Abstract
Background
Post-gastrectomy weight loss is associated with deterioration in quality of life, and influences the long-term prognosis of gastric cancer patients. We conducted a prospective, randomized controlled, open-label study to examine whether an oral elemental diet (Elental®, Ajinomoto Pharmaceuticals, Tokyo, Japan; hereafter referred to as ED) prevents postoperative weight loss in post-gastrectomy patients.
Methods
Patients were randomly divided to receive the ED or control diet. The ED group received 300 kcal of ED plus their regular diet for 6–8 weeks after surgery, starting from the day the patient started a soft rice or equivalent diet after surgery, while the control group received the regular diet alone. The primary endpoint was the percentage of body weight loss (%BWL) from the presurgical body weight to that at 6–8 weeks after surgery. Secondary endpoints were dietary adherence, nutrition-related blood parameters, and adverse events.
Results
This study included 112 patients in eight hospitals. The mean treatment compliance rate in the ED group was 68.7 ± 30.4 % (median 81.2 %). The %BWL was significantly different between the ED and control groups (4.86 ± 3.72 vs. 6.60 ± 4.90 %, respectively; p = 0.047). In patients who underwent total gastrectomy, the %BWL was significantly different between the two groups (5.03 ± 3.65 vs. 9.13 ± 5.43 %, respectively; p = 0.012). In multivariate analysis, ED treatment, surgery type, and preoperative performance status were independently associated with %BWL. No significant differences were observed in the other clinical variables.
Conclusions
ED supplementation reduced postoperative weight loss in gastric cancer patients undergoing gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prognosis of patients with gastric cancer has improved in recent years because of progress in its diagnosis and treatment. Five-year survival rates of >90 % have been reported in patients with stage I disease thanks to curative gastrectomy.1 However, a major complication of gastrectomy is weight loss, which is associated with marked deteriorations in quality of life (QOL) and has an adverse impact on the long-term prognosis of gastric cancer patients.2
Several recent nutritional intervention studies of post-gastrectomy patients have assessed the effects of immunonutrition on postoperative complications or early postoperative enteral nutrition on the velocity of recovery time compared with total parenteral nutrition (TPN); however, the effects of these oral nutritional interventions were inconsistent.3–6
Post-gastrectomy patients often experience organic changes, such as diminished food intake because of depressed gastric reservoir function, and functional changes, including a reduced ability to digest and absorb lipids. These changes may be the main causes of protracted postoperative undernutrition and weight loss.7
Elental® (Ajinomoto Pharmaceuticals, Tokyo, Japan; hereafter referred to as ED) is an orally available elemental diet that contains essential amino acids as the sole source of nitrogen with a low fat content. Consequently, it does not cause indigestion and allows the gastrointestinal tract to rest; therefore, patients could start taking ED soon after gastrectomy. It was also recently reported to reduce the severity of adverse reactions, particularly stomatitis and oral mucositis, following chemotherapy for esophageal or colorectal cancer.8,9
Based on these properties of ED, we hypothesized that its administration could aid body weight management following gastrectomy. Therefore, we conducted a multicenter, prospective, randomized, controlled, open-label, clinical trial to examine whether early, continuous oral nutritional support with ED could prevent post-gastrectomy weight loss in patients with impaired digestive and absorptive functions.
Methods
Ethical Considerations
This trial was conducted in accordance with the World Medical Association Declaration of Helsinki and was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000008056). The study protocol was approved by the Institutional Review Board of each participating hospital, and written informed consent was obtained from all patients.
Patients
Patients were eligible for this study if they satisfied the following inclusion criteria: previously untreated (other than gastrectomy) and histopathologically confirmed gastric adenocarcinoma; age ≥ 20 years; clinical stage I, II, or III disease; Eastern Cooperative Oncology Group performance status (PS) of 0–2; curative resection at the end of surgery; ability for oral intake; provided written informed consent before randomization; and did not develop any severe postoperative complications between surgery and randomization. Exclusion criteria are listed in the electronic supplementary material (ESM). All patients underwent curative distal gastrectomy (DG) or total gastrectomy (TG) for gastric adenocarcinoma at a participating hospital between September 2011 and July 2012. Patients were invited to participate in the study after they underwent surgery. The surgical procedures are briefly described in the ESM.
Study Design and Nutritional Support
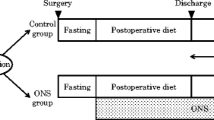
After confirming postoperative eligibility, patients were randomly assigned to the ED group or the control diet group. A coordinating center (Sakai Municipal Hospital Clinical Trial Center) generated the treatment allocation codes using a computer-generated randomization table, and patients were stratified according to surgical method (TG/DG), clinical stage (≤IA/>IA), and presurgical body mass index (BMI; <18.5/≥18.5 kg/m2).
During the study period, patients in both groups consumed a regular diet without any restrictions. Patients assigned to the ED group were also provided ED at a dose of 300 mL/day (300 kcal/day), in addition to their regular diet, for 6–8 weeks, beginning from the day the patients started a soft rice or equivalent diet after surgery. Patients assigned to the control group continued their regular diet alone during the study. Treatments were administered in an open-label manner. Patients recorded their daily dietary intake using a simple dietary survey throughout the study period. Adjuvant chemotherapy is usually started within 6 weeks after surgery.10,11 Considering adjuvant chemotherapy may be delayed depending on the patient’s circumstances, we set the observation time to 6–8 weeks to avoid potential confounding effects of adjuvant chemotherapy on the study endpoints.
Clinical Efficacy and Safety Evaluations
The primary endpoint of this study was the percentage of body weight loss (%BWL) between the patient’s presurgical body weight and that at 6–8 weeks after starting the soft rice or equivalent diet. Secondary endpoints included adherence to ED based on the doses recorded in a diary, changes in nutrition-related blood parameters (serum albumin, serum total protein, serum total cholesterol, and total lymphocyte count), and frequency and severity of adverse events. Adverse events were recorded according to the Common Toxicity Criteria of the National Cancer Institute (version 4.0). Blood samples for evaluation of efficacy and safety were regularly collected during the study and were analyzed by the biochemical laboratory at the patient’s hospital.
Statistical Analysis
The methods for statistical analyses and calculation of the sample size are presented in the ESM.
Results
Patient Characteristics
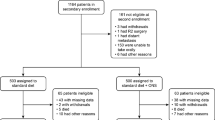
Between September 2011 and August 2012, a total of 112 non-consecutive patients who underwent TG or DG at eight participating hospitals were enrolled. Overall, 58 and 54 patients were randomly assigned to the ED and control groups, respectively (electronic supplementary Fig. S1). Body weight was measured at 6–8 weeks postoperatively in 53 patients in the ED group and 47 patients in the control group (Fig. 1); patients without body weight data (five in the ED group and six in the control group) at weeks 6–8 were excluded from the analyses of body weight.
Table 1 summarizes the preoperative characteristics of the 111 patients included in the intention-to-treat (ITT) analysis. Both groups were evenly matched in terms of their baseline characteristics, including the mean time from gastrectomy to the measurement of body weight to calculate %BWL, and the time from gastrectomy to starting a soft rice or equivalent diet.
Treatment Compliance Rate of Elental® (ED)
In the ED group, the degree of compliance with ED was determined based on the patients’ self-reported diaries as the percentage of the prescribed dose of ED consumed each day. The mean treatment compliance rate in the ED group was 68.7 ± 30.4 %, with a median value of 81.2 % (electronic supplementary Fig. S2).
Impact of ED on Percentage of Body Weight Loss
Body weight was not recorded at 6–8 weeks after starting the soft rice diet in five patients in the ED group and six patients in the control group. Therefore, %BWL was determined in 53 and 46 patients in the ED and control groups, respectively. The mean ± standard deviation change in body weight from baseline until 6–8 weeks after starting the soft rice diet was significantly smaller in the ED group than in the control group (−2.88 ± 2.47 vs. −4.06 ± 3.36 kg, respectively; p = 0.046). The %BWL was also significantly smaller in the ED group than in the control group (4.86 ± 3.72 % [95 % CI −5.86 to −3.83] vs. 6.60 ± 4.90 % [95 % CI −8.04 to −5.16], respectively; p = 0.047) (Fig. 1a). In subgroup analyses, the %BWL was significantly lower in the ED group than in the control group among patients who underwent TG (n = 19 and 16, respectively; 5.03 ± 3.65 % [95 % CI −6.79 to −3.26] vs. 9.13 ± 5.43 % [95 % CI −12.02 to −6.24], respectively; p = 0.012), but not in patients who underwent DG (n = 34 and 31, respectively; 4.77 ± 3.81 % [95 % CI −6.10 to −3.44] vs. 5.29 ± 4.12 % [95 % CI −6.80 to −3.78], respectively; p = 0.596) (Fig. 1b). Multiple regression analysis revealed that ED, surgical type, and preoperative PS were independently associated with %BWL. No association was found between BMI and %BWL (Fig. 2).
Impact of ED on Hematological and Biochemical Parameters
No significant differences were observed between the ED and control groups in terms of the hematological and biochemical parameters (Table 2), except for lymphocyte count, which increased in the ED group and decreased in the control group (64.45 ± 508.90/mm3 [95 % CI 46.93–81.97/mm3] vs. −183.12 ± 792.18/mm3 [95 % CI −199.91 to −166.33/mm3, respectively; p = 0.050).
Adverse Events
Overall, 58 and 53 patients in the ED and control groups, respectively, were included in the safety analyses (Table 3). There were a total of 25 adverse events of grade 2 or higher—15 in the ED group and 10 in the control group. The incidences of hematological and non-hematological adverse events (grade 2 or higher) were not significantly different between the two groups (p = 0.836 for hematological events and p = 0.290 for non-hematological events). One patient in the ED group died 22 days after surgery (7 days after starting ED) because of gastrointestinal hemorrhage, which was probably caused by a ruptured suture. In accordance with the study protocol, this case was immediately reported to the Efficacy and Safety Committee, which determined that the death was causally unrelated to ED. The %BWL and compliance rate were not measured in this patient.
Discussion
To our knowledge, this is the first report to show that an oral elemental nutritional supplement could attenuate body weight loss following gastrectomy in gastric cancer patients as the %BWL was significantly lower in the ED group than in the control group. Moreover, a subgroup analysis showed that the %BWL was significantly lower in the ED group than in the control group among patients who underwent TG, although the difference between the two groups was much smaller and not statistically significant among patients who underwent DG. In this study, the magnitude of postoperative weight loss in the control group was less than that in previous studies.12 These results suggest that nutritional guidance and the use of a diary to record body weight, dietary intake, and compliance with ED may affect the degree of weight loss after gastrectomy.
Several studies have shown that postoperative weight loss is influenced by patient demographic characteristics, operative procedures, cancer stage, and the extent of regional lymphadenectomy.12–18 When comparing TG and DG, postoperative weight loss was greater in patients who underwent TG.14,15 It was also reported that postoperative weight loss is smaller in patients who underwent laparoscopic gastrectomy than in patients who underwent open gastrectomy.19 Furthermore, factors such as the extent of regional lymphadenectomy, cancer stage, and other concurrent organ resections have also been reported to influence the postoperative %BWL.18 In the present study, both groups were evenly matched in terms of the type of gastrectomy (TG/DG), operative procedure (open/laparoscopic), reconstruction method, regional lymphadenectomy, and cancer stage, which suggests that these factors are unlikely to contribute to the difference in %BWL between the ED and control groups.
We also performed multiple regression analysis to identify which factors, including ED, were associated with %BWL. Of note, ED was the strongest independent predictor of %BWL, followed by the surgical procedure (TG vs. DG) and preoperative PS (0 vs. 1–2), whereas other factors, including age, sex, and BMI, were not associated with %BWL. Our results are concordant with previously published results12–19 and suggest that patients with advanced gastric cancer and those who undergo highly invasive surgical procedures, such as TG, are likely to experience severe weight loss and will require greater nutritional interventions.
Regarding preoperative PS, patients with a preoperative PS of 1–2 may have shown significant body weight loss before surgery. Indeed, in an earlier report, the mean %BWL of patients with solid tumors was 3.3, 8.6, and 14.4 % in patients with a PS of 0, 1, and 2, respectively.20
The aims of previous studies were to examine the benefits of nutritional interventions relative to TPN in terms of reducing the incidence and severity of postoperative complications and shortening hospital stays in gastric cancer patients.21 However, few studies have examined what type of nutritional intervention might be effective for reducing protracted postoperative weight loss in gastric cancer patients.3–6 In patients who undergo gastrectomy, body weight decreases rapidly immediately after surgery and continues for approximately 3 months. Although the body weight is maintained thereafter, it rarely recovers to the preoperative level.3 Postoperative weight loss is a potentially major complication of gastrectomy and is associated with deteriorations in QOL; it may also have an adverse effect on the long-term prognosis of patients.2 In this study, administration of ED attenuated the %BWL compared with the control group, and might help to prevent deterioration in QOL or long-term prognosis.
A recent retrospective analysis by Aoyama et al.22 revealed that weight loss >15 % at 4 weeks after gastrectomy is a risk factor for premature interruption of S-1 therapy. Accordingly, administration of ED and reduction of %BWL in the early postoperative stage may support adherence to S-1 therapy. We are now performing a phase II study to determine whether postoperative administration of ED increases adherence to S-1 therapy in gastric cancer patients.
Although we demonstrated that ED attenuated %BWL, comparative trials are needed to determine the most suitable composition of the nutritional agent. It is also important to consider a composition suitable for post-gastrectomy patients that takes into account the functional changes that occur following gastrectomy. Organic and functional changes are expected to occur after gastric resection. Organic changes include a physical reduction in the luminal capacity of the stomach due to resection, anorexia due to a sense of abdominal distension from depressed food-retaining capacity, and gastric dumping syndrome caused by a change in temporal regulation of gastric emptying.7,18 Functional changes may include impaired lipid digestion due to reduced lipase secretion, pancreatic exocrine insufficiency associated with concurrent pancreatic resection in patients requiring extensive surgery, diarrhea, and steatorrhea.7 An elemental diet with a low fat content that contains amino acids, which do not require digestion as a source of nitrogen, may be suitable for patients with these organic and functional problems. Moreover, this elemental diet was associated with more rapid gastric emptying and fewer episodes of aspiration than standard liquid diets in bedridden gastrostomy-fed patients.23
Patients in the ED and control groups freely consumed an ordinary diet in our study. Although a simple diet survey was conducted, the data could not be used to calculate the dietary caloric intake. Therefore, it is unclear whether the total dietary caloric intake or composition differed between the two groups. This limits our ability to predict the mechanism involved in the effects of ED on %BWL. Future studies should include more precise diet records to overcome this limitation. Furthermore, future studies should compare different nutritional formulations to determine the optimal nutritional intervention for reducing %BWL in post-gastrectomy patients.
Adachi et al. examined the effects of intravenous ghrelin peptide administration on post-TG weight loss.24 An increase in food intake of approximately 210 kcal was observed in the ghrelin-treated group, and postoperative weight loss was significantly lower in the ghrelin-treated group than in the control group. The mean treatment compliance rate of 68.7 ± 30.4 % (median 81.2 %) in the ED group suggests that this group received approximately 210 kcal/day of enteral nutrition in addition to their usual food intake. Therefore, it is reasonable to suggest that the inhibition of early postoperative weight loss in patients who underwent TG was due to the administration of ED.
Enteral nutrition with an eicosapentaenoic acid (EPA)-enriched diet was reported to preserve lean body mass after esophageal cancer surgery, suggesting that EPA has benefits on body composition in patients who undergo major surgery.25 A phase III trial is currently ongoing to evaluate whether perioperative administration of an EPA-enriched supplement prevents body weight loss after TG for gastric cancer.26 The results of that study may reveal differences in the effects of specific nutrients on body weight loss after gastric surgery.
The incidence of adverse events (grade ≥ 2) was not significantly different between the ED and control groups; However, one patient in the ED group died because of gastrointestinal hemorrhage, which was possibly caused by a ruptured suture. This event was determined by the Efficacy and Safety Committee to not BE causally related to ED treatment. Grade 2 diarrhea occurred in the ED group but not in the control group, which suggests that clinicians should take precautions when prescribing oral ED to post-gastrectomy patients in clinical practice. Nevertheless, diarrhea has already been reported in patients receiving early postoperative enteral nutrition, and the incidence and severity of diarrhea were not greater in the present study than in a prior study.27
Conclusion
This study revealed that daily nutritional intervention with ED, an oral elemental diet, at 300 kcal per day for 6–8 weeks attenuated %BWL in post-gastrectomy patients.
References
Onodera H, Tokunaga A, Yoshiyuki T, et al. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51:82–5.
Kong H, Kwon OK, Yu W. Changes of quality of life after gastric cancer surgery. J Gastric Cancer. 2012;12:194–200.
Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Dig Dis. 2012;13:401–6.
Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99:621–9.
Li J, Ji Z, Yuan C, et al. Limited efficacy of early enteral nutrition in patients after total gastrectomy. J Invest Surg. 2011;24:103–8.
Kim HU, Chung JB, Kim CB. The comparison between early enteral nutrition and total parenteral nutrition after total gastrectomy in patients with gastric cancer: the randomized prospective study [in Korean]. Korean J Gastroenterol. 2012;59:407–13.
Rogers C. Postgastrectomy nutrition. Nutr Clin Pract. 2011;26:126–36.
Fukui T, Itoh Y, Orihara M, et al. Elental prevented and reduced oral mucositis during chemotherapy in patients esophageal cancer [in Japanese]. Gan To Kagaku Ryoho 2011;38:2597–601.
Ogata Y, Takeuchi M, Ishibashi N, et al. Efficacy of Elental on prevention for chemotherapy-induced oral mucositis in colorectal cancer patients [in Japanese]. Gan To Kagaku Ryoho. 2012;39:583–7.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Park HS, Jung M, Kim HS, et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015;22(1):224–31.
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Liedman B, Andersson H, Bosaeus I, Hugosson I, Lundell L. Changes in body composition after gastrectomy: results of a controlled, prospective clinical trial. World J Surg. 1997;21:416–20.
Takachi K, Doki Y, Ishikawa O, et al. Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. J Surg Res. 2006;130:1–7.
Kiyama T, Mizutani T, Okuda T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313–9.
Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14:144–9.
Hirao M, Takiguchi S, Imamura H, et al. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591–7.
Kurokawa Y, Sasako M, Sano T, et al. Functional outcomes after extended surgery for gastric cancer. Br J Surg. 2011;98:239–45.
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10.
Bozzetti F, SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. 2009;17:279–84.
Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29:242–8.
Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Horiuchi A, Nakayama Y, Sakai R, Suzuki M, Kajiyama M, Tanaka N. Elemental diets may reduce the risk of aspiration pneumonia in bedridden gastrostomy-fed patients. Am J Gastroenterol. 2013;108:804–10.
Adachi S, Takiguchi S, Okada K, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–20.
Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
Yoshikawa T, Hiki N, Taguri M, et al. A phase III trial to evaluate the effect of perioperative nutrition enriched with eicosapentaenoic acid on body weight loss after total gastrectomy for T2-T4a gastric cancer. Jpn J Clin Oncol. 2012;42:459–62.
Papapietro K, Díaz E, Csendes A, et al. Early enteral nutrition in cancer patients subjected to a total gastrectomy. Rev Med Chil. 2002;130:1125–30.
Acknowledgment
The authors would like to thank all KSES collaborators, investigators, and patients for their participation and contribution to this study; Manabu Suzuki, PhD, and Yoshiki Kurose, employees of the Medical Science Department, Ajinomoto Pharmaceuticals Co., Ltd, for providing technical help in data management and writing assistance; Prof. Setsuko Anami, PhD, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Doshisha Women’s College of Liberal Arts, Kyotanabe, Kyoto, Japan, for her clinical review of adverse events, and proofing and approving the final version of manuscript; Ajinomoto Pharmaceuticals Co., Ltd for providing meeting room facilities; and Nicholas D. Smith, PhD, of Edanz Group Ltd, for providing editorial assistance.
Author Contributions
Conceived and designed the study: Hiroshi Imamura and Ryohei Kawabata. Participated in data acquisition: Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Kentaro Inoue, Jin Matsuyama, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Junji Kawada, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, and Atsushi Takeno. Statistical analysis and interpretation of data: Hiroshi Imamura. Statistical analysis of data: Toshio Shimokawa. Drafted the article: Hiroshi Imamura. Proofed and approved the final manuscript: Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, Atsushi Takeno, and Toshio Shimokawa. All authors had access to the data and jointly decided to submit the manuscript.
Disclosures
Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Kentaro Inoue, Jin Matsuyama, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Junji Kawada, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, Atsushi Takeno, and Toshio Shimokawa declare they have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration
UMIN000008056 (University Hospital Medical Information Network Clinical Trials Registry).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imamura, H., Nishikawa, K., Kishi, K. et al. Effects of an Oral Elemental Nutritional Supplement on Post-gastrectomy Body Weight Loss in Gastric Cancer Patients: A Randomized Controlled Clinical Trial. Ann Surg Oncol 23, 2928–2935 (2016). https://doi.org/10.1245/s10434-016-5221-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5221-4