Abstract
Background
For patients with peritoneal carcinomatosis, extent of disease and completeness of cytoreductive surgery (CRS) are major prognostic factors for long-term survival. Assessment of these factors could be improved using imaging agents. Pegsitacianine is a pH-sensitive polymeric micelle conjugated to the fluorophore indocyanine green. The micelle disassembles in acidic microenvironments, such as tumors, resulting in localized fluorescence unmasking. We assessed the utility of pegsitacianine in detecting residual disease following CRS.
Patients and Methods
NCT04950166 was a phase II, non-randomized, open-label, multicenter US study. Patients eligible for CRS were administered an intravenous dose of pegsitacianine at 1 mg/kg 24–72 h before surgery. Following CRS, the peritoneal cavity was reexamined under near-infrared (NIR) illumination to evaluate for fluorescent tissue. Fluorescent tissue identified was excised and evaluated by histopathology. The primary outcome was the rate of clinically significant events (CSE), defined as detection of histologically confirmed residual disease excised with pegsitacianine or a revision in the assessment of completeness of CRS. Secondary outcomes included acceptable safety and pegsitacianine performance.
Results
A total of 53 patients were screened, 50 enrolled, and 40 were evaluable for CSE across six primary tumor types. Residual disease was detected with pegsitacianine in 20 of 40 (50%) patients. Pegsitacianine showed high sensitivity and was well tolerated with no serious adverse events (SAEs). Transient treatment-related, non-anaphylactic infusion reactions occurred in 28% of patients.
Conclusions
Pegsitacianine was well tolerated and facilitated the recognition of occult residual disease following CRS. The high rate of residual disease detected suggests that the use of pegsitacianine augmented surgeon assessment and performance during CRS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cytoreductive surgery (CRS) remains an essential component of modern treatment algorithms for peritoneal carcinomatosis from a wide range of primary malignancies, including those of gastrointestinal (appendiceal, colorectal, gastric), gynecologic, or peritoneal (mesothelioma, primary peritoneal carcinomatosis) origin.1 Across these diverse disease sites, the extent of peritoneal disease, quantified by the peritoneal carcinomatosis index (PCI), and the amount of residual disease following cytoreduction, quantified by the completeness of cytoreduction score (CC score), are consistently identified as critical prognostic factors for patients undergoing CRS.2 Patients with complete cytoreduction have demonstrated improved overall survival as compared with patients with incomplete cytoreduction.3 Accurate determination of the PCI and CC scores is of paramount importance in selecting appropriate candidates for surgical or adjuvant therapies and driving expectations regarding oncologic outcome. Given the inherent complexity in achieving optimal outcomes in CRS, any methods to enhance the accuracy of PCI or CC scoring could meaningfully impact the care of patients with peritoneal surface malignancies.
Intraoperative molecular imaging of malignant tissue has been an expanding area of innovation in cancer surgery.4,5 Most strategies in current development rely on passive fluorescent probes that accumulate preferentially in tumors, or active probes that are conjugated to molecular targeting moieties resulting in specific uptake and concentration in target tissues. For example, the near-infrared imaging agent, Cytalux, which targets folate receptor expressing cells, was recently approved by the Food and Drug Administration (FDA) for use as an imaging agent for the detection of ovarian and lung malignancies (https://cytalux.com/wp-content/uploads/2023/01/221216_CYTALUX-Prescribing-Information.pdf).
Pegsitacianine is an activatable fluorescent micellar nanoparticle targeting the acidic pH characteristic of most solid tumors, which was designed for use as an intraoperative adjunct to oncologic surgery. This agent is composed of a hydrophilic polyethylene glycol (PEG) exterior, with an inner hydrophobic polymethyl methacrylate (PMMA) core to which the near-infrared (NIR) fluorophore, indocyanine green (ICG), is covalently conjugated.6 At physiologic pH, the fluorophore is quenched within the nanoparticle core, keeping it in a fluorescence-off state. Exposure to acidic conditions, such as the tumor microenvironment, causes the micelle to dissociate and release fluorescent quenching, enabling visualization upon excitation with near-infrared light. Previous studies have evaluated the preliminary safety and efficacy of pegsitacianine. A phase I study examining the use of this imaging agent as a single IV infusion prior to oncologic surgery in 30 patients with solid tumors demonstrated both a favorable safety profile as well as clinical utility in detecting positive margins in nine cases and occult additional disease in two cases.7
In this phase II clinical trial, we examined the use of pegsitacianine as an adjunct for detection of disease during CRS. We hypothesized that pegsitacianine could enhance detection of residual disease following CRS, enabling surgeons to either perform additional disease resection to achieve a more complete cytoreduction, or to enhance the accuracy of CC score assessment. The outcomes of interest were the frequency with which the residual disease was detected or CC assessment influenced by the use of pegsitacianine, as well as the safety profile and overall performance characteristics of this agent.
Patients and Methods
NCT04950166 was a phase II, non-randomized, open-label, multicenter US study. Eligible study participants were adults with imaging- and/or biopsy-confirmed metastatic disease of peritoneal origin, and a suspected PCI ≥ 10, who were eligible surgical candidates for complete cytoreductive surgery. Patients were enrolled in two groups. Group 1 included patients with solid metastatic disease (tumors containing ≤ to 50% mucin), and group 2 included patients with mucinous disease (> 50% mucin content). No predetermined allocation of patients to either group was implemented, rather, enrollment was open first to group 1 with the opportunity to open group 2 for enrollment following the demonstration of satisfactory pegsitacianine sensitivity and specificity values of 70% or greater in group 1. Patients were not eligible if they had a known allergy to any components of pegsitacianine or had excessive and generalized metastatic disease that was deemed inoperable by the surgeon.
This study was performed in compliance with all regulations, guidelines, and applicable laws for the USA, where the study was conducted. Protocol and informed consent were approved by institutional review boards and ethics committees at each site. All enrolled subjects were provided informed consent before trial conduct. This study was conducted at five institutions in the USA from November 2021 until April 2023. Treatment-related and treatment-emergent adverse events were coded by Common Terminology Criteria for Adverse Events (CTCAE) (v5.01).
Trial procedures were conducted as depicted in Fig. 1. Patients were infused intravenously with 1 mg/kg of pegsitacianine 24–72 (± 8) h prior to surgery, using a 3 mg/mL solution at a maximum rate of 10 ml/min. During standard-of-care (SOC) cytoreductive surgery (CRS), surgeons imaged up to ten individual representative suspected tumor specimens and five normal specimens throughout the peritoneal cavity for fluorescence analysis; specimens were imaged on a background of non-neoplastic mesenteric tissue within the peritoneal cavity, and the surgeon determined a binary fluorescence status. At the completion of planned CRS (SOC resection), the surgeon determined the CC score, after which a NIR camera was used to evaluate each region of the peritoneal cavity under pegsitacianine guidance for evidence of undocumented residual disease. The CC score was reevaluated on the basis of fluorescence imaging, and any additional fluorescent deposits could be resected at the discretion of the surgeon (post-SOC resection). The fluorescent status of each specimen (SOC and post-SOC) was determined by the surgeon and correlated to final pathology. Patients were monitored for safety for 28 days following infusion of pegsitacianine.
NIR imaging was performed with NIR camera systems compatible with indocyanine green (ICG), which included: EleVision IR (Medtronic, Minnesota), SPY PHI (Stryker, Michigan), Explorer Air II (Surgvision, Netherlands) and PDE Neo II (Hamamatsu, New Jersey).
The primary objective of this study was to determine if administration of pegsitacianine facilitated the detection of residual disease following standard of care resection of peritoneal metastases. This was assessed by determining the proportion of patients that had at least one additional lesion detected under pegsitacianine guidance following CRS that was confirmed as positive for disease by pathological evaluation. Secondary objectives included demonstration of an acceptable safety profile of pegsitacianine and reliable sensitivity, specificity, negative predictive values, and positive predictive values of the imaging agent at the level of individual specimens. Exploratory objectives included the assessment of specimen to background ratios and the impact on accuracy of pegsitacianine in differentiating metastatic disease from normal healthy tissue.
The safety and intent-to-treat (ITT) populations included all patients who received at least one dose (partial or full) of pegsitacianine. The efficacy-evaluable population included all patients who received > 75% of the intended dose of pegsitacianine, had a minimum of one image collected during their procedure, and had the opportunity for post-SOC evaluation of the peritoneal cavity. All efficacy analyses were conducted on this population. All SOC lesions (suspected tumor and suspected normal) and any post-SOC, fluorescence-guided specimens were included in analysis of secondary and exploratory endpoints. Sensitivity was defined as the ratio (multiplied by 100) of the number of tumor specimens correctly identified as fluorescent with pegsitacianine (true positive specimens) over the total number of all pathologically confirmed tumor specimens (true positive + false negative). Specificity was defined as the ratio (multiplied by 100) of healthy non-cancerous tissue specimens correctly identified as negative for fluorescence (true negative) over all non-cancerous healthy tissue specimens (true negative + false positive). Only areas of residual disease identified with fluorescence guidance were resected post-SOC. Therefore, no true negative specimens were collected post-SOC, and specificity could only be calculated for SOC specimens. Two-sided 95% confidence intervals were calculated for each rate via the Clopper Pearson method as well as via a cluster bootstrap to compare the effects of within-subject clustering. Receiver operator characteristic (ROC) curves were constructed from a mixed model that accounted for within-subject correlation and compared with the area under the curve of standard ROC curve. Since the number of specimens each subject had was variable, to keep the same number of specimens in the bootstrapped sample, the algorithm was stratified initially by the number of specimens a subject had, then resampled by first choosing a subject within that strata, and then resampling the same number of specimens that subject originally had. All resampling was done with replacement. Specimen-to-background ratios were defined as the mean fluorescence intensity of the specimen divided by the mean fluorescence intensity of normal background tissue within the peritoneal cavity (typically a region of uninvolved mesentery). One-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons was used to compare specimen-to-background ratios (Prism, version 9.5.1 or later). All other computations for statistical analyses were performed using SAS® software, version 9.4 or later.
Results
A total of 53 subjects were screened, and 50 were deemed eligible and were administered pegsitacianine. These 50 patients are included in the safety-evaluable patient population (Fig. 2). A total of ten patients were excluded from evaluation on the basis of incomplete dosing of pegsitacianine (three), physician decision to terminate cytoreductive surgery at staging laparoscopy due to excessive disease (four), no opportunity to evaluate the peritoneal cavity due to aborted surgery (one), and no evidence of any disease at pathology (two) (Supplemental Table 1). Thus, 40 patients were included in efficacy-evaluable population for analysis across five study sites, comprising a diverse array of primary tumor types. Baseline patient characteristics are presented in Table 1. Disease volume, as assessed by PCI, ranged from 10 to 36, with a median of 14.5. Prior to assessment with fluorescence imaging following SOC cytoreductive surgery, initial surgeon assessment showed that 22 patients underwent a CC0 (55%) 17 underwent a CC1 (42%) cytoreduction, with a single patient scored as CC2 (2.5%).
The primary clinical outcome was the rate of resection of initially undetected residual disease or revision of the completeness of cytoreduction assessment (i.e., a change in the CC score) following the post-CRS imaging of the peritoneal cavity, which were defined as clinically significant events. Residual disease was detected following intended CRS in 20/40 (50%) patients. Across these 20 patients with residual disease, a total of 33 additional specimens that were resected following reimaging were confirmed to be malignant by a pathologist, and a total of 13 patients (32.5%) had their CC score revised on the basis of the size of detected lesions confirmed at final pathology (from 0 to 2 in three cases, 1 to 2 in four cases, and 0 to 1 in six cases). Overall specimen size (size of resected tissue including tumor and any resected surrounding tissue) among those resected post-SOC ranged (longest diameter) from 0.3 cm to 10 cm, with a median specimen size of 1.2 cm. Of the 22 patients who had initially been assessed to have a CC0 following standard of care resection, 9 (40.9%) had additional disease detected under pegsitacianine guidance that increased the CC score assessment. Patient distribution of clinically significant events is presented in Supplemental Table 1. Images of residual disease resected under pegsitacianine guidance are shown in Fig. 3. The distribution of “missed” lesions throughout the peritoneal cavity is summarized in Supplemental Fig. 1, although no discernable pattern emerged from this analysis, as lesions were identified throughout the entirety of the peritoneal cavity.8 Residual disease was identified across all six primary tumor types evaluated with pegsitacianine.
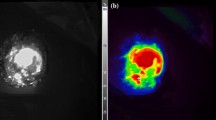
In situ images of residual disease detected on the lower curvature of the stomach under pegsitacianine guidance depicted in A white light and B near-infrared fluorescence heat map, in low-grade mucinous appendiceal cancer; C specimen-to-background ratios across pathologically confirmed resected standard of care tumor and normal tissue specimens; D tumor-to-background ratios calculated across tumor types
Secondary objectives in this study included the evaluation of safety and performance of pegsitacianine. A total of 650 different specimens collected from SOC resection and post-SOC fluorescence-guided resection from the 40 included patients were examined across four near-infrared camera systems. All specimens were imaged using an ex vivo, intraoperative technique in which excised tissue specimens were placed against adjacent non-neoplastic mesenteric tissue within the peritoneal cavity for use as anatomically relevant background during imaging, prior to being designated by the surgeon as fluorescent or non-fluorescent, and then sent for histologic assessment. The true positive rate or sensitivity, defined as specimens designated to be fluorescent by the surgeon and confirmed to contain malignant tissue by the pathologist out of the total number of all malignant specimens, was 78.6% (282/359). The true negative rate or specificity, defined as those specimens designated to be non-fluorescent by the surgeon and negative for malignancy by the pathologist out of the total number of all negative specimens, was 56.4% (164/291). Discordant results consisted of false-positive samples (fluorescent but negative for malignancy), 43.6% (127/291), or false-negative samples (malignant but non-fluorescent), 21.4% (77/359). Specific performance data and receiver operator characteristic curves accounting for within-patient clustering bias can be found in Table 2 and Supplemental Fig. 2, respectively. The patient-level false-positive rate, wherein all post-SOC fluorescence-guided specimens were determined to be negative for malignancy on final pathology, was 20% (8/40), or 12.5% if lymph node resected specimens are excluded.
Performance characteristics of the fluorescent probe and imaging platform were also assessed using specimen-to-background ratio (SBR), with adjacent non-neoplastic mesenteric tissue serving as the background. All SBR data were analyzed by an unbiased third-party reviewer trained in image analysis, blinded to designated fluorescent status and pathology. True positive and false-positive specimens had significantly higher SBRs as compared with true negative and false-negative SBR (Fig. 3C). True positive specimens had significantly higher SBRs compared with false positives (p = 0.0001). No clinically significant variation was observed in performance by primary tumor type, nor between mucinous and non-mucinous pathology (Fig. 3D).
Pegsitacianine was well tolerated, with no treatment-related serious adverse events occurring in any patient. All adverse events are summarized in Table 3. Infusion-related reactions were the most common treatment emergent adverse event (TEAE) and were experienced by 14 of 50 (28%) patients, all of which were considered grade 1 or grade 2 adverse events. No relationship was observed between infusion-related reactions and tumor type or disease burden. These infusion-related reactions were generally transient, with a median duration of 16 min, and self-limited. Most common symptoms included flushing, pain, and dizziness. All symptoms are summarized in Table 4. Symptomology, onset, and duration were consistent with previous studies.
Discussion
Molecular imaging agents hold great promise in improving oncologic outcomes for patients undergoing cancer surgery. By augmenting the surgeon’s ability to detect tumors, imaging agents could theoretically improve the ability to accurately stage patients with cancer, to detect occult lesions, achieve negative margins during surgery, or to quantify residual disease at the conclusion of surgery. CRS for peritoneal carcinomatosis serves as an ideal test case for the use of molecular imaging agents, since a vast number of individual tumors can be analyzed from a much smaller number of patients, thereby increasing the efficiency and statistical power to assess sensitivity and specificity of these agents. Patients with peritoneal surface malignancies also stand to benefit from molecular imaging strategies, since they could enhance assessment of several key variables influencing oncologic outcome in these patients, namely disease volume (PCI) and completeness of cytoreduction (CC score).
The results of this phase II clinical trial examining the use of pegsitacianine in patients undergoing CRS for a variety of indications further supported the favorable safety and tolerability profile previously demonstrated in phase 1 and 2 studies in other solid tumor types.7 We found that the administration of this agent 24–72 h prior to surgery, when combined with intraoperative imaging, resulted in a detection of residual disease in 50% of cases. Similar studies with fluorescence imaging agents have considered a 3–10% event rate as a threshold indicating clinical benefit.9,10 Given these established metrics for success, the high rate of detection of residual disease in this trial provides a compelling case for the clinical utility of pegsitacianine. The completeness of surgery has been widely cited as a key prognostic factor for outcomes in patients undergoing CRS.11 Complete resection (CC0, no visible disease remaining in the peritoneal cavity) remains the objective whenever possible. Patients undergoing CRS for a wide variety of primary tumor types consistently achieve improved overall survival based on completeness of cytoreduction, including clear separation of outcomes between CC0 and CC1 resections, a distinction that was revised with the assistance of fluorescence imaging in many of the patients treated in this study.2,12,13 Furthermore, while computed tomography (CT) and magnetic resonance imaging (MRI) scans are used for initial staging of disease, the sensitivity for detection of smaller lesions is poor, ranging from 11% to 28% in lesions less than 0.5 cm across previous studies, with variance in sensitivity across tumor types and pathology.14,15 Thus, in addition to utility in assessing the post-CRS status of the peritoneal cavity, molecular imaging agents could also contribute meaningfully to the critical task of optimal patient selection prior to CRS.
Our experiences in this clinical trial identified a number of barriers to wide adaptation of this technique. First, it will be important to develop strategies to mitigate off-target fluorescence caused by clearance of pegsitacianine in normal tissues such as liver, small bowel, and lymph nodes, which may confound interpretation of fluorescent signal. It will also be necessary to refine the quantitative metrics used to distinguish fluorescent versus non-fluorescent specimens to aid in reducing false positives and false negatives and improve consistency of detection. While complete resection is prioritized in CRS, tissue sparing may instead be prioritized in other indications where high false-positive rates could lead to excessive removal of critical tissue. The binary and somewhat subjective determination made by investigators in this study would ideally be replaced with empirically established thresholds derived from the SBR or other numerical metrics of fluorescence. True positive specimens in this study had significantly higher SBR than negative specimens, which supports the development of thresholding to distinguish fluorescence.
While this study allowed for the use of any ICG-compatible camera system, some systems are better optimized for tumor imaging, with heat map overlay, autogain features, and improved sensitivity that may aid in better detection and determination of fluorescence status. Future studies will likely focus on the camera systems best suited to this particular use case, as this may help reduce variability in detection capabilities. Ultimately, even with established thresholds and optimal camera systems, fluorescence guidance should serve as an ancillary tool to the surgeon’s standard of care tools—palpation and visualization—to draw attention to areas in the surgical cavity that may have been missed, allowing for reassessment. Finally, from the patient’s perspective, the preoperative infusion of pegsitacianine does require an extra procedural visit, with attendant cost and inconvenience.
With additional experience, it may be possible to identify subsets of patients in whom molecular imaging could contribute to better outcomes during CRS. For example, while no statistically significant pharmacodynamic differences were observed across mucin content in this study, mucinous tumors trended toward a higher false-positive rate. This could be attributed to the lower tumor cell content in mucinous tumors. Future studies should investigate the impact of tumor cell content of resected specimens to determine if there is a threshold that impacts detection. Since this phase II study was limited in size, additional studies in larger patient cohorts should further evaluate the role of primary tumor type, mucin content, lesion size, camera system, lesion depth, and prior therapies to better define the clinical utility of molecular imaging in CRS.
Beyond CRS, other scenarios in the care of patients with peritoneal metastases or other malignancies may be excellent use cases for molecular imaging. The limitations of staging laparoscopy are well documented in the literature, and the utility of ICG in the assessment of vascular perfusion, biliary anatomy, and even hepatic tumor imaging, has been described.16,17,18 On the basis of the findings of the current study, pegsitacianine could augment the sensitivity of peritoneal disease detection during staging laparoscopy by highlighting areas for focused tissue sampling. Moreover, patients being considered for CRS in the context of carcinomatosis often undergo serial laparoscopic assessments to determine feasibility of complete cytoreduction. PCI estimates in this context can be inaccurate; underestimation of the PCI could lead to non-therapeutic laparotomy, while overestimation could lead to exclusion of candidates who may actually benefit from surgery. Pegsitacianine may also provide potential utility in minimally invasive cytoreduction of ovarian cancer, which has remained controversial due to concerns over impaired assessment and completeness of resection.19,20 Finally, for less experienced centers or training centers for CRS, use of this agent may enable surgeons to gain confidence in malignant tissue detection and completeness assessment during the learning curve for CRS.
Overall, this study demonstrated that pegsitacianine was well tolerated and facilitated the recognition of occult residual disease following CRS in half of the evaluated cases. The high rate of residual disease detected warrants further clinical investigation and suggests that pegsitacianine augmented the surgeon’s performance and clinical outcomes during CRS.
References
Cortes-Guiral D, Hubner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers. 2021;7(1):91. https://doi.org/10.1038/s41572-021-00326-6.
Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–18. https://doi.org/10.1002/cncr.25356.
Levine EA, Stewart JHT, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218(4):573–85. https://doi.org/10.1016/j.jamcollsurg.2013.12.013.
Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation–a new cutting edge. Nat Rev Cancer. 2013;13(9):653–62. https://doi.org/10.1038/nrc3566.
Nagaya T, Nakamura YA, Choyke PL, Kobayashi H. Fluorescence-guided surgery. Front Oncol. 2017;7:314. https://doi.org/10.3389/fonc.2017.00314.
Ma X, Wang Y, Zhao T, et al. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J Am Chem Soc. 2014;136(31):11085–92. https://doi.org/10.1021/ja5053158.
Voskuil FJ, Steinkamp PJ, Zhao T, et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat Comm. 2020. https://doi.org/10.1038/s41467-020-16814-4.
Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2(1):3. https://doi.org/10.1186/1477-7800-2-3.
Tanyi JL, Randall LM, Chambers SK, et al. A phase III study of pafolacianine injection (OTL38) for intraoperative imaging of folate receptor-positive ovarian cancer (study 006). J Clin Oncol. 2022. https://doi.org/10.1200/JCO.22.00291.
Smith BL, Hunt KK, Carr D, et al. Intraoperative fluorescence guidance for breast cancer lumpectomy surgery. NEJM Evidence. 2023. https://doi.org/10.1056/EVIDoa2200333.
Foster JM, Zhang C, Rehman S, Sharma P, Alexander HR. The contemporary management of peritoneal metastasis: a journey from the cold past of treatment futility to a warm present and a bright future. CA Cancer J Clin. 2023;73(1):49–71. https://doi.org/10.3322/caac.21749.
Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113(2):315–25.
Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining “optimal” residual disease. Gynecol Oncol. 2012;125(2):483–92. https://doi.org/10.1016/j.ygyno.2012.02.024.
Sugarbaker PH. Preoperative assessment of cancer patients with peritoneal metastases for complete cytoreduction. Indian J Surg Oncol. 2016;7(3):295–302. https://doi.org/10.1007/s13193-016-0518-0.
Jacquet PF, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72(5):1631–6.
Abo T, Nanashima A, Tobinaga S, et al. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging. Eur J Surg Oncol (EJSO). 2015;41(2):257–64. https://doi.org/10.1016/j.ejso.2014.09.008.
Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29(7):2046–55. https://doi.org/10.1007/s00464-014-3895-x.
Li K, Zhang Z, Nicoli F, et al. Application of indocyanine green in flap surgery: a systematic review. J Reconstr Microsurg. 2018;34(2):77–86. https://doi.org/10.1055/s-0037-1606536.
Nitecki R, Rauh-Hain JA, Melamed A, et al. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int J Gynecol Cancer. 2020;30(9):1450–4. https://doi.org/10.1136/ijgc-2020-001584.
Melamed A, Rauh-Hain JA. Minimally invasive interval cytoreductive surgery: it’s time for a randomized trial. Int J Gynecol Cancer. 2019;29(9):1339–40. https://doi.org/10.1136/ijgc-2019-000971.
Acknowledgment
The authors thank the patients that agreed to participate in this study, as well as all investigators, pathologists, and clinical staff who supported this study. The authors acknowledge that the development of pegsitacianine has been supported through grants from the Cancer Prevention Research Institute of Texas (CPRIT), OncoNano Medicine. Additionally, the authors thank Ron Allen, Ph.D., for conducting image analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Shannon Westin received grants/contracts to institution from Astra Zeneca, AvengeBio, Bayer, Bio-Path, Clovis Oncology, GSK, Jazz Pharmaceuticals, Mereo, Novartis, Nuvectis, Roche/Genentech, and Zentalis and consulted for AstraZeneca, Caris, Clovis Oncology, Eisai, EQRX, Gilead, GSK, Immunocore, ImmunoGen, Lilly, Merck, Mereo, Mersana, NGM Bio, Nuvectis, Roche/Genentech, SeaGen, Verastem, Vincerx, Zentalis, and ZielBio; Dr. Giorgos Karakousis received research support from industry to fund clinical trial at institution; Mr. Brian Madajewski is the holder of intellectual property in the form of patents and copyrights (Hall K.A.; Madajewski B; Kaplan H; “pH Responsive Compositions, Formulations, and Methods of Imaging a Tumor” 2020, United States patent appl.62/937141, 18 November 2020); Dr. Charles Balch is the chair of the Scientific Advisory Board of OncoNano Medicine and invested in OncoNano stock; and Dr. David Bartlett was responsible for the clinical trial patient enrollment fee being negotiated between AHN and OncoNano. The authors acknowledge support from the Cancer Research and Prevention Institute of Texas, OncoNano Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wagner, P., Levine, E.A., Kim, A.C. et al. Detection of Residual Peritoneal Metastases Following Cytoreductive Surgery Using Pegsitacianine, a pH-Sensitive Imaging Agent: Final Results from a Phase II Study. Ann Surg Oncol 31, 4726–4734 (2024). https://doi.org/10.1245/s10434-024-15165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15165-4