Abstract
Background
Weight loss (WL) after gastrectomy for gastric cancer is associated with both decreased compliance with adjuvant chemotherapy and impaired survival. This study examined the effects of administering oral nutritional supplements (ONS) for 3 months after gastrectomy in terms of compliance with adjuvant chemotherapy and survival outcomes.
Methods
This large-scale, multicenter, open-label, randomized controlled trial enrolled 1,003 gastric cancer patients undergoing curative gastrectomy. Patients were assigned to the control group (n = 503) or ONS group (n = 500). In the ONS group, 400 kcal/day of ONS was recommended in addition to a regular diet for 3 months after gastrectomy. Compliance with adjuvant chemotherapy and survival outcomes were compared between the two groups.
Results
Compared with the control group, the ONS group showed significantly decreased WL at 3 months after gastrectomy (8.6 ± 6.1 vs. 7.2 ± 5.7%, respectively, P = 0.0004). The control and ONS groups did not differ regarding the induction rate of adjuvant chemotherapy (84.9 vs. 82.8%, respectively, P = 0.614) or the continuation rate at 3 months postoperatively (75.3 vs. 76.6%, respectively, P = 0.809). Oral nutritional supplements for 3 months showed no survival benefit; the 3- and 5-year overall survival (OS) rates were 91.3% and 87.6% in the control group and 89.6% and 86.4% in the ONS group, respectively, indicating no significant difference (P = 0.548). Subgroup analysis could not detect a population in which ONS administration increased OS.
Conclusions
Administration of ONS for 3 months after gastrectomy was not associated with increased compliance with adjuvant chemotherapy or with improved prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fifth most common cancer worldwide, and the fourth leading cause of cancer deaths.1 While curative gastrectomy is essential for the treatment of gastric cancer, weight loss (WL) remains one of the major complaints postoperatively.2 Weight loss is associated with not only a remarkable deterioration in quality of life but also with reduced immune function and worse prognosis because of decreased compliance with adjuvant chemotherapy.3,4,5,6
Weight loss after gastrectomy is known to be caused by the following factors: increased catabolism due to surgical stress and inflammation; decreased food storage capacity; malabsorption resulting from decreased pancreatic enzyme and gastric acid secretion; and decreased ghrelin secretion in the stomach.7,8,9 Several surgical approaches have been used to reduce WL, including procedures that avoid total gastrectomy as much as possible, such as subtotal gastrectomy with a very small remnant stomach,10,11 and minimally invasive surgery, such as laparoscopic and robotic surgery.12,13,14 However, the issue of WL after gastrectomy has not been resolved.
Weight loss after gastrectomy is known to be time-dependent and is most pronounced during the first 3 months postoperatively. Overall, 10–20% of body weight was reported to be lost after gastrectomy; more than 80% of this WL was observed within the first 3 months postoperatively, while the remaining 20% occurred slowly over time.15
The objective of the current study was to elucidate the long-term effects of ONS for 3 months after gastrectomy using the clinical data of patients who participated in a previously described large-scale (n = 1,003), multicenter, open-label, randomized controlled trial that was conducted to evaluate the clinical impact of administering oral nutritional supplements (ONS) for 3 months after gastrectomy.16 The results showed that WL at 3 months was significantly reduced in the ONS group than in the control group, but the difference became nonsignificant at 1 year postoperatively. However, the post-gastrectomy reduction of WL was maintained for up to 1 year in patients who received ≥ 200 kcal/day of ONS.
Methods
Study Population and Design
In this study, we examined patients enrolled in the Osaka University Clinical Research Group for Gastroenterological Study, a large-scale, multicenter, open-label, phase III randomized controlled trial at 22 hospitals, in which curative distal, proximal, and total gastrectomy (DG, PG, and TG, respectively) were performed between November 11, 2013, and July 13, 2017 for histologically proven primary gastric cancer. Details regarding the eligibility criteria and the 2-stage enrollment system of the original trial have been reported previously.16 The study protocol was approved by the institutional review board of each participating hospital before study initiation. This study was performed in accordance with both the Japanese Ethical Guidelines for Clinical Research and the international ethical recommendations documented in the Declaration of Helsinki. All patients provided written, informed consent before randomization.
Surgical Procedure
Patients underwent standard gastrectomy and lymph node dissection according to the Japanese Gastric Cancer Treatment Guidelines 2014.17 In most cases, D1 plus lymphadenectomy (D1 + dissection) was performed in patients with cT1 tumors without regional lymph node metastasis, while D2 lymphadenectomy was performed in patients with cT1 tumors with regional lymph node metastasis and in patients with cT2–4 tumors. The surgical approach (i.e., open or laparoscopic) and reconstruction method were not prescribed in the protocol and depended on the gastric cancer treatment strategy at each institution. Surgical data and pathology results were recorded according to the 14th edition of the Japanese Classification of Gastric Carcinoma.18 Postoperative management, including the resumption of oral intake other than ONS, was generally performed according to the clinical policies of each participating institution.
Intervention
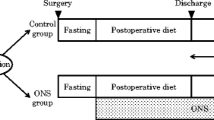
As previously described, enrolled patients were randomly assigned (1:1) to either the ONS group or the control group, on the basis of stratification factors such as institution, disease stage, and type of gastrectomy. In addition to the regular diet, it was recommended that patients in the ONS group, but not the control group, receive 400 mL/day (400 kcal/day) of Racol® NF for 3 months beginning within 3 days after resumption of the regular oral diet.
Postoperative Adjuvant Chemotherapy
Regarding postoperative adjuvant chemotherapy, oral administration of S-1 for 1 year was planned for patients with pathological stage II or III gastric cancer according to the Japanese Gastric Cancer Treatment Guidelines 2014 (ver. 4).17,19 Postoperative chemotherapy for stage III gastric cancer also included capecitabine plus oxaliplatin treatment for 6 months or 6 months of S-1 plus docetaxel followed by 6 months of S-1.20,21 In addition, intraperitoneal chemotherapy and other systematic chemotherapies were used depending on the policies and clinical trials of each participating institution.22
Surveillance
The enrolled patients received surveillance at each institution’s outpatient clinics on the basis of the principles of the Japanese Gastric Cancer Treatment Guidelines 2014 (ver. 4).17 Surveillance included physical examinations and blood tests (such as serum albumin level, C-reactive protein (CRP) level, and levels of tumor markers including carcinoembryonic antigen and carbohydrate antigen 19-9) at 1 and 2 months postoperatively and then every 3 months for the first year postoperatively and every 6 months beyond the first year. Imaging tests, such as computed tomography scans, were recommended every 6 months until 5 years postoperatively.
Statistical Analysis
Overall survival (OS) was defined as the period between surgery and death from any cause, and relapse-free survival (RFS) was defined as the period between surgery and recurrence or between surgery and death if recurrence did not occur. Overall survival and RFS curves were calculated using the Kaplan–Meier method and were statistically compared between the ONS and control group using the log-rank test. Comparisons of OS curves between the two groups were examined according to each pathological stage. Additionally, for patients whose caloric intake of ONS was available from patient reports (n = 403), the ONS group was divided into two subgroups: ≥ 200 kcal/day (based on half of the recommended amount of 400 kcal/day) versus < 200 kcal/day. An analysis was performed to determine how OS was affected by the administration of ≥ 200 kcal/day of ONS. A subgroup analysis was performed with a proportional hazards model for OS to evaluate the statistical interactions between the treatment groups and seven prespecified subgroups. Continuous numerical data are expressed as the mean and standard deviation (SD), and the distribution of dichotomous data is presented as the percentage with the 95% confidence interval (CI). The χ2 test was used to compare binary variables, and the Student t-test was used to compare continuous variables. All P values < 0.05 were judged as statistically significant. Statistical analysis was performed using JMP software version 17.0.0 (SAS Institute, Cary, NC). The trial is registered at the UMIN Clinical Trials Registry (UMIN = CTR) (UMIN000011919).
Results
Patient Baseline Characteristics
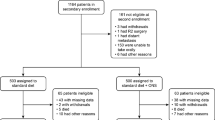
The Trial Consort Diagram was presented in a previous report.16 Briefly, a total of 1,167 patients were enrolled in this study, and after the second-stage randomization and exclusion based on several criteria, 1,003 patients were randomly assigned to the two groups (503 to the control group and 500 to the ONS group) (Fig. 1). The background characteristics of the patients in the two groups were well balanced (Table 1).
Serum Albumin Level and WL at 3 Months After Gastrectomy
The mean serum albumin level and mean WL were compared between the two groups at 3 months after gastrectomy, because the ONS intervention period was 3 months. The mean serum albumin level was not significantly different between the ONS and control groups (4.02 ± 0.36 vs. 3.99 ± 0.38, respectively, P = 0.181). By contrast, the mean WL in the ONS group was significantly reduced than that in the control group (7.2 ± 5.7% vs. 8.6 ± 6.1%, respectively, P = 0.0004), although the difference was only 1.4%.
Postoperative Adjuvant Chemotherapy
Among patients with pathological stage IIA–IIIC (excluding T3N0 and T1N2–3) who received postoperative adjuvant chemotherapy, Table 2 compares the ONS and control groups in terms of induction rates and regimens, as well as continuation rates at 3, 6, and 12 months after gastrectomy. The treatment regimens were similar between the two groups. The ONS group, compared with the control group, did not have a higher induction rate (82.8 vs. 84.9%, respectively, P = 0.614), or higher continuation rates at 3 months (76.6 vs. 75.3%, respectively, P = 0.809), 6 months (66.9 vs. 66.4%, respectively, P = 0.933), or 12 months (51.7 vs. 47.3%, respectively, P = 0.446).
Prognosis
Full Analysis Set
Figure 2 shows RFS and OS curves. The 3- and 5-year OS rates were 91.3% and 87.6% in the control group and 89.6% and 86.4%% in the ONS group, respectively, with no significant differences between the two groups (hazard ratio 0.899, 95% CI 0.633–1.273, P = 0.548). Figure 3 shows that there were no significant differences in OS curves between the control and ONS groups when stratified by pathological stage, and ONS administration was not associated with any survival benefit in more advanced cancers.
OS curves after surgery by pathological stage. OS curve of the control group (blue line) and ONS group (red line). a OS curve of the control group (n = 242) and ONS group (n = 239) in stage IA (P = 0.665). b OS curve of the control group (n = 60) and ONS group (n = 58) in stage IB (P = 0.208). c OS curve of the control group (n = 62) and ONS group (n = 57) in stage IIA (P = 0.899). d OS curve of the control group (n = 37) and ONS group (n = 37) in stage IIB (P = 0.191). e OS curve of the control group (n = 33) and ONS group (n = 35) in stage IIIA (P = 0.802). f OS curve of the control group (n = 34) and ONS group (n = 36) in stage IIIB (P = 0.682). g OS curve of the control group (n = 25) and ONS group (n = 26) in stage IIIIC (P = 0.174). h OS curve of the control group (n = 10) and ONS group (n = 12) in stage IV (P = 0.056). OS, overall survival
Effect of the Administration of ≥ 200 kcal/day of ONS
Our previous report showed that the administration of ≥ 200 kcal/day of ONS suppressed WL for up to 1 year postoperatively. Therefore in this study, we compared the OS of patients who consumed ≥ 200 kcal/day of ONS with the OS of patients who consumed < 200 kcal/day of ONS and patients in the control group. The OS curve of the ≥ 200 kcal/day ONS group (n = 221) did not differ from those of the < 200 kcal/day ONS group (n = 182) or the control group (n = 503) (Fig. 4). The 3- and 5-year OS rates of patients in the ≥ 200 kcal/day ONS group were 90.5% and 87.4%, respectively.
Subgroup Analysis of OS
Subgroup analysis was performed with a proportional hazards model for OS to evaluate statistical interactions between treatment groups and backgrounds (Fig. 5). The subgroups were defined on the basis of the following seven factors: age, sex, body mass index, serum albumin level, surgical approach, operative procedure, and pathological stage. There was no subgroup in which ONS administration was significantly associated with longer OS after gastrectomy.
Subgroup analysis was performed with a proportional hazards model for OS to evaluate statistical interactions between the treatment group and background. 95%CI, 95% confidence interval. BMI, body mass index; Alb, albumin; Lap, laparoscopic; TG, total gastrectomy; ONS, oral nutritional supplements; HR, hazard ratio
Discussion
In this study, a survival analysis of data from a large RCT was performed to evaluate the effectiveness of ONS after gastric cancer surgery. The results showed that the administration of ONS for 3 months after surgery for gastric cancer did not affect compliance with adjuvant chemotherapy or prognosis.
Several retrospective analyses showed that postoperative WL was associated with both decreased compliance with adjuvant chemotherapy and impaired survival outcomes,5,6,23 and it has been hypothesized that gastric cancer treatment outcomes might improve if WL could be suppressed through perioperative nutritional support. An RCT examined the effects of eicosapentaenoic acid–rich ONS on prognosis after gastrectomy for gastric cancer.24 No clear survival benefit was observed, but the trial did not have a large enough sample size, and the primary endpoint, namely WL after gastrectomy, was not demonstrated in the trial.25 The current study led to the same conclusion, namely the absence of a survival benefit of ONS in this setting. This study analyzed the data from the first RCT on this topic to be conducted with a sufficient sample size, and the first in which postoperative ONS administration was shown to be effective in suppressing postoperative WL.16 Subgroup analyses in this study did not detect populations in which ONS was beneficial for OS after gastrectomy. The benefits of ONS were expected to be greater in patients with lower preoperative BMI, malnutrition, total gastrectomy (which is associated with higher WL), or more advanced disease for which postoperative chemotherapy is more important, but in fact ONS exhibited reduced benefits in these groups.
It has already been reported that preoperative nutritional supports in gastric cancer patients with severe malnutrition reduce postoperative surgical site infection. In this randomized controlled trial,26 there were only 26 patients (2.6%) (data not shown) of severe preoperative malnutrition that require nutritional support according to the ESPEN guidelines,27 and the details of preoperative nutritional supports were not investigated. Additionally, 63 of 1003 enrolled patients received neoadjuvant chemotherapy, however neoadjuvant chemotherapy for advanced gastric cancer is now being actively employed in clinical trials and daily practice in Japan.22,28 Therefore, preoperative nutritional supports with ONS for advanced gastric cancer may reduce postoperative complications and improve prognosis than administering it postoperatively.
The EFFORT trial examined whether nutritional intervention improved disease outcomes and prognosis in patients with a variety of conditions not limited to gastric cancer.29 While individualized nutritional support for medical patients at nutritional risk significantly reduced short-term mortality, there was no legacy effect on longer-term outcomes.30 These results suggest that nutritional interventions for patients at nutritional risk, including those undergoing gastric cancer surgery, are effective during the interventions, but the effects will wane after the interventions are discontinued. Long-term interventions lasting several years, or other techniques that have not yet been developed, might be required to improve prognosis.
In terms of novel, alternative approaches, enforced enteral feeding and pharmacological interventions are possible candidates. Regarding enteral nutrition, in our study the difference in the mean WL rate with or without 3 months of ONS administration (average intake 208 kcal/day) was only approximately 1.4%. By contrast, in total gastrectomy patients reported by Komatsu et al. the difference after 3 months of nighttime home enteral nutrition (1,200 kcal/day) was quite large, at 11.2%, and this treatment significantly increased the compliance with postoperative adjuvant chemotherapy.31 The enteral feeding tube were placed intraoperatively in only nine patients (2 after total gastrectomy and 7 after distal gastrectomy, data not shown) in this study. The large, sustained difference in WL caused by enforced enteral feeding might improve the prognosis of gastric cancer. One potential pharmacological intervention is ghrelin.32 Ghrelin is a hormone secreted from the stomach that increases appetite and lean body weight.33,34 Adachi et al. reported that ghrelin administration after total gastrectomy for gastric cancer significantly suppressed WL and lean body mass loss.35 Anamorelin is an orally active ghrelin receptor agonist that can be used in Japan for cases of non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer that are accompanied by cachexia,36,37 but it cannot be used for WL after radical gastrectomy for gastric cancer. Expanding its indications remains a future challenge. Furthermore, because exercise therapy in addition to nutritional therapy may help to preserve or increase lean body mass from the previous report, so multimodal intervention, including exercise therapy, is possible candidate approach in the future trial.2
This study had several limitations. First, the total nutritional intake in the ONS group was unclear, because the study did not assess caloric intake in the regular diet. Second, ONS administration was limited to 3 months, and ONS did not improve patients’ nutritional status or reduce WL beyond 1 year after surgery. Third, the adjuvant chemotherapy continuation rate was compared between the two groups in the current study; however, the ratio of chemotherapy dose to the total planned dose of adjuvant chemotherapy could not be compared because of detail of adjuvant chemotherapy were not recorded in case report form. Nevertheless, this study was part of a large, multicenter RCT, and the survival analysis was conducted in a cohort that showed a significant reduction in WL after 3 months of ONS administration.
Conclusions
Oral nutritional supplements administration for 3 months after radical gastrectomy for gastric cancer significantly suppressed WL but did not lead to increased compliance with adjuvant chemotherapy or improved survival outcomes. Different approaches should be investigated in future prospective trials to identify nutritional interventions with larger, longer-lasting impacts on reducing WL after gastrectomy in gastric cancer patients.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Ida S, Kumagai K, Nunobe S. Current status of perioperative nutritional intervention and exercise in gastric cancer surgery: a review. Ann Gastroenterol Surg. 2022;6(2):197–203.
Climent M, Munarriz M, Blazeby JM, et al. Weight loss and quality of life in patients surviving 2 years after gastric cancer resection. Eur J Surg Oncol. 2017;43(7):1337–43.
Tsuburaya A, Noguchi Y, Yoshikawa T, et al. Long-term effect of radical gastrectomy on nutrition and immunity. Surg Today. 1993;23(4):320–4.
Aoyama T, Kawabe T, Fujikawa H, et al. Loss of lean body mass as an independent risk factor for continuation of s-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015;22(8):2560–6.
Aoyama T, Yoshikawa T, Maezawa Y, et al. The postoperative lean body mass loss at one month leads to a poor survival in patients with locally advanced gastric cancer. J Cancer. 2019;10(11):2450–6.
Braga M, Zuliani W, Foppa L, Di Carlo V, Cristallo M. Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg. 1988;75(5):477–80.
Friess H, Böhm J, Müller MW, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91(2):341–7.
Doki Y, Takachi K, Ishikawa O, et al. Ghrelin reduction after esophageal substitution and its correlation to postoperative body weight loss in esophageal cancer patients. Surgery. 2006;139(6):797–805.
Nunobe S, Takahashi M, Kinami S, et al. Evaluation of postgastrectomy symptoms and daily lives of small remnant distal gastrectomy for upper-third gastric cancer using a large-scale questionnaire survey. Ann Gastroenterol Surg. 2022;6(3):355–65.
Furukawa H, Kurokawa Y, Takiguchi S, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. 2018;21(3):500–7.
Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14(2):144–9.
Lee S, Kim HH. Minimally invasive surgery in advanced gastric cancer. Ann Gastroenterol Surg. 2022;6(3):336–43.
Takeoka T, Yamamoto K, Kurokawa Y, et al. Comparison of the effects of open and laparoscopic approach on body composition in gastrectomy for gastric cancer: a propensity score-matched study. Ann Gastroenterol Surg. 2024;8(1):40–50.
Lee JH, Hyung WJ, Kim HI, et al. Method of reconstruction governs iron metabolism after gastrectomy for patients with gastric cancer. Ann Surg. 2013;258(6):964–9.
Miyazaki Y, Omori T, Fujitani K, et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer. 2021;24(5):1150–9.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–12. https://doi.org/10.1007/s10120-011-0041-5
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Yanagimoto Y, Kurokawa Y, Doki Y. Essential updates 2021/2022: perioperative and surgical treatments for gastric and esophagogastric junction cancer. Ann Gastroenterol Surg. 2023;7(5):698–708.
Aoyama T, Sato T, Maezawa Y, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22(3):476–83.
Aoyama T, Yoshikawa T, Ida S, et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on the long-term oncological outcomes after total gastrectomy for gastric cancer. Oncol Lett. 2022;23(5):151.
Ida S, Hiki N, Cho H, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017;104(4):377–83.
Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol. 2015;22(Suppl 3):S778–85.
Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–50.
Kurokawa Y, Kawase T, Takeno A, et al. Phase 2 trial of neoadjuvant docetaxel, oxaliplatin, and S-1 for clinical stage III gastric or esophagogastric junction adenocarcinoma. Ann Gastroenterol Surg. 2023;7(2):247–54.
Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–21.
Kaegi-Braun N, Tribolet P, Gomes F, et al. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: secondary analysis of a prospective randomized trial. Clin Nutr. 2021;40(3):812–9.
Komatsu S, Konishi T, Matsubara D, et al. Night home enteral nutrition as a novel enforced and physiologically effective nutrition therapy following total gastrectomy for gastric cancer. Sci Rep. 2022;12(1):14922.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60.
Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8.
Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601–11.
Adachi S, Takiguchi S, Okada K, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138(4):1312–20.
Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–31.
Hamauchi S, Furuse J, Takano T, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer. 2019;125(23):4294–302.
Acknowledgment
The data management for this study was outsourced to the Supporting Center for Clinical Research and Education (SCCRE) data center, which is funded by donations from multiple hospitals. The electronic data capture system was provided by Medical Edge, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
KY contributed to data interpretation and critical revision of the manuscript for important intellectual content. TO conducted data collection and wrote the initial draft of the manuscript. YK, YM, KF, RK, HI, AT, YY, TT, TS, HE, and YD contributed to data collection and interpretation and critical review of the manuscript. All authors read and approved the final version of the manuscript and have agreed to the accountability of all aspects of the study, thereby ensuring that any queries related to the accuracy or integrity of any part of the work are answerable.
Corresponding author
Ethics declarations
DISCLOSURE
We declare that we have no conflicts of interest.
Ethical Approval
All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent for study inclusion, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omori, T., Yamamoto, K., Kurokawa, Y. et al. Long-Term Effects of Oral Nutritional Supplements After Gastrectomy for Gastric Cancer: A Survival Analysis from a Multicenter, Open-Label, Randomized Controlled Trial. Ann Surg Oncol 31, 6909–6917 (2024). https://doi.org/10.1245/s10434-024-15667-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15667-1