Abstract
Background
Neoadjuvant chemoradiation therapy (nCRT) is recommended when lymph node metastasis is evident or strongly suspected on preoperative imaging studies, even for a completely resectable (cT1-2) tumor with minimal lymph node involvement (cN1). We evaluated the validity of upfront surgical approach in this patient group.
Methods
We retrospectively reviewed data from 247 patients with cT1-2 esophageal squamous cell carcinoma (ESCC) who underwent upfront radical esophagectomy followed by the pathology-based adjuvant treatment. Oncologic outcomes of cN1 patients were compared with those of cN0 patients.
Results
There were 203 cN0 and 44 cN1 patients. The lymph node yield was 62.0 (interquartile range [IQR], 51.0–76.0) in cN0 and 65.5 (IQR, 57.5–85.0) in cN1 patients (p = 0.033). The size of metastatic node was 0.6 cm (IQR, 0.4–0.9 cm) in cN0 and 0.8 cm (IQR, 0.5–1.3 cm) in cN1 patients (p = 0.001). Nodal upstaging was identified in 29.1% of cN0 and 40.9% of cN1 patients, whereas 18.2% of the cN1 had no actual lymph node metastasis (pN0). The 5-year disease-free survival rate was not significantly different between the groups (cN0, 74.4%; cN1, 71.8%; p = 0.529). Survival rates were closely correlated with pN stage, and a multivariate analysis revealed that pN2-3 stage was a risk factor for poor disease-free survival.
Conclusions
Upfront radical surgery provided accurate nodal staging information, potentially sparing some cN1 patients from unnecessary nCRT while demonstrating comparable survival rates. It might be a valid option for the treatment of cT1-2N1 ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal cancer is a highly aggressive disease, with the prognosis remaining poor after surgery alone for patients with locally advanced cancer. The phase III Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS) trial has demonstrated the survival benefits of neoadjuvant chemoradiation therapy (nCRT) followed by surgery over surgery alone in patients with cT1N1 or cT2-3N0-1 esophageal or esophagogastric junction cancer.1,2 The Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010) trial has confirmed the benefits of nCRT in patients with cT1-4N1 or cT4N0 esophageal squamous cell carcinoma (ESCC).3 Thus, the current guidelines recommend nCRT followed by surgery (nCRT + S) for most resectable tumors, except for cT1N0 cases.4,5
Although this approach seems rational given that lymph node metastasis is considered advanced disease,6 the previous trials did not consider the number of metastatic lymph nodes, which is an independent prognostic factor of oncologic outcome.7,8 This is because they used the 6th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system, which only indicated the presence or absence of lymph node involvement.9 Rice et al. reported a distinct survival difference between patients with ≤ 2 metastatic nodes and those with ≥ 3 metastatic nodes.10 Peyre et al. showed that patients with ≥ 3 metastatic nodes have a > 50% risk of developing systemic disease.6 Indeed, the 7th and 8th editions of the AJCC/UICC staging system have acknowledged the importance of the number of involved lymph nodes by revising the N category to a numerical classification ranging from N0 to N3.11,12
This study began with the question of whether nCRT is absolutely necessary for patients with minimal lymph node involvement (N1), particularly in those with completely resectable T1 or T2 tumors. We have been performing upfront radical esophagectomy for this subgroup of patients based on the following rationales: (1) ESCC can be treated until a certain stage of progression by radical surgery; (2) current modalities for preoperative nodal staging are suboptimal, potentially leading to over- or understaging; and (3) deconditioning issues after nCRT. We employ adjuvant therapy based on the final pathological report.
We hypothesized that upfront radical esophagectomy could be a valid option for cT1-2 ESCC patients with minimal lymph node involvement (cN1) if oncologic outcomes were comparable to cT1-2 N0 ESCC patients. If the results support this hypothesis, we could suggest conducting a randomized, clinical trial (RCT) in this subgroup of patients.
Methods
Data from 424 consecutive patients who underwent transthoracic esophagectomy between 2015 and 2020 were prospectively collected and reviewed (Fig. 1). Among them, data from 264 patients with cT1 or cT2 tumors were included in the analysis. The exclusion criteria were: (1) cN2 or N3 diseases and (2) patients referred after receiving nCRT. The study included 247 patients for further analysis and was approved by the institutional review board (IRB-4-2023-0559). The requirement for informed consent was waived because of the retrospective nature of the analysis.
All patients underwent a staging workup with esophagogastroduodenoscopy, endoscopic ultrasonography (EUS), contrast-enhanced chest and abdomino-pelvic computed tomography (CT), and positron emission tomography (PET)-CT. The tumor stage was determined based on the 7th edition of the AJCC cancer staging manual.11 The lymph node stations were classified according to the 11th edition of the Japanese Classification of Esophageal Cancer.13
In a previous report, we provided a detailed description of the surgical procedure.14 Transthoracic esophagectomy was performed with total mediastinal lymphadenectomy that included the dissection of bilateral recurrent laryngeal nerves. In addition, upper abdominal lymph node dissection was performed including the resection of No. 7, 8a, 9, and 11p nodes. A narrow gastric tube was then created and pulled up through the retrosternal route, and a handsewn esophagogastric anastomosis was made. Bilateral neck node dissection, including No. 101 and 104 stations, was added in patients with suspicious cervical lymph node metastasis or upper thoracic ESCC. We have been routinely performing en bloc esophagectomy since 2017 with three-field lymphadenectomy in all patients, regardless of tumor location or cervical lymph node involvement.15
Adjuvant therapy was administered based on the pathological report. Cisplatin-based chemotherapy was primarily reserved for patients with multiple lymph node metastasis. For patients with superficial ESCC and solitary lymph node metastasis to No. 1, 2, or 106recR, radical surgery alone was considered appropriate because these lymph nodes are recognized as proximal regional lymph nodes that can be effectively treated.16,17,18 Radiation therapy was administered to patients with incomplete resection, and adjuvant chemoradiation therapy was administered to pT3 N+ patients.
The patients were scheduled to visit the outpatient clinic for chest and abdomino-pelvic CT scans every 4–6 months in the first 2 years and every 6 months thereafter. Locoregional recurrence was defined as tumor recurrence in the surgical field or at the anastomosis site, whereas distant recurrence was defined as metastasis to distant organs or nonregional lymph nodes, such as the retroperitoneal abdominal para-aortic nodes. Recurrence was diagnosed based on clinical grounds and confirmed with either PET scan or biopsy. Disease-free survival (DFS) was calculated as the time from the date of surgery to the first occurrence of tumor recurrence or death from any cause, whereas recurrence-free interval (RFI) was calculated as the time from the date of surgery to the first occurrence of tumor recurrence.
Continuous variables are presented as mean ± standard deviation (SD), and the intergroup difference was analyzed using the Student’s t test. Variables that were not normally distributed are presented as medians (interquartile range [IQR]) and were compared by using the Mann–Whitney U test. Differences in categorical variables were analyzed by using the χ2 test or Fisher’s exact test. Survival curves were estimated by using the Kaplan–Meier method and compared by using the log-rank test. Statistical significance was set at p < 0.05. Multivariable Cox proportional hazards regression was conducted to identify risk factors for DFS. Variables with p < 0.20 on univariate analysis were used as input variables for the multivariable Cox regression analysis. All statistical analyses were performed by using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY).
Results
The study included 247 patients with cT1-2 ESCC, of which 203 were in the cN0 group and 44 were in the cN1 group. The clinical profiles of the patients are presented in Table 1. Significant differences in age, sex, and tumor location were not observed between the two groups. However, cT2 tumors were more prevalent in the cN1 group (40.9% vs. 16.7% in cN0, p = 0.001). Although no significant difference was noted in the operation data, bilateral neck node dissection tended to be more frequently performed in the cN1 group than in the cN0 group (p = 0.068). The lymph node yield was higher in the cN1 group (65.5, IQR 57.5–85.0) than in the cN0 group (62.0, IQR 51.0–76.0) (p = 0.033). Incomplete resection was identified in one patient in each group who had upper thoracic ESCC: the circumferential margin was positive in one cN0 patient with a pT3 tumor, and the proximal resection margin was positive in one cN1 patient with a pT2 tumor. Meanwhile, 90-day mortality was observed in three patients: two in the cN0 group due to pneumonia and conduit necrosis, and one in the cN1 group due to pneumonia.
Table 2 outlines the pathological data. The cN1 group exhibited more aggressive histopathological features, including longer tumor length and higher rates of lymphatic permeation, vascular invasion, and perineural invasion. Lymph node metastasis was present in 29.1% (59/203) of cN0 patients and 81.8% (36/44) of cN1 patients, with the mediastinal nodes being the most frequent metastasis site in both groups. We analyzed 202 metastatic lymph nodes, and the size of metastatic focus was 0.3 cm (IQR, 0.2–0.6 cm) in cN0 patients and 0.6 cm (IQR, 0.2–1.2 cm) in cN1 patients (p < 0.001). In the cN1 group, 18.2% (8/44) showed downstaging to pN0, whereas 40.9% (18/44) showed upstaging to pN2 or N3. Adjuvant therapy was administered to 9.4% (19/203) of cN0 patients (chemotherapy, n = 11; chemoradiation, n = 5; radiation, n = 3) and 45.4% (20/44) of cN1 patients (chemotherapy, n = 17; chemoradiation, n = 2; radiation, n = 1) based on the pathological report.
The median follow-up time was similar in both the cN0 and cN1 groups (36.0 and 37.5 months, respectively; p = 0.984). Among the 203 cN0 patients, 24 (11.8%) experienced recurrences: six (3.0%) were locoregional, fourteen (6.9%) were distant, and four (2.0%) were combined. Among the 44 cN1 patients, eight (18.2%) had recurrences: two (4.5%) were locoregional, five (11.4%) were distant, and one (2.3%) was combined. The incidence (p = 0.255) and pattern of recurrence (p = 0.704) did not differ significantly between the two groups.
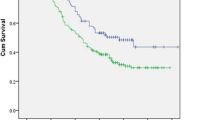
During the follow-up period, the mortality rates for the cN0 and cN1 groups were 16.7% (34/203) and 11.4% (5/44), respectively (p = 0.496). Figure 2 reveals no significant differences in the 5-year overall survival (OS), DFS, and RFI between the two groups. Further survival analysis was conducted for all patients based on the pathological nodal stage (Fig. 3), indicating a better correlation with the pN stage rather than the cN stage. When three or more lymph node metastases occurred (pN2 or N3), the 5-year survival rates were significantly decreased (< 50%) compared with those of pN0 or pN1 patients. Cox’s proportional hazards model identified that pN2 or N3 stage and incomplete resection were significant risk factors for DFS (Table 3).
Discussion
The principal finding of this study was the comparable survival rates of cT1-2 N1 patients who underwent upfront radical esophagectomy. The 5-year OS, DFS, and RFI were 84.9%, 71.8%, and 80.7%, respectively. The cT1-2 N1 tumor comprises a relatively small portion among esophageal cancer. It is distinguished from more advanced cases (cT3-4 or cN2-3), because the primary tumor was completely resectable, and the lymphatic metastasis occurred at only one or two regional lymph nodes. Because the CROSS and NEOCRTEC trials demonstrated better oncological outcomes in the nCRT + S arm compared with the S alone arm, the current standard treatment for cT1-2 N1 patients is nCRT + S.4,5 If nCRT + S is preferred according to those guidelines, the following points must be considered: (1) Are those preoperative nodal staging modalities highly reliable to prevent overstaging?; (2) Are the findings of those RCTs directly translatable to cT1-2 N1 ESCC patients?; and (3) If we intend to perform an upfront esophagectomy, what are the benefits of the approach?
Clinical Nodal Staging and the Risk of Overstaging in cT1-2 N1 Patients
The accurate measurement of lymph node metastasis is crucial in these patients, because nCRT is unnecessary if there is no actual lymph node metastasis. In the present study, understaging was noted in 29.1% of the cN0 and 40.7% of the cN1 patients, whereas overstaging was identified in 18.2% of the cN1 patients. Although we employed CT, EUS, and PET-CT scan in all patients, accurate nodal staging was possible in only 70.9% of the cN0 and 40.9% of the cN1 patients, because the avidity of the primary tumor may have affected the detectability of lymph nodal metastases. If the primary ESCC lesion presents with a low 18F-FDG uptake, as in cases of cT1 or T2, PET-CT may have a limited role for initial nodal staging because of low sensitivity.19 Manabe et al.19 reported that the sensitivity was only 15.2% when the 18F-FDG uptake of the primary lesion was low. Another reason is the small metastatic focus size (0.3 cm in the cN0 and 0.6 cm in the cN1, p < 0.001). We reported earlier that a significant proportion of nodal metastases were extremely small to detect via PET-CT imaging, and meticulous lymph node dissection could provide an accurate pathological stage.20 In our series, 5.9% (12/203) and 40.7% (18/44) of the patients with cN0 and cN1, respectively, had pN2 or N3 disease and demonstrated a significantly reduced survival rates compared with those with pN0 or N1 disease. These patients received adjuvant chemotherapy; however, they may benefit from neoadjuvant chemotherapy if we could detect N2 or N3 disease on the initial staging work-up studies.
Overstaging is more problematic, because unnecessary CRT will be applied to the pN0 patients, whilst there is a chance of adjuvant treatments in the understaging cases. In the present study, 18.2% (8/44) of the cN1 patients were reported as pN0, for whom nCRT was unnecessary or even harmful. In an institute where nCRT is actively employed to cN1 patients, histologic diagnosis by ultrasonography, EUS, or thoracoscopic sampling should be considered to prevent overstaging.
Interpretation of the Previous RCTs for the Management of cT1-2 N1 ESCC
Another crucial issue is the interpretation of the results of recent RCTs comparing nCRT + S with S alone1,3,21; these studies used the 5th or 6th edition of the TNM classification (Table 4). The patients enrolled in these RCTs may include cN2 or N3 cases if the 7th or 8th edition of the TNM classification was applied. For example, in the French Francophone de Cancérologie Digestive (FFCD) 9901 trial, the ranges of the numbers of metastatic lymph nodes were 0–10 in the nCRT + S arm and 0–25 in the S alone arm.21 Thus, information on the effects of nCRT in cT1-2 N1 patients is limited. Thus far, the FFCD 9901 trial was the only RCT that enrolled relatively early-stage patients (cT1-2 N0-1 or cT3N0). They reported that nCRT with cisplatin plus fluorouracil did not improve the R0 resection rate or DFS (35.6% in the nCRT + S arm and 27.7% in the S alone arm, p = 0.648) but enhanced postoperative mortality.21 Two further RCTs (CROSS and NEOCRTEC) enrolled more advanced-stage patients (more than 80% of the patients had cT3 or T4 tumors), for whom nCRT is undoubtedly required to enhance resectability and loco-regional control. In addition, the CROSS trial included an extremely heterogenous population, in terms of tumor histology and location, and allowed a transhiatal approach that has been regarded as a noncurative surgery for thoracic ESCC. Moreover, although 78% had T3 or deeper tumor and 76% exhibited lymph node metastasis in the S alone arm, these patients did not receive any further adjuvant treatment. To some extent, the CROSS trial compared the nCRT group to an undertreated group. RCTs that focused on cT1-2N1 ESCC are warranted to assess whether nCRT improves outcomes for this subgroup of patients.
Potential Benefits of Upfront Radical Esophagectomy in cT1-2 N1 Patients
Adequate lymph node dissection undoubtedly contributes to the accuracy of staging. Upfront radical esophagectomy could provide accurate pathological staging information, which serves as both the strongest prognostic factor and a guide to adjuvant therapy. In the present study, the 5-year survival rates were more closely correlated with the pN stage than the cN stage. Once three or more lymph nodes were involved, the 5-year DFS rate was significantly decreased to 35.3%. A multivariate analysis also confirmed that pN2-3 stage was a significant risk factor for DFS. Regarding adjuvant therapy, there have been several reports that support its effectiveness on prolonging survival. In the Japan Clinical Oncology Group Study—JCOG 9204 trial, the 5-year DFS rate was 45% with surgery alone and 55% with surgery plus chemotherapy (p = 0.037).22 Rice et al. and Hwang et al. reported in their propensity-matched analysis that the addition of CRT to surgery significantly prolonged survival time.23,24 Additionally, Chen et al. compared 562 patients with locally advanced ESCC and reported that there was no survival difference between nCRT + S and S + adjuvant CRT.25 A favorable survival in our series could be attributed, in part, to the addition of adjuvant therapy based on the pathological report after radical esophagectomy.
It is still difficult to generalize that radical esophagectomy could increase the cure rate in patients with lymph node metastasis. Although some believe that lymph node metastasis equals systemic disease and discard the surgical option for such cases, we believe that radical esophagectomy with extensive lymph node dissection could cure the disease or prolong DFS until certain stages of progression. In the present study, we performed two- or three-field lymphadenectomy, including meticulous dissection along the bilateral recurrent laryngeal nerve chains in all patients. This is reflected in the large number of lymph node yields in our series (62.0 in the cN0 and 65.5 in the cN1), which is higher than that in other RCT series.1,3,21 In our series, 71.8% of the cN1 patients were in disease-free status at 5 years after surgery, although 81.8% of the cN1 cases had pathological stage II or III. In addition, the 5-year DFS rate of the pN1 patients was similar to that in pN0 patients (Fig. 3). This finding suggests that in cases with one or two lymph node metastasis, radical surgery plus adjuvant treatment may be useful and effective to increase the cure rate and prolong survival.
There are a few limitations to this study. This was a retrospective, observational study to evaluate the feasibility and validity of an upfront surgical approach in patients with cT1-2N1 disease. Because this subgroup comprises a relatively small portion among the entire population, the inclusion of only a small number of patients was inevitable in a single-center setting. In addition, direct comparison with nCRT was not possible because nCRT has been reserved for patients with cT3 or multiple lymph node metastasis in our institute.
Conclusions
Upfront radical esophagectomy could be a valid option for the treatment of cT1-2N1 ESCC. It provided accurate pathological staging and demonstrated a comparable survival rate to cT1-2N0 cases. Future RCTs comparing our approach with nCRT + S in patients with cT1-2N1 are warranted.
References
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8.
Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–803.
Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9(8):830–87.
Obermannova R, Alsina M, Cervantes A, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004.
Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248(6):976.
Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247(2):365–71.
Zhang HL, Chen LQ, Liu RL, et al. The number of lymph node metastases influences survival and International Union Against Cancer tumor-node-metastasis classification for esophageal squamous cell carcinoma. Dis Esophagus. 2010;23(1):53–8.
Greene FLePDLeFIDeFAGeBCMeHDGeMMe. AJCC Cancer Staging Manual[electronic resource]. 2002.
Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125(5):1103–13.
Compton CCeBDReG-AJeKSHeOAeWMKe. AJCC Cancer Staging Atlas[electronic resource] :A Companion to the Seventh Editions of the AJCC Cancer Staging Manual and Handbook/edited by Carolyn C. Compton, David R. Byrd, Julio Garcia-Aguilar, Scott H. Kurtzman, Alexander Olawaiye, Mary Kay Washington. 2012.
Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42.
Japan Esophageal Society office. Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14(1):1–36.
Kim DJ, Park SY, Lee S, Kim HI, Hyung WJ. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc. 2014;28(6):1866–73.
Kim HE, Yang YH, Park BJ, Park SY, Min IK, Kim DJ. Skeletonizing en bloc esophagectomy revisited: oncologic outcome in association with the presence of thoracic duct lymph nodes. Ann Surg Oncol. 2022;29(8):4909–17.
Matsubara T, Ueda M, Abe T, Akimori T, Kokudo N, Takahashi T. Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic oesophagus. Br J Surg. 1999;86(5):669–73.
Kunisaki C, Makino H, Kimura J, et al. Therapeutic strategy for esophageal cancer based on solitary lymph node metastasis. Hepatogastroenterology. 2011;58(110–111):1561–5.
Park SY, Kim DJ, Son T, et al. Extent of mediastinal lymphadenectomy and survival in superficial esophageal squamous cell carcinoma. J Gastrointest Surg. 2017;21(10):1584–90.
Manabe O, Hattori N, Hirata K, et al. Diagnostic accuracy of lymph node metastasis depends on metabolic activity of the primary lesion in thoracic squamous esophageal cancer. J Nucl Med. 2013;54(5):670–6.
Park SY, Kim DJ, Jung HS, Yun MJ, Lee JW, Park CK. Relationship between the size of metastatic lymph nodes and positron emission tomographic/computer tomographic findings in patients with esophageal squamous cell carcinoma. World J Surg. 2015;39(12):2948–54.
Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–22.
Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan clinical oncology group study–JCOG9204. J Clin Oncol. 2003;21(24):4592–6.
Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126(5):1590–6.
Hwang JY, Chen HS, Hsu PK, et al. A propensity-matched analysis comparing survival after esophagectomy followed by adjuvant chemoradiation to surgery alone for esophageal squamous cell carcinoma. Ann Surg. 2016;264(1):100–6.
Chen HS, Hsu PK, Liu CC, Wu SC. Upfront surgery and pathological stage-based adjuvant chemoradiation strategy in locally advanced esophageal squamous cell carcinoma. Sci Rep. 2018;8(1):2180.
Yang H, Liu H, Chen Y, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 2021;156(8):721–9.
Acknowledgment
All authors declare that they have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y.H., Park, B.J., Kim, H.E. et al. Completely Resectable (cT1-2) Esophageal Squamous Cell Carcinoma with Minimal Lymph Node Involvement (cN1): Is Neoadjuvant Chemoradiation Therapy the Only Viable Treatment Option?. Ann Surg Oncol 31, 2490–2498 (2024). https://doi.org/10.1245/s10434-023-14756-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14756-x