making it more complex than other models and increasing the potential for overfitting. Furthermore, this model lacks internal validation, external validation, as well as a DCA curve, limiting its clinical utility. In contrast, our model has undergone rigorous external validation and demonstrated a net benefit.
Another recently developed model is the GRANT score. However, it differs from our new models, as evidenced by its suboptimal predictive performance for DFS and OS in RCC (with c-indices of 0.589 and 0.613, respectively). Notably, this model was developed based on a population with a relatively high risk of recurrence after RN,29 potentially making it less suitable for patients undergoing PN for cT1-ccRCC.
This study has several limitations. Becaues of the late adoption of robot-assisted surgery in the participating institutions, the number of patients undergoing robot-assisted PN was limited. Additionally, patients who underwent robot-assisted surgery lacked an adequate follow-up period, leading to the exclusion of robot-assisted surgery as a covariate in our study. Nevertheless, previous investigations have shown that robot-assisted surgery does not increase the risk of poor oncological outcomes compared to laparoscopic surgery. Therefore, we hypothesize that the novel nomograms can be effectively applied to patients undergoing robot-assisted PN. Moreover, potential racial biases exist; patients treated at the participating institutions are predominantly Asian, posing challenges in collecting adequate samples from other racial groups. However, existing studies have not demonstrated any differences in ccRCC prognosis between Asian and other racial groups.30 Consequently, we believe that this model can be applied to other races Finally, external validation in a large population is still needed.
Despite the above limitations, our novel nomograms effectively address several deficiencies present in existing models. For instance, we successfully mitigate confounding factors arising from multicollinearity during predictor selection. Additionally, our nomograms are better suited to contemporary populations compared with older models, and the confirmed net benefit of our nomograms further enhances their utility.
Conclusions
After external validation, our novel nomograms demonstrated commendable predictive capacity for forecasting 2-year and 5-year oncological outcomes in patients with cT1-ccRCC who have undergone PN. These nomograms offer simplicity and convenience in clinical application, substantiated by their tangible clinical net benefit. We recommend these new nomograms specifically for patients with cT1-ccRCC after PN to predict their oncological outcomes. These tools are valuable for patient counseling, personalized follow-up strategies, informed decision-making, and interpreting clinical trial findings. However, it remains imperative to externally validate these novel nomograms in a confirm population to substantiate their role as reliable prognostic predictive tools in clinical practice.
Data availability
The data sets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA Guideline: Part I. J Urol. 2021;206(2):199–208. https://doi.org/10.1097/JU.0000000000001911.
Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906. https://doi.org/10.1016/S0140-6736(15)00046-X.
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(1):71–90. https://doi.org/10.6004/jnccn.2022.0001.
Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. https://doi.org/10.1016/j.eururo.2022.03.006.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–400. https://doi.org/10.1016/S0022-5347(05)64153-5.
Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97(7):1663–71. https://doi.org/10.1002/cncr.11234.
Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25(11):1316–22. https://doi.org/10.1200/JCO.2006.06.1218.
Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60(4):644–61. https://doi.org/10.1016/j.eururo.2011.06.041.
Parker WP, Cheville JC, Frank I, et al. Application of the Stage, Size, Grade, and Necrosis (SSIGN) score for clear cell renal cell carcinoma in contemporary patients. Eur Urol. 2017;71(4):665–73. https://doi.org/10.1016/j.eururo.2016.05.034.
Usher-Smith JA, Li L, Roberts L, et al. Risk models for recurrence and survival after kidney cancer: a systematic review. BJU Int. 2022;130(5):562–79. https://doi.org/10.1111/bju.15673.
Buti S, Puligandla M, Bersanelli M, et al. Validation of a new prognostic model to easily predict outcome in renal cell carcinoma: the GRANT score applied to the ASSURE trial population. Ann Oncol. 2018;29(7):1604. https://doi.org/10.1093/annonc/mdx799.
Leibovich BC, Lohse CM, Cheville JC, et al. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur Urol. 2018;73(5):772–80. https://doi.org/10.1016/j.eururo.2018.01.005.
Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165. https://doi.org/10.1016/j.ijsu.2021.106165.
Andrade HS, Zargar H, Caputo PA, et al. Five-year oncologic outcomes after transperitoneal robotic partial nephrectomy for renal cell carcinoma. Eur Urol. 2016;69(6):1149–54. https://doi.org/10.1016/j.eururo.2015.12.004.
Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019;74(1):4–17. https://doi.org/10.1111/his.13735.
Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiation. Cancer Immunol Res. 2015;3(12):1303–7. https://doi.org/10.1158/2326-6066.CIR-15-0150.
Amin MB, Greene FL, Edge SB, et al. The Eighth Edi tion AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. https://doi.org/10.3322/caac.21388.
Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–66. https://doi.org/10.1200/JCO.2002.05.111.
Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71(4):606–17. https://doi.org/10.1016/j.eururo.2016.08.060.
Banegas MP, Harlan LC, Mann B, Yabroff KR. Toward greater adoption of minimally invasive and nephron-sparing surgical techniques for renal cell cancer in the United States. Urol Oncol. 2016;34(10):433 e9-433 e17. https://doi.org/10.1016/j.urolonc.2016.05.021.
Qu L, Chen H, Chen Q, et al. Development and validation of a prognostic model incorporating tumor thrombus grading for nonmetastatic clear cell renal cell carcinoma with tumor thrombus: A multicohort study. MedComm (2020). 2023;4(4):e300. https://doi.org/10.1002/mco2.300.
Gandaglia G, Ploussard G, Valerio M, et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol. 2019;75(3):506–14. https://doi.org/10.1016/j.eururo.2018.10.012.
Mazzone E, Gandaglia G, Ploussard G, et al. Risk stratification of patients candidate to radical prostatectomy based on clinical and multiparametric magnetic resonance imaging parameters: development and external validation of novel risk groups. Eur Urol. 2022;81(2):193–203. https://doi.org/10.1016/j.eururo.2021.07.027.
Bhat KRS, CovasMoschovas M, Sandri M, et al. A predictive preoperative and postoperative nomogram for postoperative potency recovery after robot-assisted radical prostatectomy. J Urol. 2021;206(4):942–51. https://doi.org/10.1097/JU.0000000000001895.
Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943–52. https://doi.org/10.1007/s00345-018-2309-4.
Lee BH, Feifer A, Feuerstein MA, et al. Validation of a postoperative nomogram predicting recurrence in patients with conventional clear cell renal cell carcinoma. Eur Urol Focus. 2018;4(1):100–5. https://doi.org/10.1016/j.euf.2016.07.006.
Tan MH, Kanesvaran R, Li H, et al. Comparison of the UCLA Integrated Staging System and the Leibovich score in survival prediction for patients with nonmetastatic clear cell renal cell carcinoma. Urology. 2010;75(6):1365–70. https://doi.org/10.1016/j.urology.2009.07.1289.
Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J Clin Oncol. 2019;37(23):2062–71. https://doi.org/10.1200/JCO.19.00107.
Buti S, Puligandla M, Bersanelli M, et al. Validation of a new prognostic model to easily predict outcome in renal cell carcinoma: the GRANT score applied to the ASSURE trial population. Ann Oncol. 2017;28(11):2747–53. https://doi.org/10.1093/annonc/mdx492.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. https://doi.org/10.3322/caac.21820.
Acknowledgment
Haozhe Xu contributed to the conceptualization, methodology, data curation, software development, validation, formal analysis, and writing of the original draft. Zhuo Xing was involved in investigation and formal analysis. Jie Wang and Zhengtong Lv provided resources and supervision. Piye Deng was involved in the investigation and visualization. Yuan Li was involved in conceptualization, writing, reviewing, editing, supervision, and project administration. This study was supported by the National Natural Science Foundation of China (82373435 and 82172878).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Xing, Z., Wang, J. et al. Development and External Validation of Nomograms for Predicting Disease-Free Survival and Overall Survival in Patients with cT1-ccRCC After Partial Nephrectomy: A Multicenter Retrospective Study. Ann Surg Oncol 31, 5827–5838 (2024). https://doi.org/10.1245/s10434-024-15718-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15718-7