Abstract
Purpose
Recently, it has been reported that sarcopenia and nutritional evaluation are associated with the prognosis of patients with cancer; however, there are only a few detailed reports on oral cancer. This single-center retrospective study aimed to analyze the relationship between computed tomography (CT)-assessed sarcopenia (CT-SP), immunocompetence, nutritional status, and the prognosis of patients with oral squamous cell carcinoma (OSCC).
Methods
This retrospective study included patients who underwent radical therapy with surgery for OSCC between January 2014 and January 2021. Skeletal muscle in the third cervical vertebra (C3) was measured using preoperative cervical CT, and the skeletal muscle index (SMI) was calculated. Nutritional status were investigated using blood tests. The correlation between each parameter and prognosis was analyzed. The primary predictor variables were SMI, ECOG performance status, BMI, and nutritional status. The primary outcome variable was the 5-year overall survival rate (OS) and the secondary outcome variable was 5-year disease-specific survival rate (DSS).
Results
One hundred sixty-three patients were registered retrospectively. The number of patients with CT-SP was 76 (52%). In the univariate analysis, CT-SP, prognostic nutritional index (PNI), and lymphocyte-monocyte ratio (LMR) were associated with poor prognosis, with statistically significant differences in OS and DSS. In the multivariate analysis, only CT-SP was identified as an independent prognostic factor for DSS. CT-SP was significantly correlated with the PNI.
Conclusion
CT-SP was associated with a significant decrease in survival rate in patients with OSCC. Furthermore, CT-SP was correlated with the PNI.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The annual incidence of oral cancer worldwide is 377,713 cases with 177, 757 deaths [1]. Oral cancer can be treated curatively due to advances in reconstruction methods; however, despite the development of various drug therapies for advanced and recurrent cases, the prognosis is poor. Many patients with oral cancer present with advanced disease and malnutrition or dysfunction before surgery.
Sarcopenia is a condition characterized by the loss of skeletal muscle mass and function and is strongly correlated with physical disability, poor quality of life, and death [2]. In recent years, there have been reports that sarcopenia is a poor prognostic factor for head and neck cancer; therefore, preoperative evaluation of sarcopenia is very important. However, there are only a few reports specifically on oral cancer. Sarcopenia is often diagnosed by measuring the skeletal muscle in the third lumber vertebra (L3) region. Computed tomography (CT), including for the lumbar spine, is not usually performed on patients with oral cancer. Recently, sarcopenia has been diagnosed in patients with oral cancer by measuring the skeletal muscle mass in the third cervical vertebra (C3) region [3].
The prognostic nutritional index (PNI), which incorporates the serum albumin level and peripheral blood lymphocyte count, serves as an indicator of immunocompetence and nutritional status. The PNI is often used as a biomarker for various carcinomas, including esophageal, gastric, and lung cancers [4]. Similarly, the PNI is a marker of poor prognosis in patients with oral and head and neck cancers [4, 5]. Moreover, the association of prognosis with nutritional status, modified Glasgow Prognostic Score (mGPS), C-reactive protein-albumin ratio (CAR), lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), and neutrophil–lymphocyte ratio (NLR) as indicators of immunocompetence has been reported in cancer patients [4, 6,7,8,9]. It has been suggested that skeletal muscle destruction may occur due to malnutrition and inflammatory reactions and is associated with sarcopenia [10]. However, there have been no reports on the association of immunocompetence and nutritional status with sarcopenia from the perspective of preoperative nutritional evaluation in patients with oral cancer. In this study, we investigated the correlation between the prognosis of CT-assessed sarcopenia (CT-SP) in the C3 region and that of various immuno-nutritional indicators in patients who underwent radical therapy with surgery for oral squamous cell carcinoma (OSCC) at a single institution.
Subjects and methods
Study design
In this retrospective study, patients with OSCC who underwent radical therapy with surgery for stage I–IV at Shizuoka city Shizuoka Hospital between January 2014 and January 2021 were included. Patients whose blood samples were not collected due to outpatient surgery or who did not undergo contrast-enhanced CT or magnetic resonance imaging (MRI) were excluded. Skeletal muscle in the third cervical vertebra (C3) was measured using preoperative cervical CT, and the skeletal muscle index (SMI) was calculated. Nutritional status were investigated using blood tests. The correlation between each parameter and prognosis was analyzed. The primary predictor variables were SMI, Eastern Cooperative Oncology Group performance status (PS), body mass index (BMI), and nutritional status. The primary outcome variable was the 5-year overall survival rate (OS) and the secondary outcome variable was 5-year disease-specific survival rate (DSS).
Treatment
Patients with advanced-stage oral cancer received preoperative chemoradiotherapy (2 Gy per day and low-dose pepleomycin [2.5 mg/day] subcutaneously as a 100-h continuous injection daily for approximately 3 weeks) [11]. Other patients received a combination of TS1 or cetuximab and radiation therapy or a combination of radiation therapy and super selective intra-arterial infusion chemotherapy. Postoperative treatment included concurrent chemoradiation therapy (cisplatin or carboplatin) or radiotherapy (RT) alone.
Evaluation of CT-SP and immunocompetence and nutritional status
For skeletal muscle evaluation, preoperative cervical contrast-enhanced CT was used to select the slice in which the transverse and spinous processes of the third cervical spine were most widely secured. All images were analyzed by a single researcher using the open-source software ImageJ (Fig. 1). The sternocleidomastoid and paravertebral muscles were measured as described by Lee et al. [12]. (Fig. 1) The internal jugular and common carotid arteries were excluded. When a simple CT scan was obtained, the measurement was performed using the blood vessel information of the same MRI slice. The total cross-sectional area (CSA) (cm2) of the sternocleidomastoid and paravertebral muscles was calculated, and the CSA of L3 was estimated using the formula proposed by Lee et al. [12] [Eq. (1)]. The skeletal muscle index (SMI) was calculated by adjusting the patient’s height [Eq. (2)].
Measurements of computed tomography (CT)- assessed sarcopenia. The total cross-sectional areas (CSA) of the sternocleidomastoid and paravertebral muscles were calculated by preoperative cervical CT using the open-source software ImageJ. CT slices of the CT-SP-positive patient (a) and CT-SP-negative patient (b). The area surrounded by the yellow line is the muscle to be measured. Numbers indicate muscle blocks
The blood samples of the patients were examined at the first visit or near the time of the first visit. The following parameters were evaluated: platelet count, C-reactive protein (CRP) level, albumin (Alb) level, and white blood cell count, including neutrophil, lymphocyte, and monocyte counts. The mGPS is a modified version of the GPS reported by McMillan et al. [13]: CRP ≤ 0.5 mg/dL and Alb ≥ 3.5 g/dL indicate an mGPS score of 0, CRP > 0.5 mg/dL or Alb < 3.5 g/dL indicates an mGPS score of 1, and CRP > 0.5 mg/dL and Alb < 3.5 g/dL indicates an mGPS score of 2. The prognostic nutritional index (PNI), neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and C-reactive protein-albumin ratio (CAR) were examined as the indicators of immunocompetence and nutritional status [4, 6,7,8,9]. The PNI was calculated as (10 × the serum Alb level) + (0.05 × the lymphocyte count). The NLR was calculated by dividing the neutrophil count by the lymphocyte count. The PLR was calculated by dividing the platelet count by the lymphocyte count. The LMR was calculated by dividing the lymphocyte count by the monocyte count. The CAR was calculated by dividing serum CRP by serum Alb level.
The optimal cutoff values for immunocompetence and nutritional status were determined using receiver operating characteristic (ROC) curve analysis based on Youden’s index. The cut-off values are presented in Table S1. Based on these cut-off values, the patients were classified into two groups: Those with a BMI (mass (kg)/height2 (m)) < 18.5 were classified as underweight, and those with a BMI ≥ 25 were classified as overweight [14].
Prognostic factors
PS, CT-SP, BMI (underweight vs. normal weight and overweight vs. normal weight), immunocompetence, and nutritional status (mGPS, NLR, PNI, PLR, LMR, and CAR) were examined as the prognostic factors for the constitution and condition of the patients (Table 3). Age, sex, clinical stage, pathological lymph node metastasis (pN), neoadjuvant therapy, Yamamoto–Kohama criteria (YK), World Health Organization (WHO) differentiation classification, vascular invasion, lymphatic invasion, and perineural invasion were examined as the prognostic factors for cancer. The YK classification was used to determine the grade of invasion [15]. Grade 1 denotes a tumor with a well-defined border, Grade 2 denotes a tumor with a less-marked border, Grade 3 denotes a group of cells and no distinct border, and Grade 4 denotes diffuse invasion. Grade 4 was sub-classified into Grades 4C and 4D. Grade 4C refers to diffuse invasion with cord-like pattern. Tumors of this type invade deeply in the form of a cord-shaped microtumor nest. Grade 4D refers to diffuse invasion. Tumor cells of this type invade the deeper portion diffusely as a single or a few cells [15]. As per the WHO differentiation classification, there are three degrees of differentiation: well-differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3).
Statistical analysis
The primary outcome variable was the 5-year overall survival rate (OS) and the secondary outcome variable was 5-year disease-specific survival rate (DSS). The statistical software EZR 2.7–1' (Easy R) was used for the statistical analysis [16]. Survival rates were calculated using the Kaplan–Meier method. Log-rank and chi-square tests were used to compare survival rates between groups, and multivariate analysis was performed using the Cox proportional hazards model. Multivariate analysis was performed using prognostic factors that met a cut-off p value of < 0.05 on univariate analysis. Statistical significance was set at p < 0.05. The association between CT-SP and nutritional index was analyzed using logistic regression analysis.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee Shizuoka City Shizuoka Hospital (Registry No.20–40) and was performed in accordance with the latest version of the Declaration of Helsinki.
Results
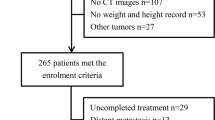
This retrospective study included 163 patients with oral squamous cell carcinoma (OSCC) who underwent radical primary surgery for stage I–IV between January 2014 and January 2021. Patients were excluded if 1) their blood samples were not collected due to outpatient surgery (10 patients) or if 2) they did not undergo contrast-enhanced CT or MRI (seven patients). Therefore, a total of 146 patients (67 males and 79 females) were subjected to CT assessment and prognostic analyses. The age of the patients ranged from 20 to 94 years (average: 69.9 years). Patient characteristics are shown in Table 1. The CT-SP measurements based on the CSAs of the slice in the C3 area of the positive (Fig. 1a) and negative cases (Fig. 1b) are shown in Fig. 1. The CT-SP-positive patient was an 84-year-old woman with stage I lower gingival carcinoma (SMI: 18.63 cm2/m2). The CT-SP-negative patient was an 81-year-old man with stage III oral floor carcinoma (SMI: 64.8 cm2/m2). The treatment and outcomes are shown in Table 2. The 5-year OS and DSS were 74.9% and 82.2%, respectively. The prognostic factors are listed in Table S2. Univariate analysis showed that pN, YK, vascular invasion, lymphatic invasion, and perineural invasion were significantly correlated with prognosis (Table S2). The prognostic factors for the constitution and condition of the patients are listed in Table 3. Univariate analysis of PS, CT-SP, nutritional index, BMI (underweight vs. normal weight), and BMI (overweight vs. normal weight) revealed that CT-SP, PNI, and LMR are correlated with DSS and OS (Table 3). Figure 2 shows the Kaplan–Meier curves for OS and DSS with and without CT-SP. DSS as well as OS of patients without CT-SP was significantly better than those of patients with CT-SP. In the multivariate analysis of the prognosis factors for nutritional status and PS, only CT-SP was identified as an independent prognostic factor for DSS (Table 4). Logistic analysis was performed to analyze the relationship among CT-SP, PNI, and LMR, as they were found to be correlated with both OS and DSS in the univariate analysis. It was found that the PNI was independently and significantly correlated with CT-SP (Table 5).
Discussion
Sarcopenia is a syndrome characterized by the progressive and generalized loss of skeletal muscle mass and strength, with a risk of adverse outcomes, such as physical disability, poor quality of life, and death [17]. To date, there is no consensus on the assessment of sarcopenia in routine clinical practice [18, 19]. The concept of using a single sentinel muscle for the diagnosis of sarcopenia was proposed by a group of experts; however, the assessment of a single muscle that best reflects systematic sarcopenia is controversial [20,21,22]. At the L3 vertebral body level, radiologically measured CSA is linearly related to the total body muscle mass, making it an excellent surrogate for detecting sarcopenia [23]. However, in the field of oral surgery, there are few opportunities to perform abdominal CT, and evaluation of sarcopenia in the L3 region is not considered useful. In recent years, there have been some reports on the diagnosis of sarcopenia by measuring skeletal muscle mass in the C3 region using CT [23]. In addition, as it has been reported that L3 and C3 are correlated, and sarcopenia evaluation based on the C3 region is possible [24]. The C3 method is simple and useful for the diagnosis of sarcopenia because it can be determined by head and neck CT imaging of the C3 region.
Sarcopenia is correlated with the prognosis of cancer patients [17], and the postoperative survival rates with sarcopenia of those various carcinomas, such as gastric cancer [25], colorectal cancer, [26] lung cancer, [27] and head and neck cancer, are poor [23]. However, there are few reports on the association between sarcopenia and oral cancer. In our study, CT-SP was correlated with the prognosis of oral cancer. CT-SP served as a strong predictor of DSS in patients with oral cancer in the multivariate analysis. Yoshimura et al. reported that a low quantity of cervical muscle was statistically associated with short survival in the multivariate analysis [18]. Lee et al. reported that CT-SP was independently associated with poor outcomes in the multivariate analysis of OS.
mGPS, CAR, PNI, LMR, PLR, and NLR have been reported to function as nutritional indicators, inflammation-based prognostic scores, and indicators of systemic inflammatory response (SIR), and they can affect the outcome of cancer treatment [4, 6,7,8,9]. The mGPS and CAR both include C-reactive protein and serum albumin and are useful prognostic tools for malignant tumors [6, 7]. The mGPS correlates with clinical outcomes in patients with head and neck cancers [28]. High CAR predicts early recurrence and poor prognosis in patients with gastric cancer treated with curative intent [6]. NLR and PLR are correlated with head and neck cancer prognosis as systemic inflammation-based blood leukocyte indices [29]. Furthermore, preoperative NLR, PLR, and LMR are associated with poor prognosis in patients with oral cancer [5, 30]. The PNI derived from serum albumin level and absolute lymphocyte count was first established for preoperative nutritional status and postoperative complications in patients with gastrointestinal malignancies [31]. Fang et al. demonstrated that the PNI predicted the prognosis of patients with oral cancer [5]. In the present study, PNI and LMR, but not mGPS, NLR, or CAR, were associated with poor diagnosis of DDS and OS in the univariate analysis. In many of the studies, blood was collected 1 week before surgery, and in our study, blood was collected at a time close to the first visit, which could be the reason for the variation in the inflammation index and albumin level. These data suggest that skeletal muscle deteriorates in the presence of malnutrition and inflammatory reactions, and the SIR is associated with weight and muscle loss and poor outcomes in patients with cancer [32]. The combined effects of sarcopenia and SIR on the survival outcomes of patients with colorectal and esophageal cancers have been previously evaluated [32,33,34,35]. However, to the best of our knowledge, only mGPS has been reported to correlate with sarcopenia in oral cancer [3]. In the present study, we found that the PNI and CT-SP were correlated with prognosis and with each other. Therefore, it is important not only to consider PNI as a prognostic factor but also to consider it as a preoperative indicator of malnutrition and pro-inflammatory state and to correctly manage anti-inflammatory treatments and nutrition before surgery to improve the CT-SP and prognosis.
Perioperative rehabilitation is expected to improve physical fitness, promote early mobilization, and reduce postoperative pulmonary complications in patients with esophageal cancer [36]. Preoperative physical therapy improves pulmonary function and endurance in patients undergoing upper abdominal surgery [37]. Perioperative rehabilitation and nutrition management for sarcopenia patients with oral cancer is expected to improve not only malnutrition and loss of skeletal muscle mass, but also prognosis.
Our study has some limitations. First, it was a retrospective cohort study dating back up to 8 years; some treatments include preoperative chemoradiotherapy, which is not the current standard therapy. However, this study aimed to identify the prognostic value of the CT-SP. Therefore, the differences in treatment may not have significantly affected our results. No correlation was observed between the C3 and L3 levels. However, CT-SP based on C3 indicated the prognosis, and oral cancer patients may not undergo L3 imaging; therefore, the lack of a correlation between them is not considered a major issue. However, true sarcopenia could not be consistently evaluated. Since the cut-off value for sarcopenia has not been determined, it is not viable for clinical use in the future. Sarcopenia detection using C3 is excellent for preoperative evaluation; however, postoperative follow-up evaluation is difficult because the sternocleidomastoid muscle may be resected in patients who have undergone neck dissection as a first-line treatment. Therefore, evaluation of sarcopenia outside the scope of neck dissection is important for future investigations.
In this study, we found that CT-SP at the C3 correlated with oral cancer prognosis and the PNI. To contribute to the improvement of treatment outcomes in patients with oral cancer, we intend to conduct further research in this area in the future.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, (in eng). CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia, (in eng). Clin Cases Miner Bone Metab 11(3):177–180
Lin SC et al (2020) Sarcopenia results in poor survival rates in oral cavity cancer patients, (in eng). Clin Otolaryngol 45(3):327–333. https://doi.org/10.1111/coa.13481
Luan CW, Tsai YT, Yang HY, Chen KY, Chen PH, Chou HH (2021) Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: a systematic review and meta-analysis, (in eng). Sci Rep 11(1):17117. https://doi.org/10.1038/s41598-021-96598-9
Fang KH et al (2021) Preoperative prognostic nutritional index predicts prognosis of patients with oral cavity cancer, (in eng). Oral Dis. https://doi.org/10.1111/odi.13840
Toiyama Y et al (2016) Clinical burden of C-Reactive protein/albumin ratio before curative surgery for patients with gastric cancer, (in eng). Anticancer Res 36(12):6491–6498. https://doi.org/10.21873/anticanres.11248
Toiyama Y, Miki C, Inoue Y, Tanaka K, Mohri Y, Kusunoki M (2011) "Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer, (in eng). Exp Ther Med 2(1):95–101. https://doi.org/10.3892/etm.2010.175
Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD (2018) The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis, (in eng). Eur Arch Otorhinolaryngol 275(7):1663–1670. https://doi.org/10.1007/s00405-018-4972-x
Kanda M et al (2016) Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer, (in eng). Medicine (Baltimore) 95(24):e3781. https://doi.org/10.1097/MD.0000000000003781
Kalinkovich A, Livshits G (2017) Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis, (in eng). Ageing Res Rev 35:200–221. https://doi.org/10.1016/j.arr.2016.09.008
Katagiri T, Ohyama Y, Miyamoto H, Egawa Y, Moriki T, Hasegawa K (2020) Pathological responses to low-dose irradiation and Pepleomycin in Oral squamous cell carcinoma are predictive of Locoregional control, (in eng). BMC Cancer 20(1):1216. https://doi.org/10.1186/s12885-020-07707-2
Lee J et al (2021) Sarcopenia and systemic inflammation synergistically impact survival in oral cavity cancer, (in eng). Laryngoscope 131(5):E1530–E1538. https://doi.org/10.1002/lary.29221
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer, (in eng). Proc Nutr Soc 67(3):257–262. https://doi.org/10.1017/S0029665108007131
Fukuhara-Makiyama N et al (2021) Personality traits and BMI trends over three years in Japanese university students, (in eng). PLoS ONE 16(3):e0248833. https://doi.org/10.1371/journal.pone.0248833
Yamamoto E, Kohama G, Sunakawa H, Iwai M, Hiratsuka H (1983) Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity, (in eng). Cancer 51(12):2175–2180. https://doi.org/10.1002/1097-0142(19830615)51:12%3c2175::aid-cncr2820511205%3e3.0.co;2-m
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics, (in eng). Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Cruz-Jentoft AJ et al (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People, (in eng). Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Yoshimura T et al (2021) Prognostic role of preoperative sarcopenia evaluation of cervical muscles with long-term outcomes of patients with oral squamous cell carcinoma, (in eng). Cancers (Basel) 13(18):4725. https://doi.org/10.3390/cancers13184725
Cruz-Jentoft AJ et al (2019) Sarcopenia: revised European consensus on definition and diagnosis, (in eng). Age Ageing 48(4):601. https://doi.org/10.1093/ageing/afz046
Cesari M et al (2012) Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia, (in eng). J Cachexia Sarcopenia Muscle 3(3):181–190. https://doi.org/10.1007/s13539-012-0078-2
Baracos VE (2017) Psoas as a sentinel muscle for sarcopenia: a flawed premise, (in eng). J Cachexia Sarcopenia Muscle 8(4):527–528. https://doi.org/10.1002/jcsm.12221
Maeda K, Koga T, Akagi J (2016) Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia, (in eng). Clin Nutr 35(5):1147–52. https://doi.org/10.1016/j.clnu.2015.09.011
Wong A, Zhu D, Kraus D, Tham T (2021) Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis, (in eng). Laryngoscope 131(2):333–341. https://doi.org/10.1002/lary.28616
Bril SI et al (2019) Interobserver agreement of skeletal muscle mass measurement on head and neck CT imaging at the level of the third cervical vertebra, (in eng). Eur Arch Otorhinolaryngol 276(4):1175–1182. https://doi.org/10.1007/s00405-019-05307-w
Kawamura T et al (2018) Long-term outcomes of gastric cancer patients with preoperative sarcopenia, (in eng). Ann Surg Oncol 25(6):1625–1632. https://doi.org/10.1245/s10434-018-6452-3
Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N (2020) Sarcopenia in patients with colorectal cancer: A comprehensive review, (in eng). World J Clin Cases 8(7):1188–1202. https://doi.org/10.12998/wjcc.v8.i7.1188
Shinohara S, Otsuki R, Kobayashi K, Sugaya M, Matsuo M, Nakagawa M (2020) Impact of sarcopenia on surgical outcomes in non-small cell lung cancer, (in eng). Ann Surg Oncol 27(7):2427–2435. https://doi.org/10.1245/s10434-020-08224-z
Terazawa K, Ohashi T, Shibata H, Ishihara T, Ogawa T (2022) Immune-modified Glasgow prognostic score: A new prognostic marker for head and neck cancer, (in eng). Head Neck. https://doi.org/10.1002/hed.27170
Szilasi Z et al (2020) Neutrophil-To-Lymphocyte and Platelet-To-Lymphocyte ratios as prognostic markers of survival in patients with head and neck tumours-results of a retrospective multicentric study, (in eng). Int J Environ Res Public Health 17(5):1742. https://doi.org/10.3390/ijerph17051742
Ruiz-Ranz M et al (2022) Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment, (in eng). Front Immunol 13:941351. https://doi.org/10.3389/fimmu.2022.941351
Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF (1980) Prognostic nutritional index in gastrointestinal surgery, (in eng). Am J Surg 139(1):160–167. https://doi.org/10.1016/0002-9610(80)90246-9
Abbass T, Dolan RD, Laird BJ, McMillan DC (2019) The relationship between imaging-based body composition analysis and the systemic inflammatory response in patients with cancer: a systematic review, (in eng). Cancers (Basel) 11(9):1304. https://doi.org/10.3390/cancers11091304
Feliciano EMC et al (2017) Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study, (in eng). JAMA Oncol 3(12):e172319. https://doi.org/10.1001/jamaoncol.2017.2319
Sugawara K et al (2020) Associations of systemic inflammation and sarcopenia with survival of esophageal carcinoma patients, (in eng). Ann Thorac Surg 110(2):374–382. https://doi.org/10.1016/j.athoracsur.2020.03.013
Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC (2019) The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer, (in eng). J Cachexia Sarcopenia Muscle 10(1):111–122. https://doi.org/10.1002/jcsm.12357
Inoue T et al (2016) Changes in exercise capacity, muscle strength, and health-related quality of life in esophageal cancer patients undergoing esophagectomy, (in eng). BMC Sports Sci Med Rehabil 8:34. https://doi.org/10.1186/s13102-016-0060-y
Soares SM, Nucci LB, da Silva MM, Campacci TC (2013) Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial, (in eng). Clin Rehabil 27(7):616–627. https://doi.org/10.1177/0269215512471063
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by Yoshio Ohyama, Yoshinori Inaba, Tomoki Kanemaru, and Mako Kubota. The first draft of the manuscript was written by Yoshio Ohyama and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Research Ethics Committee of Shizuoka City Shizuoka Hospital (Registry No.20–40) and was performed in accordance with the latest version of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ohyama, Y., Inaba, Y., Kubota, M. et al. CT-assessed sarcopenia and prognostic nutritional index are associated with poor prognosis in oral squamous cell carcinoma. Oral Maxillofac Surg 28, 659–666 (2024). https://doi.org/10.1007/s10006-023-01191-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-023-01191-1