Abstract
Background
The objective of this meta-analysis was to assess the association of sarcopenia defined on computed tomography (CT) head and neck with survival in head and neck cancer patients.
Methods
Following a PROSPERO-registered protocol, two blinded reviewers extracted data and evaluated the quality of the included studies using the Quality In Prognostic Studies (QUIPS) tool, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. A meta-analysis was conducted using maximally adjusted hazard ratios (HRs) with the random-effects model. Heterogeneity was measured using the I2 statistic and was investigated using meta-regression and subgroup analyses where appropriate.
Results
From 37 studies (11,181 participants), sarcopenia was associated with poorer overall survival (HR 2.11, 95% confidence interval [CI] 1.81–2.45; p < 0.01), disease-free survival (HR 1.76, 95% CI 1.38–2.24; p < 0.01), disease-specific survival (HR 2.65, 95% CI 1.80–3.90; p < 0.01), progression-free survival (HR 2.24, 95% CI 1.21–4.13; p < 0.01) and increased chemotherapy or radiotherapy toxicity (risk ratio 2.28, 95% CI 1.31–3.95; p < 0.01). The observed association between sarcopenia and overall survival remained significant across different locations of cancer, treatment modality, tumor stages and geographical region, and did not differ between univariate and multivariate HRs. Statistically significant correlations were observed between the C3 and L3 cross-sectional area, skeletal muscle mass, and skeletal muscle index.
Conclusions
Among patients with head and neck cancers, CT-defined sarcopenia was consistently associated with poorer survival and greater toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sarcopenia is an age-related syndrome characterized by loss of skeletal muscle mass (SMM), strength and function, and is associated with frailty, functional decline, falls, and mortality.1 Clinically, sarcopenia is defined by low muscle strength, low muscle quantity or quality, and/or low physical performance.1 Sarcopenia can also be quantified through imaging methods such as dual-energy x-ray absorptiometry (DEXA), computed tomography (CT), and magnetic resonance imaging (MRI).2,3 Imaging modalities allow precise measurements that enable segmental and total muscle mass to be calculated and assessment of a muscle’s fat infiltration, which influences force development and muscle quality. Current radiological definitions of sarcopenia recommended by the European, Asian, and international working groups utilize DEXA of the limbs, which is favored due to its low ionizing radiation dose and ability to investigate specific regions.1
Apart from DEXA, other methods, including CT and MRI, may be used in quantifying sarcopenia. Despite MRI being a promising technique, its use is limited in clinical practice. It is confined to research due to its high cost, lengthy acquisition, and inadequate cut-off values and standardized protocol.2 Therefore, CT is commonly the first-line diagnostic modality that is frequently utilized in both oncological and non-oncological settings.3 It has been demonstrated that CT can provide estimated quantification of skeletal muscle as there is a strong correlation between CT-derived values obtained from a single CT cross-sectional image and whole-body skeletal muscle.4
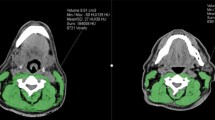
SMM may be assessed on CT imaging at the L3 vertebra level, with the L3 cross-sectional muscle area (CSMA) demonstrating excellent correlation with whole-body SMM.5,6 In practice however, such abdominal scans are not routinely performed in head and neck cancer (HNC) patients and are often available only in locally advanced disease, with up to 93% of HNC patients lacking abdominal CT imaging.7,8 Therefore, measurement of SMM at the third cervical vertebrae has been developed to overcome this limitation, demonstrating good correlation with CSA at the L3 level and providing a reliable approximation of total SMM.9,10 Such an assessment may provide a means of screening for SMM and sarcopenia in HNC patients, without generating additional costs or burden for the individual patient.
Published data have reported an association between radiologically defined sarcopenia and adverse oncologic outcomes, including poorer survival in HNC patients.2,11 Since then, several studies have sought to investigate the prognostic value of CT-defined sarcopenia at the C3 vertebra level for HNCs.12,13,14 Such an assessment of SMM may provide a cost effective, efficient means of prognosticating outcomes in HNC patients. Therefore, this study aims to investigate the prognostic value of CT-defined sarcopenia at the C3 vertebra level in HNC patients. The hypothesis is that CT-defined sarcopenia at the C3 vertebra level and above is only prognostic of survival and unfavorable outcomes in HNCs.
Methods
The prespecified protocol for this review was registered on PROSPERO (CRD42023393555). With reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a search was conducted on the Medline, Embase and Cochrane databases for studies published before 30 December 2023.15 The search strategy used a combination of the following search terms: (sarcopenia) AND ((CT head) or (CT neck)). The full search strategy is included in Online Resource 1. The references of included articles were also screened manually for a comprehensive search.
Study Selection
Three authors (JHK, CL and LTPT) independently screened abstracts in a blinded manner to check the eligibility for inclusion, with disputes being resolved through consensus from a fourth independent author (BYQT). Retrospective and prospective cohort studies, cross-sectional studies, and randomized controlled trials were considered for inclusion. The inclusion criteria were (1) clinical studies that used CT head or CT neck for the measurement of sarcopenia in patients with HNC; (2) full-text studies; (3) published in a peer-reviewed journal; and (4) written in English. Only studies that used muscle assessments at the C3 vertebrae level, or converted C3 measurements to an estimated L3 measurement, were included.
The exclusion criteria were (1) clinical studies that used MRI head or MRI neck for the measurement of sarcopenia in patients with HNC; (2) animal studies; (3) cadaver studies; (4) case reports; (5) in vitro studies; and (6) reviews.
Data Extraction
Relevant data from included articles were extracted by a pair of independent authors (CYJL and LTPT) in a double-blinded fashion into a structured proforma. Study characteristics including first author, year of study completion, stage of cancer, region of cancer, treatment modality, definition of sarcopenia, sample size, mean age, sex, BMI, Charlson Comorbidity Index (CCI) score, and smoking status were extracted. The primary outcomes of interest were overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and disease-specific survival (DSS), while secondary outcomes included chemotherapy or radiotherapy toxicity, prevalence of sarcopenia, and correlations of different measurements of sarcopenia.
Quality Assessment
Quality assessment of the included articles was performed using the Quality In Prognostic Studies (QUIPS) tool. The QUIPS rates the risk of bias of cohort studies on the premises of appropriateness of sample frame, sampling method, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, comparability of cohorts, methods for assessment of outcomes, duration of follow-up, and adequacy of follow-up.16 Publication bias was assessed by visual inspection of the respective funnel plots and Egger’s test.17,18
Statistical Analysis
All analyses were conducted in R Studio (version 4.2.2; The R Foundation for Statistical Computing, Vienna, Austria) using the meta package.19 Descriptive statistics were presented as means and standard deviations for continuous variables, and counts for categorical variables. Medians and interquartile ranges were converted to means and standard deviations using the published methods of Wan et al.20
A conventional pairwise meta-analysis was conducted using maximally adjusted hazard ratios (HRs) using the inverse variance method. Risk ratios were calculated for the categorical outcomes of chemotherapy and radiotherapy toxicities. Statistical heterogeneity was assessed via I2 and Cochran Q test values, where an I2 value of < 25% representing low heterogeneity and an I2 value ≥ 25% representing moderate to high heterogeneity.21,22 A Cochran Q test with a p value of ≤ 0.10 was considered significant for heterogeneity. Random-effects models were used in all analyses regardless of heterogeneity, as published evidence suggests that it provides more robust outcome measures compared with the alternative fixed-effects models.23 When three of more studies were available, 95% prediction intervals (PIs) were computed to estimate the potential range of true effect sizes across individual studies, given that the 95% confidence interval (CI) only accounts for the uncertainty of the mean effect size, not the uncertainty of interstudy variance.24 A meta-analysis of correlation coefficients was performed using the DerSimonian–Laird random-effects model.
Where 10 or more studies were available for a particular outcome, additional analyses were conducted to evaluate potential sources of heterogeneity between studies.25 Apart from subgroup analyses, univariate random-effects meta-regression were conducted, and effect moderators were confirmed using permutation testing with 1000 iterations to eliminate spurious results.26,27 Statistical significance was considered for outcomes with a p value ≤ 0.05. Publication bias was assessed through visual inspection of the funnel plots, with missing studies imputed using the trim-and-fill method.28 Leave-one-out influence analyses were performed to examine the influence of individual studies on the overall findings. Cumulative meta-analyses were performed, ranked by year published, to examine the stability of published data over time.
Certainty of Evidence
The quality of pooled evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.29
Results
Literature Search
Details of the study selection process are summarized in Fig. 1. Overall, 1625 articles were included in the initial search after removal of duplicates, of which 61 were selected for full-text review; 37 articles met the final inclusion criteria.
Study Characteristics
Of the 37 included studies, 34 were retrospective cohort studies9,12,13,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56 and 3 were prospective cohort studies.14,57,58 The total sample size was 11,181 patients. The mean age, BMI, and follow-up time was 58.56 ± 12.46 years, 21.61 ± 7.99 kg/m2, and 36.43 ± 27.69 months; 70% of patients were male. 22 studies measured sarcopenia at the C3 vertebra level,12,13,14,30,31,32,33,34,35,36,37,38,39,41,42,43,51,53,54,55,57,58 while 15 studies measured sarcopenia at the L3 vertebra level as converted from C3 skeletal muscle measurements.9,40,44,45,46,47,48,49,50,52,56,59,60,61,62 Cut-off points varied by study and are found in Tables 1 and 2, which also contain a summary of the key characteristics for included articles. Within the included studies, cut-off values for defining sarcopenia were calculated from receiver operating characteristic (ROC) analyses or through published definitions of sarcopenia.63,64 Details of the quality assessment of the included articles are shown in Online Resource 2.
Meta-Analysis for Overall Survival
The OS was reported in 21 studies (7562 participants).12,13,14,35,36,39,40,41,44,45,47,48,51,52,54,55,57,59,60,61,62 Based on the random-effects model, OS was significantly lower in patients with sarcopenia compared with patients without sarcopenia (HR 2.11, 95% CI 1.81–2.45; p < 0.01, I2 = 45%) (see Fig. 2). Covariates adjusted for included age (13 studies),12,13,14,35,40,41,44,45,47,48,54,59,62 sex (4 studies),35,40,45,54 stage of cancer (13 studies),12,13,14,35,36,39,40,44,47,48,51,54,62 site of cancer (7 studies),14,47,48,51,52,54,62 and BMI (5 studies).35,40,41,45,51
Given that the meta-analysis of sarcopenia and OS contained sufficient studies for further analyses, meta-regression was also performed to examine the influence of study-level covariates on OS. Meta-regression found that higher mean BMI significantly weakened the association between sarcopenia and OS, accounting for 100% of heterogeneity and leaving low (0.00%) residual heterogeneity. The pooled HR decreased by a factor of 0.25 (95% CI −0.36 to −0.14) per 1 kg/m2 increase in mean BMI. The bubble plot is shown in Online Resource 3. Other characteristics, including mean age, year of study completion, sex (percentage of male patients), smoking status, and mean follow-up duration were not significant effect moderators of OS. The results of the meta-regression are shown in Online Resource 4.
Results of the subgroup analyses are shown in Online Resource 5. While the pooled association of sarcopenia with OS remained significant across studies with a mean BMI < 25 kg/m2 (HR 3.00, 95% CI 2.40–3.75, I2 = 0%)13,14,35,40,51,52,55 and studies with a mean BMI ≥ 25 kg/m2 (HR 1.52, 95% CI 1.29–1.79; I2 = 0%),36,39,41,44,45,47,48,54,57 OS was significantly lower in studies with a mean BMI < 25 kg/m2 (p < 0.01 for test of subgroup differences). The pooled association of sarcopenia with OS remained significant and similar across all subgroups of stage of cancer, including those for stage II–IV (HR 2.76, 95% CI 2.06–3.70; I2 = 0%),12,13,35 stage III–IV (HR 2.51, 95% CI 1.76–3.58; I2 = 50%),14,36,51 and stage I–IV cancers (HR 1.80, 95% CI 1.54–2.11; I2 = 13%).40,41,44,45,47,48,52,57 The pooled association also remained significant among studies that adjusted for stage of cancer as a covariate (HR 2.15, 95% CI 1.78–2.61; I2 = 51%)14,35,36,39,40,44,47,51,54 and site of cancer as a covariate (HR 2.48, 95% CI 1.68 to 3.68; I2 = 69%).14,47,51,54 Adjustment for age, sex, and BMI did not influence the statistical significance of effect size of the pooled association, as shown from sensitivity analyses excluding studies that did not adjust for age, sex, or BMI. Further subgroup analyses demonstrated that the observed association of sarcopenia with OS remained consistent across treatment modalities, geographical regions, use of prediction models for quantifying sarcopenia, cut-off values for sarcopenia (either ROC analysis or published definitions) and sites of cancer, which included nasopharyngeal, oral cavity, and mixed sites.
While visual inspection suggested funnel plot asymmetry, this was not suggested by Egger’s test (intercept = 0.2268, 95% CI −1.10 to 0.32; t = 0.52, p = 0.56). Trim and fill imputed 10 studies (Online Resource 6) with minimal change to the pooled effect size (HR 2.31, 95% CI 1.85–2.76; I2 = 57%). Leave-one-out influence analysis showed that no single study had a drastic change on the pooled HR (Online Resource 7), and cumulative meta-analysis showed a significant and stable pooled effect size since 2017 (Online Resource 8).
Disease-Free, Disease-Specific, and Progression-Free Survival
The DFS was reported in seven studies (3348 participants).35,36,39,43,48,52,54 Based on the random-effects model, DFS was significantly lower in patients with sarcopenia compared with patients without sarcopenia (HR 1.76, 95% CI 1.38–2.24; p < 0.01, I2 = 62%) (see Fig. 3). Covariates adjusted for included age (two studies),35,54 sex (two studies),35,54 stage of cancer (four studies),35,36,39,54 and site of cancer (one study).54
Results of the subgroup analyses are shown in Online Resource 9. The pooled association of sarcopenia with DFS remained significant among studies that adjusted for age, sex, stage of cancer, site of cancer, and BMI stage of cancer as a covariate.
The DSS and PFS were reported in four (885 participants)45,54,55,61 and two (297 participants)12,37 studies, respectively. Based on the random-effects model, DSS (HR 2.65, 95% CI 1.80–3.90; p < 0.01, I2 = 0%) and PFS (HR 2.24, 95% CI 1.21–4.13; p < 0.01, I2 = 0%) were significantly lower in patients with sarcopenia compared with patients without sarcopenia (see Fig. 4). Covariates adjusted for included age (three studies),45,54,55 sex (two studies),45,54 stage of cancer (three studies),12,45,55 and site of cancer (one study).55 Adjustment for age, sex, stage of cancer, and site of cancer did not influence the statistical significance of effect size of the pooled association (see Online Resource 9).
Chemotherapy or Radiotherapy Toxicity
The chemotherapy or radiotherapy toxicity was reported in five studies (808 participants).12,37,42,51,58 Based on the random-effects model, the risk of chemotherapy or radiotherapy toxicity was significantly higher in patients with sarcopenia compared with patients without sarcopenia (risk ratio 2.28, 95% CI 1.31–3.95; p < 0.01, I2 = 84%) (see Fig. 4).
Prevalence of Sarcopenia
The prevalence of sarcopenia among HNC patients was reported in 21 studies (6780 participants).12,13,33,35,36,37,38,45,47,48,49,51,52,53,55,56,58,59,60,61,62 Based on the random-effects model, the prevalence of sarcopenia in HNC patients was 50.78% (95% CI 42.52–59.02, I2 = 98%) (see Fig. 5).
Meta-regression found that study-level characteristics, including mean age, year of study completion, sex (percentage of male patients), mean BMI, mean follow-up duration, percentage of Asian patients, and percentage of Caucasian patients were not significant effect moderators of the prevalence of sarcopenia. The results of the meta-regression are shown in Online Resource 4.
Results of the subgroup analyses are shown in Online Resource 10. No significant differences in the pooled prevalence of sarcopenia were observed across all subgroups of stage of cancer, geographical region, site of cancer, and treatment modality.
While visual inspection suggested funnel plot asymmetry, this was not suggested by Egger’s test (intercept = 0.5568, 95% CI −0.8812 to 0.7706; t = 1.46, p = 0.17). Trim and fill imputed nine studies (Online Resource 11) with minimal change to the pooled effect size (39.56, 95% CI 22.55–43.43; I2 = 52%). Leave-one-out influence analysis showed that no single study had a drastic change on the pooled prevalence (Online Resource 12), and cumulative meta-analysis showed a stable pooled effect size since 2017 (Online Resource 13).
Meta-Analysis of Correlations
The results of the meta-analysis of correlations are shown in Table 3. Statistically significant correlations were observed between C3 CSA and L3 CSA, and C3 SMI and L3 SMI.
Grading of Recommendations, Assessment, Development and Evaluations (GRADE) Quality of Evidence
The certainty of evidence for OS (moderate), DFS (low), PFS (low), DSS (low), chemotherapy or radiotherapy toxicity (low), and prevalence of sarcopenia (low) were assessed using the GRADE framework. The results of this assessment are shown in Online Resource 14.
Discussion
In this systematic review and meta-analysis of 37 studies with 11,181 participants, with overall moderate-quality evidence, CT-defined sarcopenia was associated with poorer OS and DFS in patients with HNC. This association was consistent regardless of cancer stage, cancer site, treatment modality, and study location, but was weaker in studies with a higher mean BMI. One in every two patients with HNC were sarcopenic, and these patients also had double the risks of chemotherapy or radiotherapy toxicity compared with patients without sarcopenia.
In 2021, Wong et al. investigated the relationship between sarcopenia and OS in HNC patients, with sarcopenia defined at either the C3 or L3 level using either CT or MRI imaging.11 Their study similarly revealed that radiologically defined sarcopenia was a negative predictor of OS in patients with HNC. Our meta-analysis updates the review by Wong et al. and further provides meta-analyses of the association of sarcopenia with DFS and PFS.11 We have also included only clinical studies that quantified sarcopenia at the C3 vertebrae level. Our meta-analysis also demonstrated that the association of sarcopenia with OS remained significant across different subgroups of cancer stage, cancer location, geographical region, and treatment modality. Additional sensitivity analyses demonstrated that these findings were robust to publication bias, leave-one-out analyses, and cumulative analyses.
While radiographic sarcopenia has been traditionally diagnosed using L3 SMI, newer methods such as those proposed by Swartz et al. have converted C3 CSA to an estimated L3 SMI. As most HNC patients will receive a pretreatment CT of the head and neck, this represents an expedient means of screening for sarcopenia in HNC patients. This method has been further validated in multiple other studies.31,51,65,66 In this meta-analysis, most included studies utilized CT imaging to diagnose sarcopenia with the prediction model. There were studies that employed experimental methods, such as using C3 SMI alone, L3 psoas muscle index, or C3 sternocleidomastoid muscle index. However, it should be noted that the utility of these methodologies remain debated as there is currently no evidence supporting that the mass of a single muscle correlates with whole-body composition.
Investigation of sarcopenia in oncology patients is relevant to clinical practice given its potential value for prognostication of disease outcomes. Several reasons may explain why sarcopenia is a suitable prognostic indicator for patients with HNC. First, the vast majority of HNC patients receive a CT scan of the head and neck for disease staging and surveillance, therefore increasing the convenience of sarcopenia screening. Second, HNC patients also demonstrate poorer oncological outcomes in terms of decreased OS, DFS, and chemotherapy and radiotherapy toxicity.11,67,68 Third, this meta-analysis has demonstrated a high prevalence of sarcopenia in patients with HNC, hence treatment of sarcopenia has the potential to improve patient outcomes in this field. Given that sarcopenia is a potentially modifiable risk factor in patients with HNCs, identification of sarcopenic patients may allow for early interventions to minimize treatment delays and improve outcomes.
Several mechanisms may explain how sarcopenia reduces OS in patients with cancer. Radiologically defined sarcopenia has been found to be a significant predictor of higher mortality and lower OS in other malignancies, including those of the breast, esophagus, and stomach. In breast cancer, sarcopenia was an independent predictor of negative outcomes, such as a twofold increased risk of dying from breast cancer-related pathologies.69 A possible mechanism is greater drug toxicity, which in turn reduces effective oncologic therapy doses and increases the risk of postoperative complications, including infection and immobility.11 Sarcopenia may also serve as an indicator of a patient’s nutritional status. Sarcopenia correlates with poor nutrition, which itself predicts worse outcomes, increased complications, reduced quality of life, and, consequently, increased mortality. Factors including lack of exercise, malnutrition, hormone and cytokine imbalances, and developmental influences may also play a role in the development of sarcopenia.70
Notably, subgroup analysis found that although sarcopenia was associated with poorer OS in chemoradiotherapy versus surgery, this did not reach statistical significance. These results suggest that sarcopenia is predictive of mortality in patients with HNC independent of treatment modality. We would like to highlight that possibly, the effect of sarcopenia on the chemoradiotherapy subgroup is underestimated as patients would likely have been selected against chemotherapy if they were deemed medically unfit to begin with, as evidenced by poor Eastern Cooperative Oncology Group and/or Glasgow scores, at multidisciplinary tumor board discussions.71,72 As such, the observed association between sarcopenia and chemoradiotherapy toxicity could have been underestimated in this analysis. Sarcopenia likely confers worse than observed survival in systemic treatment, and this phenomenon may be explained by the different indications for each treatment modality. Monotherapy with surgery or radiotherapy is commonly used for early cancers, while surgery with adjuvant chemoradiotherapy is typically reserved for locoregionally advanced cancers. It is reasonable to extrapolate that patients undergoing chemoradiotherapy may be more likely to be of an advanced stage of cancer compared with those undergoing surgery or radiotherapy only, except in selected indications such as organ-preservation in HNCs, the discussion of which is beyond the scope of our study.73 The results of this study support the need for future interventional studies and randomized trials on the impact of sarcopenia treatment on mortality in HNC patients.
Subgroup analysis by geographical region demonstrated no significant differences in the association between sarcopenia and OS between studies conducted in Asia, Europe, and North America. The significant association of sarcopenia on OS was also observed across different geographical regions. BMI is known to differ between ethnic groups, with Asian populations found to have a lower BMI than Caucasian populations, given the same level of body fat, age and sex.74 A higher BMI has also been shown to correlate with a lower incidence of sarcopenia.75 These findings therefore suggest that sarcopenia is independently predictive of OS, regardless of population differences in BMI. Interestingly, the meta-regression revealed that the association between sarcopenia and OS was weaker among studies with higher mean BMI. Similar results have been reported in the literature, demonstrating that a BMI lower than 22.5 kg/m2 is associated with an increased risk of all-cause mortality across 24 cancer types, including HNC.76 The effects of BMI on OS may also be related to cancer cachexia, a potential underlying mechanism of sarcopenia.
Meta-regression revealed that lower BMI was associated with poorer OS. Published data suggest that low BMI confers a survival disadvantage in patients with HNC, whereas being overweight up to a BMI of 30 kg/m2 confers a survival advantage, under the phenomenon known as the obesity paradox.77 Several factors may contribute to this phenomenon. Lower BMI may weaken the host immune system and hence blunt the host response to treatment, whereas higher BMI may mitigate chemotherapy toxicity and provide nutritional reserves to safeguard against pharyngeal muscle dysfunction, which may predispose to malnutrition. It is plausible that patients with lower BMI at presentation have lost significant weight due to advanced disease, hence their poor outcomes are a consequence of aggressive disease rather than low BMI alone.78 The prevalence of low BMI increases with more advanced stages of oncological disease and studies have shown that such patients experience significant muscle loss before they appear cachectic.79 Therefore, low BMI may be the late-stage manifestation of malnutrition, muscle loss, and poor physiological reserves. On the other hand, obesity is known to exacerbate sarcopenia by increasing infiltration of fat into muscle, lowering physical function, and increasing the risk of mortality, resulting in sarcopenic obesity.80 Sarcopenic obesity has been found to be associated with worse oncological long-term outcomes in some cancers.81,82 However, it is currently uncertain whether sarcopenic obesity confers a survival disadvantage in patients with HNC, and further studies may be useful in confirming this possible association. Taken together with the existing body of evidence, this meta-analysis supports current findings that BMI is a poor prognostic factor of OS in patients with HNC.
Interestingly, the meta-analysis of correlations demonstrated a statistically significant correlation between C3 CSA and SMI with their corresponding measurements at the L3 level. These results support those reported by Swartz et al., which showed that C3 muscle CSA strongly predicted L3 muscle CSA.9 However, considerable debate surrounds the agreement between C3 CSA and L3 CSA, with subsequent studies finding weak agreement between C3 CSA and L3 CSA measurements, and C3 SMM and L3 SMM measurements.49,53 It has been suggested that the results reported by Swartz et al. be attributed to the high proportion of non-sarcopenic subjects in their cohort, as their analyses was performed in a population including both HNC and trauma patients. Further high-quality studies may be useful to confirm the correlations between C3 and L3 muscle measurements.
There were appreciable limitations within this study. The majority of included studies utilized the Swartz prediction model for the conversion of C3 measures into predicted measures at L3.9 This model was formulated from a small population of patients with HNCs, with an average BMI of 24 kg/m2. Therefore, there are inherent limitations in the applicability of this model for the prediction of sarcopenia. Several included studies have used their own defined cut-off thresholds for sarcopenia using C3 measurements, such as C3 SMI. This reflects a current lack of consensus in the literature on how an acceptable C3 SMI measurement should be defined. Relatively high statistical heterogeneity was observed in the meta-analysis of prevalence of sarcopenia. There could have been incomplete adjustment or a lack of measurement for potential confounders. For instance, none of the included studies adjusted for nutrition or exercise status, which could have been a confounder of the association between sarcopenia and OS. There were also fewer studies that adjusted for tumor grade or aggressiveness compared with those that adjusted for tumor stage. Specific cancer sites and treatment modalities varied in the included studies, which may have added to the clinical heterogeneity of the included populations. To account for possible effect modifiers, the authors have therefore performed a subgroup analysis to stratify included studies by location of cancer and treatment modality.
Conclusion
This systematic review and meta-analysis of overall moderate quality of evidence found that CT-defined sarcopenia was associated with a significantly lower OS, DFS, DSS, and PFS, and increased chemotherapy or radiotherapy toxicity in patients with HNC. This association remained significant regardless of tumor stage, treatment modality, and geographical region, as demonstrated in subgroup analyses. Meta-regression found that higher BMI weakened the association between sarcopenia and OS. Our findings support the need for interventional studies and randomized trials to investigate sarcopenia as a potential modifiable risk factor for mortality in patients with HNCs. As the majority of patients undergoing treatment for HNCs would have a CT scan of the head and neck, where sarcopenia can be measured at the C3 vertebra, our findings are immediately applicable in routine clinical care.
Data availability
All articles in this manuscript are available from the Pubmed/Medline, Embase and Cochrane Library databases.
References
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169.
Chianca V, Albano D, Messina C, et al. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY). 2022;47(9):3205–16. https://doi.org/10.1007/s00261-021-03294-3.
Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. 2022;127(3):228–37. https://doi.org/10.1007/s11547-022-01450-3.
Rubbieri G, Mossello E, Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):181–4.
Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940–53. https://doi.org/10.1177/0148607114550189.
Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. https://doi.org/10.1152/japplphysiol.00744.2004.
de Bree R, Meerkerk CDA, Halmos GB, et al. Measurement of sarcopenia in head and neck cancer patients and its association with frailty. Review. Front Oncol. 2022;12:884988.
Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782–9. https://doi.org/10.1001/jamaoncol.2015.6339.
Swartz JE, Pothen AJ, Wegner I, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016;62:28–33. https://doi.org/10.1016/j.oraloncology.2016.09.006.
Bril SI, Wendrich AW, Swartz JE, et al. Interobserver agreement of skeletal muscle mass measurement on head and neck CT imaging at the level of the third cervical vertebra. Eur Arch Oto-Rhino-Laryngol. 2019;276(4):1175–82. https://doi.org/10.1007/s00405-019-05307-w.
Wong A, Zhu D, Kraus D, Tham T. Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis. Laryngoscope. 2021;131(2):333–41. https://doi.org/10.1002/lary.28616.
Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. 2019;137:117–24. https://doi.org/10.1016/j.radonc.2019.04.023.
Hua X, Li WZ, Huang X, et al. Modeling sarcopenia to predict survival for patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy. Front Oncol. 2021;11:625534. https://doi.org/10.3389/fonc.2021.625534.
Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol. 2019;95:95–9. https://doi.org/10.1016/j.oraloncology.2019.06.009.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Grooten WJA, Tseli E, Äng BO, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res. 2019;3:5. https://doi.org/10.1186/s41512-019-0050-0.
Sedgwick P. What is publication bias in a meta-analysis? BMJ. 2015;351:h4419. https://doi.org/10.1136/bmj.h4419.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629. https://doi.org/10.1136/bmj.315.7109.629.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. https://doi.org/10.1136/ebmental-2019-300117.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–6. https://doi.org/10.1136/bmj.39057.406644.68.
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. https://doi.org/10.1097/xeb.0000000000000065.
Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. https://doi.org/10.1136/bmj.d549.
Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. In: JPT Higgins, J Thomas, J Chandler, M Cumpston, T Li, MJ Page, VA Welch, editors. Cochrane handbook for systematic reviews of interventions. Wiley; 2019. p. 241–84. https://doi.org/10.1002/9781119536604.ch10.
Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. https://doi.org/10.1002/sim.1187.
Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663–82. https://doi.org/10.1002/sim.1752.
Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924. https://doi.org/10.1136/bmj.39489.470347.AD.
Bozkurt G, Elhassan HA, Mahmutoğlu AS, et al. Neck muscle mass index as a predictor of post-laryngectomy wound complications. Ann Otol Rhinol Laryngol. 2018;127(11):841–7. https://doi.org/10.1177/0003489418798660.
Bril SI, Chargi N, Wendrich AW, et al. Validation of skeletal muscle mass assessment at the level of the third cervical vertebra in patients with head and neck cancer. Oral Oncol. 2021;123:105617. https://doi.org/10.1016/j.oraloncology.2021.105617.
Casasayas M, García-Lorenzo J, Gómez-Ansón B, et al. Low skeletal muscle mass assessed directly from the 3rd cervical vertebra can predict pharyngocutaneous fistula risk after total laryngectomy in the male population. Eur Arch Otorhinolaryngol. 2022;279(2):853–63. https://doi.org/10.1007/s00405-021-07127-3.
Chang SW, Tsai YH, Hsu CM, et al. Masticatory muscle index for indicating skeletal muscle mass in patients with head and neck cancer. PLoS One. 2021;16(5):e0251455. https://doi.org/10.1371/journal.pone.0251455.
Endo K, Ueno T, Hirai N, et al. Low skeletal muscle mass is a risk factor for aspiration pneumonia during chemoradiotherapy. Laryngoscope. 2021;131(5):e1524–9. https://doi.org/10.1002/lary.29165.
Hua X, Liao JF, Huang X, et al. Sarcopenia is associated with higher toxicity and poor prognosis of nasopharyngeal carcinoma. Ther Adv Med Oncol. 2020;12:1758835920947612. https://doi.org/10.1177/1758835920947612.
Huang C-H, Lue K-H, Chen P-R, Hsieh T-C, Chou Y-F. Association between sarcopenia and immediate complications and mortality in patients with oral cavity squamous cell carcinoma undergoing surgery. Cancers. 2022;14(3):785. https://doi.org/10.3390/cancers14030785.
Jin W, Rich B, Yechieli R, et al. A single axial slice of the sternocleidomastoids and paravertebral muscles associated with worse local progression-free survival and severe toxicity in sarcopenic head and neck cancer patients undergoing radiotherapy. Cureus. 2022;14(2):e22463. https://doi.org/10.7759/cureus.22463.
Karavolia E, van Rijn-Dekker MI, Van den Bosch L, et al. Impact of sarcopenia on acute radiation-induced toxicity in head and neck cancer patients. Radiother Oncol. 2022;170:122–8. https://doi.org/10.1016/j.radonc.2022.03.009.
Lin SC, Lin YS, Kang BH, et al. Sarcopenia results in poor survival rates in oral cavity cancer patients. Clin Otolaryngol. 2020;45(3):327–33. https://doi.org/10.1111/coa.13481.
Lu X, Tian Y, Huang J, et al. Evaluating the prognosis of oral squamous cell carcinoma patients via L3 skeletal muscle index. Oral Dis. 2023;29(3):923–32. https://doi.org/10.1111/odi.14074.
McGoldrick DM, Yassin Alsabbagh A, Shaikh M, Pettit L, Bhatia SK. Masseter muscle defined sarcopenia and survival in head and neck cancer patients. Br J Oral Maxillofac Surg. 2022;60(4):454–8. https://doi.org/10.1016/j.bjoms.2021.07.020.
Morse RT, Ganju RG, Gan GN, et al. Sarcopenia and treatment toxicity in older adults undergoing chemoradiation for head and neck cancer: identifying factors to predict frailty. Cancers. 2022;14(9):2094. https://doi.org/10.3390/cancers14092094.
Nagpal P, Pruthi DS, Pandey M, Yadav A, Singh H. Impact of sarcopenia in locally advanced head and neck cancer treated with chemoradiation: an Indian tertiary care hospital experience. Oral Oncol. 2021;121:105483. https://doi.org/10.1016/j.oraloncology.2021.105483.
Naser MA, Wahid KA, Grossberg AJ, et al. Deep learning auto-segmentation of cervical neck skeletal muscle for sarcopenia analysis using pre-therapy CT in patients with head and neck cancer. medRxiv. 2021;16:50. https://doi.org/10.1101/2021.12.19.21268063.
Olson B, Edwards J, Degnin C, et al. Establishment and validation of pre-therapy cervical vertebrae muscle quantification as a prognostic marker of sarcopenia in patients with head and neck cancer. Front Oncol. 2022;12:812159. https://doi.org/10.3389/fonc.2022.812159.
Ufuk F, Herek D, Yüksel D. Diagnosis of sarcopenia in head and neck computed tomography: cervical muscle mass as a strong indicator of sarcopenia. Clin Exp Otorhinolaryngol. 2019;12(3):317–24. https://doi.org/10.21053/ceo.2018.01613.
van Heusden HC, Chargi N, Dankbaar JW, Smid EJ, de Bree R. Masseter muscle parameters can function as an alternative for skeletal muscle mass assessments on cross-sectional imaging at lumbar or cervical vertebral levels. Quant Imaging Med Surg. 2022;12(1):15–27. https://doi.org/10.21037/qims-21-43.
van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM, et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol. 2020;147:103–10. https://doi.org/10.1016/j.radonc.2020.03.014.
Vangelov B, Bauer J, Moses D, Smee R. The effectiveness of skeletal muscle evaluation at the third cervical vertebral level for computed tomography-defined sarcopenia assessment in patients with head and neck cancer. Head Neck. 2022;44(5):1047–56. https://doi.org/10.1002/hed.27000.
Vangelov B, Bauer J, Moses D, Smee R. A prediction model for skeletal muscle evaluation and computed tomography-defined sarcopenia diagnosis in a predominantly overweight cohort of patients with head and neck cancer. Eur Arch Otorhinolaryngol. 2023;280(1):321–8. https://doi.org/10.1007/s00405-022-07545-x.
Wendrich AW, Swartz JE, Bril SI, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33. https://doi.org/10.1016/j.oraloncology.2017.05.012.
Yamahara K, Mizukoshi A, Lee K, Ikegami S. Sarcopenia with inflammation as a predictor of survival in patients with head and neck cancer. Auris Nasus Larynx. 2021;48(5):1013–22. https://doi.org/10.1016/j.anl.2021.03.021.
Yoon JK, Jang JY, An YS, Lee SJ. Skeletal muscle mass at C3 may not be a strong predictor for skeletal muscle mass at L3 in sarcopenic patients with head and neck cancer. PLoS ONE. 2021;16(7):e0254844. https://doi.org/10.1371/journal.pone.0254844.
Yoshimura T, Suzuki H, Takayama H, et al. Prognostic role of preoperative sarcopenia evaluation of cervical muscles with long-term outcomes of patients with oral squamous cell carcinoma. Cancers (Basel). 2021;13(18):4725. https://doi.org/10.3390/cancers13184725.
Yunaiyama D, Okubo M, Arizono E, et al. Sarcopenia at the infrahyoid level as a prognostic factor in patients with advanced-stage non-virus-related head and neck carcinoma. Eur Arch Otorhinolaryngol. 2022;279(6):3131–7. https://doi.org/10.1007/s00405-021-07147-z.
Zwart AT, van der Hoorn A, van Ooijen PMA, Steenbakkers R, de Bock GH, Halmos GB. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle. 2019;10(5):1060–9. https://doi.org/10.1002/jcsm.12443.
Mascarella MA, Gardiner L, Patel T, et al. Cervical paraspinal skeletal muscle index outperforms frailty indices to predict postoperative adverse events in operable head and neck cancer with microvascular reconstruction. Microsurgery. 2022;42(3):209–16. https://doi.org/10.1002/micr.30848.
Mascarella MA, Patel T, Vendra V, et al. Poor treatment tolerance in head and neck cancer patients with low muscle mass. Head Neck. 2022;44(4):844–50. https://doi.org/10.1002/hed.26978.
Haehl E, Alvino L, Rühle A, et al. MO-0477 Pretherapeutic sarcopenia predicts survival of elderly patients undergoing radiation for HNSCC. Radiother Oncol. 2022;170:S427–8. https://doi.org/10.1016/S0167-8140(22)02371-4.
Morelli C, Formica V, Bossi P, et al. Untailored vs. gender- and body-mass-index-tailored skeletal muscle mass index (SMI) to assess sarcopenia in advanced head and neck squamous cell carcinoma (HNSCC). Cancers. 2023;15(19):4716. https://doi.org/10.3390/cancers15194716.
Ohyama Y, Inaba Y, Kubota M, Kanemaru T, Hasegawa K. CT-assessed sarcopenia and prognostic nutritional index are associated with poor prognosis in oral squamous cell carcinoma. Oral Maxillofac Surg. 2023. https://doi.org/10.1007/s10006-023-01191-1.
Ye Z, Saraf A, Ravipati Y, et al. Development and Validation of an Automated Image-Based Deep Learning Platform for Sarcopenia Assessment in Head and Neck Cancer. JAMA Network Open. 2023;6(8):E2328280. https://doi.org/10.1001/jamanetworkopen.2023.28280.
Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. https://doi.org/10.1016/s1470-2045(10)70218-7.
Prado CM, Lieffers JR, Bowthorpe L, Baracos VE, Mourtzakis M, McCargar LJ. Sarcopenia and physical function in overweight patients with advanced cancer. Can J Diet Pract Res. 2013;74(2):69–74. https://doi.org/10.3148/74.2.2013.69.
Muresan BT, Juan CS, Artero A, et al. Diagnosis of pre-sarcopenia from a single selectional cross at C3 region, using CT scans before radiotherapy. Nutr Hospit. 2019. https://doi.org/10.20960/nh.02422.
Vangelov B, Bauer J, Kotevski D, Smee RI. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr. 2022;127(5):722–35. https://doi.org/10.1017/S0007114521001446.
Hua X, Liu S, Liao J-F, et al. When the loss costs too much: a systematic review and meta-analysis of sarcopenia in head and neck cancer. Systematic review. Front Oncol. 2020;9:1561.
Findlay M, White K, Stapleton N, Bauer J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin Nutr. 2021;40(4):1711–8. https://doi.org/10.1016/j.clnu.2020.09.017.
Villaseñor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. https://doi.org/10.1007/s11764-012-0234-x.
Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33(1):17–26. https://doi.org/10.1016/j.cger.2016.08.002.
Hacker UT, Hasenclever D, Baber R, et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann Oncol. 2022;33(7):685–92. https://doi.org/10.1016/j.annonc.2022.03.274.
Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32(7):1135–41. https://doi.org/10.1016/0959-8049(95)00664-8.
Yamazaki H, Suzuki G, Nakamura S, et al. Radiotherapy for locally advanced resectable T3–T4 laryngeal cancer—does laryngeal preservation strategy compromise survival? J Radiat Res. 2018;59(1):77–90. https://doi.org/10.1093/jrr/rrx063.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
Liu C, Cheng KYK, Tong X, Cheung WH, Chow SKH, Law SW, Wong RMY. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front Endocrinol. 2023;14:1077255.
Tu H, McQuade JL, Davies MA, et al. Body mass index and survival after cancer diagnosis: a pan-cancer cohort study of 114 430 patients with cancer. Innovation (Camb). 2022;3(6):100344. https://doi.org/10.1016/j.xinn.2022.100344.
Hobday S, Armache M, Paquin R, et al. The body mass index paradox in head and neck cancer: a systematic review and meta-analysis. Nutr Cancer. 2023;75(1):48–60. https://doi.org/10.1080/01635581.2022.2102659.
Fattouh M, Chang GY, Ow TJ, et al. Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck. 2019;41(3):707–14. https://doi.org/10.1002/hed.25420.
Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manag. 2012;44(2):181–91. https://doi.org/10.1016/j.jpainsymman.2011.09.010.
Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–21. https://doi.org/10.1016/j.arr.2016.09.008.
Tschann P, Weigl MP, Clemens P, et al. Sarcopenic obesity is a risk factor for worse oncological long-term outcome in locally advanced rectal cancer patients: a retrospective single-center cohort study. Nutrients. 2023;15(11):2632. https://doi.org/10.3390/nu15112632.
Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29:ii1–9. https://doi.org/10.1093/annonc/mdx810.
Acknowledgment
All authors have made substantial contributions to all of the following: (1) conception and design of the study, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of this manuscript. This manuscript, including related data, figures and tables, has not been previously published and is not under consideration elsewhere.
Funding
No sources of funding were required for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: BYQT, LFT, AM. Data curation: JHK, CYJL, LTPT, EYXG. Formal analysis: JHK, CYJL LTPT. Supervision: BYQT, LFT, AM, JSYH, AJT. Writing – original draft: JHK, CYJL, LTPT. Writing – reviewing and editing: JHK, CYJL, LTPT, BKJT, EYXG, JSYH, AJT, AXS, AM, LFT, BYQT. All authors have read and approved the final version of this manuscript submitted for publication.
Corresponding author
Ethics declarations
Disclosure
Jin Hean Koh, Claire Yi Jia Lim, Lucas Tze Peng Tan, Andrew Makmur, Esther Yan Xin Gao, Jamie Sin Ying Ho, Angel Justina Tan, Anna See, Benjamin Kye Jyn Tan, Li Feng Tan, and Benjamin Yong Qiang Tan declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koh, J.H., Lim, C.Y.J., Tan, L.T.P. et al. Prevalence and Association of Sarcopenia with Mortality in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol 31, 6049–6064 (2024). https://doi.org/10.1245/s10434-024-15510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15510-7