Abstract
Background
Despite a radical operation, about half of gastric cancer (GC) patients with advanced GC experience peritoneal metastasis (PM), and the patients with PM have a poor prognosis. However, because staging laparoscopy was a highly invasive procedure for patients, identification of PM using a liquid biopsy can be useful for patients with GC.
Methods
This study analyzed two genome-wide miRNA expression profiling datasets (GSE164174 and TCGA). The study prioritized biomarkers in pretreatment plasma specimens from clinical training and validation cohorts of patients with GC. The authors developed an integrated exosomal miRNA panel and established a risk-stratification model, which was combined with the miRNA panel and currently used tumor markers (CEA, CA19-9, CA125, and CA72-4 levels).
Results
The comprehensive discovery effort identified a four-miRNA panel that robustly predicted the metastasis with excellent accuracy in the TCGA dataset (area under the curve [AUC] 0.86). A circulating exosomal miRNA panel was established successfully with remarkable diagnostic accuracy in the clinical training (AUC 0.85) and validation (AUC 0.86) cohorts. Moreover, the predictive accuracy of the panel was significantly superior to that of conventional clinical factors (P < 0.01), and the risk-stratification model was dramatically superior to the panel and currently used clinical factors for predicting PM (AUC 0.94; univariate: odds ratio [OR] 77.00 [P < 0.01]; multivariate OR 57.71 [P = 0.01]).
Conclusions
The novel risk-stratification model for predicting PM has potential for clinical translation as a liquid biopsy assay for patients with GC. The study findings highlight the potential clinical impact of the model for improved selection and management of patients with GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer (GC) is the fifth most common malignant cancer in the world and the third leading cause of cancer-related poor survival outcomes.1,2 Diagnosis of GC often is difficult due to a lack of specific symptoms. As a result, most patients have been seen in a progressive state at the time of initial diagnosis.
Cancer metastasis is an important event in the prognosis and tumor progression of GC patients. Generally, peritoneal metastasis (PM) is the most common distant metastatic pattern in GC patients and an important factor of poor survival outcomes.3 In recent years, although various treatment methods combining systemic chemotherapy and surgery have been developed to improve the survival outcomes for patients with advanced GC, the prognosis remains poor.4 Without treatment, the 5-year survival rate for patients with PM is only 2%, and the median survival time is reportedly 3–5 months.5
Despite radical operations for GC, about half of advanced GC patients have experienced PM.6 In chemotherapy for unresectable or recurrent GC with PM, the efficacy of systemic chemotherapeutic agents remains limited.7 Moreover, staging laparoscopy is the only procedure for identifying PM. However, this procedure requires general anesthesia and is highly invasive for patients with GC. Therefore, the identification of predictive markers for PM would aid clinical management and potential treatment stratification of patients with GC.
Exosomes are small extracellular vesicles covered with a membrane. Released from various types of cells, exosomes are present in a variety of biologic fluids. Accumulating reports have shown that exosomes play pivotal roles in intercellular communication through the movement of nucleic acids such as microRNAs (miRNAs), messenger RNA, DNA, and other non-coding RNAs.8,9,10 Among these nucleic acids, miRNAs are short noncoding RNAs, and some reports have shown that miRNAs in circulating exosomes are resistant to enzymatic degradation by ribonucleases, playing an important role in disease metastasis formation, and that dysregulation of miRNAs is associated with tumor progression of GC.11,12 In fact, circulating exosomal miRNAs can be used as noninvasive biomarkers for detecting tumor progression and diagnosis in various malignancies.13
Several recent reports have demonstrated that tumor-derived miRNAs are present in the human circulation in a highly stable form that is protected against endogenous ribonuclease activity and therefore focus on the diagnostic and prognostic utility of blood-based miRNA levels.14 These reports suggest that blood-based miRNAs may constitute accurate methods for the diagnosis and prognostic prediction of cancer, although a few studies to date have specifically focused on the clinical impact of noninvasive miRNAs.15,16,17,18,19,20,21
Previous reports show that the miRNA expression pattern reflects the physiologic and pathologic status of patients with cancer. Also, these studies have indicated that specific exosomal miRNA expression is involved in cancer pathogenesis and have shown their potential as circulating biomarkers for cancer malignancy. Additionally, our group previously showed that the blood-based miRNA assays allowed significant prediction of cancer recurrence and metastasis.22,23,24 By analyzing blood-based miRNAs before treatment that can be used for patients with GC, we developed a robust model and made a nomogram for predicting the probability of PM. Our novel model can predict PM and survival outcomes.
Materials and Methods
Patient Cohorts
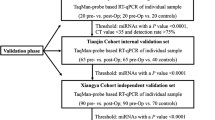
For comprehensive discovery, we analyzed the miRNA expressions from two public datasets (GSE164174 and TCGA) to establish an miRNA panel for the identification of PM in patients with GC. In the discovery phases, miRNA microarray data from the GSE164174 cohort (n = 2840; GC [n = 1423] and normal sample [n = 1417]) was analyzed to identify cancer-specific miRNAs. Then, the TCGA cohort (n = 430; non-metastatic GC [n = 393] and metastatic GC [n = 37]) was analyzed to identify PM specific miRNAs from tumor tissue samples. Then, we used 51 plasma samples from patients with GC (training cohort [n = 25; 16 without PM and 9 with PM] and validation cohort [n = 26; 16 without PM and 10 with PM]; Fig. 1A).
Genome-wide discovery and validation of the miRNA panel to predict PM in patients with GC. A Overview of the study. B Volcano plot of the expression profiles between GC and normal samples in the GSE164174 cohort. C Receiver operating characteristic curves showing the diagnostic performance of the four-miRNA panel for distinguishing patients with PM in the TCGA cohort (resectable GC [n = 393]; unresectable GC [n = 37]; AUC 0.86). miRNA microRNA, PM peritoneal metastasis, GC gastric cancer, TCGA The Cancer Genome Atlas, AUC area under the curve, CI confidence interval
All the patients underwent staging laparoscopy, and blood samples were collected the day before the surgery, between January 2015 and December 2020. At the surgery, PM or non-PM was diagnosed because the primary hypothesis was detection of PM at diagnosis. All the specimens were diagnosed as GC by pathologists at our institution according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) grading system. The follow-up protocol included a physical examination, evaluation of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels, and imaging methods such as ultrasonography, positron emission tomography (PET)-computed tomography (CT), and magnetic resonance imaging (MRI). Only regions confirmed by imaging methods were diagnosed as tumor recurrence sites.
In the PM group, primary resection was performed for all the patients at the time of tumor regression during chemotherapy according to comprehensive judgment based on such factors as negative tumor marker levels and partial or complete response to the treatment via these imaging methods. Chemotherapy was received as a fluorouracil-based regimen for all the patients with pre- and post-gastrectomy. The chemotherapy consisted of cisplatin and S-1 (CS), S-1 and oxaliplatin (SOX), or S-1 regimen. The CS therapy involved cisplatin (60 mg/m2) and S-1 (80 mg/m2 on days 1–14) via a rapid intravenous infusion every 3 weeks. The SOX therapy included S-1 (80 mg/m2 on days 1–14) and oxaliplatin (130 mg/m2) via a rapid intravenous infusion every 3 weeks. The S-1 regimen included 80 mg/m2 on days 1–14 every 3 weeks. The study was approved by the institution and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Exosome Isolation from Plasma Specimens
Total exosome isolation was performed from plasma using a total exosome isolation kit (#4484450; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, 200 μL of plasma was thawed on ice and centrifuged at 2000g for 30 min at room temperature to remove cells and debris. Next, we added 40 μL of the reagent from the kit and incubated the contents for 30 min at 4 °C. After incubation, the samples were centrifuged at 10,000g for 10 min at room temperature, and the supernatant was discarded. The exosomes were contained in the pellets, which were resuspended using 200 μL of phosphate-buffered saline. Isolated exosomes were preserved at 4 °C until RNA extraction. Then, we confirmed that the CD63 used as one of the most common surface markers of exosomes was positive. Also, the real-time PCR method was used to confirm that the amplification curve was acceptable, and the experiment was performed.
RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction from Plasma
Total RNA extraction was performed using an RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA was synthesized using a reverse transcription kit (Applied Biosystems, Thermo Fisher Scientific). All primers for the miRNAs analyzed in this study were purchased from Thermo Fisher Scientific. The catalog number for all the miRNA primers was 339306, and the assay identification numbers of the individual miRNAs were as follows: Hsa-miR-16-5p (391), Hsa-miR-21-5p (397), Hsa-miR-320 (241053), Hsa-miR-191-5p (2299), and Hsa-miR-451 (1141). The target transcripts were normalized to miR-16-5p expression as an internal control by using the 2−ΔDCt method.
Statistical Analysis
Logistic regression was used to train a classifier based on the expression of miRNAs. Once the model was established in the training cohort, the same statistical weights and cutoff thresholds were applied in the validation cohort. The clinicopathologic characteristics of the patient cohorts were compared between the training and validation groups using the Mann–Whitney U test or chi-square test for categorical data. Clinicopathologic characteristics are shown as patient number and ratio except for age and tumor markers, which are shown as median and range (Table 1). Disease-free survival and overall survival (OS) were analyzed by the Kaplan–Meier method. Uni- and multivariate Cox proportional hazard regression models were used to identify the prognostic markers. A P value lower than 0.05 was considered statistically significant. Statistical analyses were performed using MedCalc statistical software V.16.2.0 (MedCalc Software bvba, Ostend, Belgium) and JMP Pro 14 statistical software (SAS Institute Japan, Tokyo, Japan).
Results
Comprehensive miRNA Expression Showed a Four-miRNA Panel for Prediction of PM
We performed a comprehensive discovery in two independent miRNA datasets (GSE164174 and TCGA) to identify an miRNA panel for predicting PM in patients with GC (Fig. 1A). First, we analyzed miRNA expressions between the patients with GC (n = 1423) and normal samples (n = 1417) in the GSE164174 cohort. We identified differentially expressed candidates’ miRNAs with data availability (P < 0.05). These analyses were performed by the LIMMA package (Version info: R 4.2.2, Biobase 2.58.0, GEOquery 2.66.0, limma 3.54.0), which uses a moderated t statistic. Then, we selected candidate miRNAs based on the following criteria: absolute log2 fold change greater than 0.5, P value lower than 0.05, and expression at least 50 % of all cases. We excluded miRNAs from passenger strands (Fig. 1B). We then validated these candidates against the TCGA public dataset and identified four differentially expressed miRNAs (miR-21, miR-191, miR-320, and miR-451). A logistic regression model incorporating these four miRNAs to identify patients at high risk of PM resulted in the TCGA dataset (area under the curve [AUC] 0.86; 95% confidence interval [CI] 0.81–0.91; Fig. 1C), highlighting the clinical significance of our discovery effort. These results demonstrated the diagnostic accuracy of the PM identification for patients with GC.
Clinical Training Confirmed Ability of the Blood-Based miRNA Panel to Predict PM
We evaluated the biomarker panel in the clinical cohorts. Our findings confirmed that the patients in the clinical training and validation cohorts had clinicopathologic characteristics similar to those of clinical cohorts. The training cohort comprised 25 patients, including 9 patients with PM (36%) and 11 patients with recurrence (44%). The median age of the patients in this cohort was 73.4 years. The validation cohort consisted of 26 patients, including 10 patients with PM (38.5%) and 10 patients with recurrence (38.5%). The median age of the patients in this cohort was 73.0 years. The detailed clinicopathologic characteristics of the patients in these cohorts are provided in Table 1.
We performed survival analysis for OS and RFS in the independent patient cohorts. The median follow-up time was 33.6 months in the training cohort and 38.8 months in the validation cohort. The median treatment time was 11.4 months in the training cohort and 17.8 months in the validation cohort. Primary tumor resections were performed as total gastrectomy (TG), distal gastrectomy (DG), proximal gastrectomy (PG), or others (local resection) according to the tumor location, with D2 lymphadenectomy performed for 72.0% of the training cohort and 76.9% of the validation cohort. The R0 primary resection rate was 80% in training cohort and 76.9% in validation cohort.
To confirm the predictive ability of our discovery panel as a liquid biopsy, we evaluated the training and validation cohorts on the selected four miRNAs using reverse transcription-quantitative polymerase chain reaction assays in pretreatment blood specimens. Based on the coefficients of each miRNA derived from the training cohort (n = 25: 9 with PM and 16 without PM), we developed a risk score: − 2.39608*miR-191) + (3.68915*miR-21) + (1.03109*miR-320) + (− 0.63182*miR-451) − 3.42746. Our established miRNA panel showed significant performance for predicting PM (AUC 0.85; 95% CI 0.66–0.96; Fig. 2A) with a specificity of 0.78 and a sensitivity of 1.00 (Table 2).
Training and validation of the exosomal miRNA panel as a blood-based assay for identifying PM in patients with GC. A Receiver operating characteristic curve showing the diagnostic performance of the panel in the training cohort (PM [n = 3]; no PM [n = 8]; AUC 0.83). B Risk score distribution plot in the training cohort. A modified risk score was obtained by subtracting individual risk scores from the Youden’s index. C Receiver operating characteristic curve showing the diagnostic performance of the panel in the validation cohort (PM [n = 16]; no PM [n = 24]; AUC 0.83). D Risk score distribution plot in the validation cohort. miRNA microRNA, PM peritoneal metastasis, GC gastric cancer, AUC area under the curve, CI confidence interval
We dichotomized the patients into high- and low-risk groups based on Youden’s index-derived cutoff values. According to the distribution of the risk scores and metastatic status, the risk scores were notably higher for the patients with PM than for those without PM (Fig. 2B). Our results showed that we successfully evaluated blood-based parameters before treatment for predicting PM in patients with GC.
Clinical Validation Confirmed the Translational Potential of the miRNA Panel
To confirm the translational potential of the panel to identify the high-risk patients, we evaluated the performance in the clinical validation cohort (n = 26: 16 without PM and 10 with PM). We assessed the miRNA panel using the same cutoff values and coefficients from the training cohort for the validation cohort. We validated our miRNA panel for predicting PM with an AUC of 0.86 (95% CI 0.66–0.96; Fig. 2C, D), a specificity of 0.80, and a sensitivity of 0.81 (Table 2). Next, we divided the patients into high- and low-risk groups using Youden’s index and performed uni- and multivariate analyses of logistic regression. Our novel panel showed it to be an excellent predictor of PM in the clinical validation cohort (odds ratio [OR] 10.52; 95% CI 2.27–48.76; P < 0.01; Table 3).
Risk Model Combining the miRNA Panel With Clinical Factors Demonstrated Better Accuracy for Predicting PM
Given that several tumor markers (cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], cancer antigen 72-4 [CA72-4], and carcinoembryonic antigen [CEA]) are important biomarkers for malignant potential, we examined the extent to which a model combining the panel and tumor markers would improve the prediction. We compared the predictive accuracy of the miRNA panel with and without tumor markers. The risk stratification model with tumor markers had notably better prediction of PM (AUC 0.94; Fig. 3A) than the miRNA panel alone. To further evaluate the ability of the panel to predict PM when combined with tumor markers, the logistic regression model was used to establish a nomogram incorporating these features. The results showed that our miRNA panel had the greatest weight in the model (Fig. 3B).
Additional clinical validation of the risk stratification model for identifying PM in patients with GC. A Receiver operating characteristic curves showing the diagnostic performance of the novel risk stratification model (combination of the miRNA panel with CA72-4, CEA, CA125, and CA19-9) relative to indicated clinical factors in the validation cohort (AUC 0.94). B Nomogram indicating the possibility of PM in patients with gastric cancer. A line is drawn straight down to the possibility axis to obtain the probability of accurately predicting PM. C and D Comparison of C overall survival and D recurrence-free survival for distinguishing patients with high and low expression in the risk stratification model. The overall survival and disease-free survival times were calculated from the date of surgery to the date of death from any cause, recurrence, or the last follow-up visit. Overall survival and disease-free survival were estimated using the Kaplan–Meier method. PM peritoneal metastasis, GC gastric cancer, miRNA microRNA, CA72-4 cancer antigen 72-4, CEA carcinoembryonic antigen, CA125 cancer antigen 125, CA19-9 carbohydrate antigen 19-9, AUC area under the curve
Risk Stratification Model Exhibited Prognostic Potential for Predicting Survival Outcomes
To assess the survival potential of our model, we performed a survival analysis to compare OS and RFS between the patients categorized as high risk and those categorized as low risk. The results showed that the high-risk patients had significantly poorer survival outcomes than the low-risk patients in the validation cohort (OS [P < 0.05]; RFS [P = 0.07]; Fig. 3C, D). We also performed a survival analysis to compare OS and RFS between the patients categorized as PM and those categorized as non-PM. The results showed that the patients with PM had significantly poorer survival outcomes than the patients with non-PM (OS [P < 0.05]; RFS [P < 0.05]; Supplemental Digital Content 1). Furthermore, we performed a multivariate analysis to establish a risk stratification model incorporating our miRNA panel with tumor markers. The patients categorized as high risk by the RNA panel had a significantly worse prognosis than the low-risk patients in the validation cohort (OR 57.71; 95% CI 2.29–1456.43; P = 0.01; Table 3). These results indicate that our risk model also has significant prognostic potential.
Discussion
The presence of PM is an important risk factor of a poor prognosis for patients with advanced GC. The current study showed the inadequacy of clinical risk factors used in the clinical setting to identify distant metastasis in high-risk patients with GC. Our results indicated that a blood-based, noninvasive assay can be used to estimate that the high risk in preoperative status has significant potential for identification of PM and can lead to a novel strategy of surveillance and preoperative findings for patients with advanced GC. We considered the usefulness of the assay in a clinical setting.
Regarding cost-effectiveness, staging laparoscopy costs 113,200 yen under the health insurance compared with 3600 yen per case for the assay in Japan. Also, in terms of time to the result, staging laparoscopy requires 1 h for rapid diagnosis by a pathologist compared with about 3 h for the assay. Therefore, if staging laparoscopy could be eliminated, the assay using liquid biopsy would be beneficial in terms of both medical costs and patient invasiveness, although the time to the result from the assay is longer than for the surgery. Identifying the presence of PM will reduce the unnecessary patient burden, time, and costs of performing the staging laparoscopy.
In the current study, we developed a risk model and assessed the ability of our four-miRNA panel to predict PM in plasma samples before treatment. More importantly, our novel noninvasive risk model exhibited significantly better diagnostic accuracy for PM (AUC 0.94) than the currently used clinical risk models (combination of the tumor markers CEA, CA19-9, CA125, and CA72-4; AUC 0.75; Supplemental Digital Content 2).
The patients with positive ascites cytology were not included with the PM cases. Although we evaluated the ascites cytology, unfortunately, it was difficult to predict the positive ascites cytology in our established risk model (AUC 0.69; Supplemental Digital Content 3). The waterfall plots included patients with non-PM among the high-risk group in the risk model. One of the patients with non-PM in the high-risk group at diagnosis had experienced the PM during the follow-up period. This result supported the conclusion that the risk model may detect micro-metastatic seeding, which may have potential for the development of PM.
Our novel established diagnostic signature is significantly better for prediction of PM. A recent report indicated that exosomes from ascites convert mesothelial cells to fibroblasts and promote PM.25 Furthermore, GC cells have been shown to produce exosomes that promote PM formation by disrupting the mesothelial barrier.26 These results show that exosomes in ascites promote peritoneal fibrosis and may be a favorable microenvironment for seeded tumor cells.
Similarly, another study has shown that miRNAs are a strong risk factor for worse OS and peritoneal recurrence.27 Thus, the utility of liquid biopsy is crucial to understanding the factors that predict PM before radical surgery. The current study emphasized the inadequacy of the currently used clinical factors to identify patients with PM.
From a functional viewpoint, findings have shown various miRNAs in our biomarker panel to be candidates involved in cancer progression. For example, the exosomal miR-21-5p from cancer cells activated the β-catenin-signaling pathway and increased their downstream target, vascular endothelial growth factor (VEGF), which consequently promoted angiogenesis and vascular permeability. Also, exosomal miR-21-5p was involved in angiogenesis and vascular permeability in cancer cells and might be used as a potential new therapeutic target.28
Next, miR-191 was significantly upregulated in cancer cells and promoted proliferation, migration, invasion, and cell cycle progression of cancer cells, as well as tumor growth via the Wnt/β-catenin-signaling pathway.29 On the other hand, the miR-320 family was reported to be one of the most tumor suppressant families and related to the repression of epithelial-mesenchymal transition inhibition, cell proliferation, and apoptosis. Moreover, this family has been shown to regulate drug resistance, and to act as a potential biomarker for the diagnosis, prognosis, and prediction of cancer.30 Finally, miR-451 also could inhibit cell migration and invasion, promote apoptosis, and induce cell-cycle arrest.31 Accumulating results showed that candidate miRNAs have the role of cancer progression and suppression.
Our study had some potential limitations. First, we used clinical cohorts of patients from a single institution who showed similar clinicopathologic factors. Therefore, it is important to validate the selected miRNAs and our established model in clinical cohorts from other institutions to confirm the accuracy of our findings.
Second, our retrospective study might have resulted in potential selection bias. We evaluated our panel in a moderately sized clinical cohort because of the limited sample size. Therefore, a further prospective study with larger clinical cohorts is required to evaluate our model. However, as a result, local resection was performed at 12.0% in the training cohort and 7.7% in the validation cohort, but cytoreduction surgery was not performed. We will investigate the peritoneal cancer index in a future study because the peritoneal cancer index was reported to be a strong predictor of incomplete curative surgery.32
Finally, we established a risk stratification model that included exosomal miRNAs and clinical factors. Because clinical application of such a model can be complicated, further experimentation including other factors such as DNA mutation and methylation is needed to develop a more convenient and highly accurate diagnostic methods for PM. Nonetheless, our study showed the important result of detecting PM in patients with GC and was an important step toward the availability of molecular biomarkers for the management of GC.
In conclusion, we identified and validated a novel risk stratification model involving the combination of an miRNA panel and tumor markers (CA72-4, CEA, CA125, and CA19-9) for prediction of PM in a liquid biopsy assay. Our findings highlight the potential clinical impact of our model for improved selection of patients and for the management of patients with this malignancy.
Data availability
TCGA miRNA expression profiling data were downloaded from the University of California, Santa Cruz Xena Browser (https://xenabrowser.net). Normalized non-coding RNA profiling and clinical data from the GSE164174 dataset were downloaded from the Gene Expression Omnibus database.
References
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149:1153–62.
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers. 2013;5:48–63.
Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–8.
Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–94.
Kitayama J, Ishigami H, Yamaguchi H, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018;2:116–23.
Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–11.
van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–56.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Wang QX, Zhu YQ, Zhang H, Xiao J. Altered MiRNA expression in gastric cancer: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35:933–44.
Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14.
Nedaeinia R, Manian M, Jazayeri MH, et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24:48–56.
Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8.
Tsai KW, Liao YL, Wu CW, et al. Aberrant expression of miR-196a in gastric cancers and correlation with recurrence. Genes Chromosomes Cancer. 2012;51:394–401.
Wang M, Gu H, Wang S, et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep. 2012;5:1514–20.
Gorur A, Balci Fidanci S, Dogruer Unal N, et al. Determination of plasma microRNA for early detection of gastric cancer. Mol Biol Rep. 2013;40:2091–6.
Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–91.
Liu H, Zhu L, Liu B, et al. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203.
Song MY, Pan KF, Su HJ, et al. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS ONE. 2012;7:e33608.
Kim SY, Jeon TY, Choi CI, et al. Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J Mol Diagn. 2013;15(5):661–9.
Wada Y, Shimada M, Morine Y, et al. A blood-based noninvasive miRNA signature for predicting survival outcomes in patients with intrahepatic cholangiocarcinoma. Br J Cancer. 2022;126:1196–204.
Wada Y, Shimada M, Morine Y, et al. Circulating miRNA signature predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. JCO Precis Oncol. 2021;5:1788–801.
Wada Y, Shimada M, Murano T, et al. A liquid biopsy assay for noninvasive identification of lymph node metastases in T1 colorectal cancer. Gastroenterology. 2021;161:151-162.e151.
Wei M, Yang T, Chen X, et al. Malignant ascites-derived exosomes promote proliferation and induce carcinoma-associated fibroblasts transition in peritoneal mesothelial cells. Oncotarget. 2017;8:42262–71.
Deng G, Qu J, Zhang Y, et al. Gastric cancer-derived exosomes promote peritoneal metastasis by destroying the mesothelial barrier. FEBS Lett. 2017;591:2167–79.
Ohzawa H, Saito A, Kumagai Y, et al. Reduced expression of exosomal miR-29s in peritoneal fluid is a useful predictor of peritoneal recurrence after curative resection of gastric cancer with serosal involvement. Oncol Rep. 2020;43:1081–8.
He Q, Ye A, Ye W, et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576.
Wang Z, Guan W, Ma Y, et al. MicroRNA-191 regulates oral squamous cell carcinoma cells growth by targeting PLCD1 via the Wnt/β-catenin signaling pathway. BMC Cancer. 2023;23:668.
Liang Y, Li S, Tang L. MicroRNA 320, an anti-oncogene target miRNA for cancer therapy. Biomedicines. 2021;9:591.
Wang W, Zhang L, Wang Y, et al. Involvement of miR-451 in resistance to paclitaxel by regulating YWHAZ in breast cancer. Cell Death Dis. 2017;8:e3071.
Jónsdóttir B, Lomnytska M, Poromaa IS, Silins I, Stålberg K. The peritoneal cancer index is a strong predictor of incomplete cytoreductive surgery in ovarian cancer. Ann Surg Oncol. 2021;28:244–51.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac), for editing a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wada, Y., Nishi, M., Yoshikawa, K. et al. Circulating Exosomal MicroRNA Signature Predicts Peritoneal Metastasis in Patients with Advanced Gastric Cancer. Ann Surg Oncol 31, 5997–6006 (2024). https://doi.org/10.1245/s10434-024-15592-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15592-3