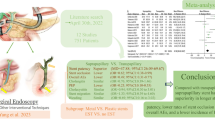
Abstract
Background
Optimal preoperative biliary drainage for patients with pancreatic cancer before pancreatoduodenectomy remains unclear. This study aimed to investigate the comparison of efficacy and safety between a metallic stent (MS) and a plastic stent (PS).
Methods
Comparative studies on the use of MS and PS for pancreatic cancer before pancreatoduodenectomy were systematically searched using the MEDLINE and Web of Science databases. Pre- and postoperative data also were extracted. Random-effects meta-analyses were performed to compare post-endoscopic retrograde cholangiopancreatography (ERCP) complications as well as intra- and postoperative outcomes between the two arms of the study, and pooled odds ratios (ORs) or mean differences (MDs) were calculated with 95 percent confidence intervals (CIs).
Results
The study analyzed 12 studies involving 683 patients. Insertion of MS was associated with a lower incidence of re-intervention (OR, 0.06; 95% CI 0.03–0.15; P < 0.001), increased post-ERCP adverse events (OR, 2.22; 95% CI 1.13–4.36; P = 0.02), and similar operation time (MD, 18.0 min; 95% CI –29.1 to 65.6 min; P = 0.46), amount of blood loss (MD, 43.0 ml; 95% CI –207.1 to 288.2 ml; P = 0.73), and surgical complication rate (OR, 0.78; 95% CI 0.53–1.15; P = 0.21). The cumulative stent patency rate after 3 months was higher in the MS group than in the PS group (70–100 % vs 30.0–45.0 %).
Conclusion
For biliary drainage in patients with pancreatic cancer during this era of multidisciplinary treatment, MS use might be the first choice because MS provides a more durable biliary drainage and a similar risk of postoperative outcomes compared with PS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic cancer (PC) is a devastating disease and one of the major causes of cancer-related death.1 To improve survival outcomes, the role of neoadjuvant treatment (NAT) for patients with borderline resectable or locally advanced PC has evolved.2 Because obstructive jaundice should be ameliorated before NAT is initiated,3 durable and secure preoperative biliary drainage (PBD) is needed. With the increasing use of NAT for patients with resectable PC, the role of PBD has increased in recent decades.4
According to previous studies on unresectable PC,5 endoscopic biliary drainage (EBD) is commonly used for biliary drainage.6 Two types of implantable devices are used for EBD: plastic stent (PS) and metal stent (MS). Plastic stents are 7 to 10 Fr in size and can easily be removed and replaced with fewer incidences of endoscopic retrograde cholangiopancreatography (ERCP)-related pancreatitis and cholecystitis.7,8
On the other hand, metal stents usually are larger than 10 mm and have a self-expanding force, a longer patency period than plastic stents and less need for re-intervention. However, use of MS has disadvantages. Previous studies have shown that the post-ERCP adverse event rate was higher after MS insertion than after PS insertion, and that self-expanding pressure led to inflammatory changes in the surrounding tissue.5,9,10,11 Therefore, selecting a stent in the preoperative setting has been a trade-off, especially for patients treated with NAT. A longer MS patency period (4–12 weeks) is required before surgery for patients undergoing upfront surgery and a much longer waiting time for patients receiving NAT,12 while local inflammation should be avoided to minimize surgical risk.
To identify which types of stents are optimal in the preoperative setting, several studies have compared MS with PS for resectable and borderline resectable PC.13,14,15,16,17,18,19,20,21,22,23,24 However, the patient populations in these studies were small, and the studies were retrospective and performed mostly at a single center. Little is known about the optimal stents for resectable and borderline resectable PC with obstructive jaundice in a preoperative setting. Therefore, this study aimed to investigate the comparison between MS and PS in terms of endoscopic re-intervention and perioperative complication rates for patients with PC who underwent subsequent surgery.
Methods
Search Strategy
This systematic review and meta-analysis were reported in accordance with PRISMA guidelines (Table S1).25 A systematic literature survey was conducted according to the recommendations of the Cochrane Collaboration.26 Searches were performed to identify all studies referring to preoperative decompression of the bile duct for PC. The MEDLINE and Web of Science databases were searched for eligible articles published between January 1989 and October 2022.
In 1989, the first clinical trial of MS deployment was reported.27 The following search terms were used: ((pancreatic neoplasm*) OR (pancreatic cancer) OR (pancreatic ductal adenocarcinoma) OR (pancreatic head cancer) OR (head of pancreas)) AND ((metal*) or (plastic)). The final electronic search was performed on 15 November 2022. No language restrictions were imposed in any of the searches. In addition, the reference lists of all articles fulfilling the eligibility criteria and other relevant articles missed in the electronic searches were examined through manual searches.
Selection Criteria
Two independent investigators (Y.E. and M.T.) reviewed all records identified in the literature search. The inclusion criteria specified only studies that compared MS with PS in terms of re-intervention, stent potency, and postoperative outcomes. The exclusion criteria ruled out reports that did not include a surgical description, reports that did not include at least two of the four components of the intra- and postoperative findings (blood loss, operation time, morbidity, and mortality), and review articles without original data and those that dealt with cell lines or animals. If the abstract was relevant, the full article was assessed for eligibility. For overlapping cohorts, the most recent or relevant publication was selected.
Data Extraction
Data were extracted independently by two authors (Y.E. and M.T.) according to a pre-specified protocol. A third reviewer (M.K.) resolved all disagreements. From each included study, the first-author information, year of publication, study type (e.g., retrospective study, prospective study, or randomized control trial), study design (e.g., single-center or multi-center study), country of origin, number of patients, types of neoadjuvant therapy, regimen of neoadjuvant chemotherapy, resectability classification (e.g., resectable, borderline resectable, and locally advanced PC) according to national comprehensive cancer network (NCCN) guidelines (version 1, 2022), periods between stent insertion and operation, stent types, rates and reasons for re-intervention, cumulative stent patency rate, and intra- and postoperative characteristics (e.g., blood loss, operation time, morbidity, and mortality) were recorded.
Re-intervention was defined as biliary drainage necessitated by the appearance of elevated hepatobiliary enzyme and total bilirubin levels (stent occlusion), concomitant cholangitis, or dislocated stents (stent migration). The study defined ERCP-related adverse effects, including cholecystitis, pancreatitis, perforation, and bleeding, as local inflammation or bleeding after ERCP. Surgical complications included all grades of the Clavien–Dindo (CD) classification.28 Two-by-two contingency tables were constructed to perform a meta-analysis. Data were extracted independently by two authors (Y.E. and M.T.) according to the pre-specified protocol.
Quality Assessment
The methodologic quality of the included studies was assessed by two authors (Y.E. and M.T.) using the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I). The ROBINS-I includes seven potential risks of bias (confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selective reporting). Each domain was assessed as low, moderate, serious, critical, or not assessable, and the overall risk of bias was evaluated for each study. Two authors (Y.E. and M.T.) independently assessed the methodologic quality of the included studies and the overall quality of evidence. In case of disagreement, a consensus was reached through discussion with a third reviewer (M.K.).
Data Synthesis and Statistical Analysis
Outcomes were either presented as originally reported or, if possible, calculated from published raw data. Quantitative analyses of at least three studies were performed. The sample mean and standard deviation were calculated using the sample median, size, and minimum and maximum values.29 Pooled odds ratios (ORs) and 95 percent confidence intervals (CIs) were calculated to compare the rates of re-intervention, surgical complications, and mortality between the MS and PS groups. Pooled weighted mean differences (MDs) and 95 % CIs were calculated for blood loss and operative times.
A random-effects model was used for the meta-analysis. A P value lower than 0.050 was considered statistically significant. The I2 and Q tests were used to assess study heterogeneity. K-mean cluster analysis was used to identify a potential subset of patients relative to 3 months stent patency and postoperative complication rates.30 Potential publication bias was assessed by visual inspection of funnel plots and application of the Harbord test if more than nine studies were analyzed.31 For statistical analysis, R version 4.2.0 (R Project for Statistical Computing, Vienna, Austria) and the meta-analysis package (Meta 5.1.1 and estmeansd 0.2.1) were used.
Results
Overview of Literature Search
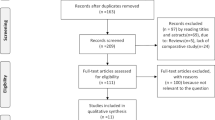
The electronic database search identified 5300 articles. From these articles 742 duplicates were removed, after which 4370 articles were excluded after screening of the titles and abstracts. The remaining 188 studies met the pre-specified inclusion criteria and were evaluated using full-text analysis. The analysis included 12 studies (Fig. 1).13,14,15,16,17,18,19,20,21,22,23
Characteristics of Included Studies
The included studies were conducted in three countries (Table 1). Nine studies were conducted in a single center, and two studies were multi-center investigations. The studies included two randomized controlled trials,21,22 one prospective cohort study,15 and nine retrospective cohort studies.13,14,16,17,18,19,20,23,24 Nine studies, all from Japan, referred to NAT and resectability. Of the 683 participants assessed, 286 were treated with MS, and 397 were treated with PS. The stent types in the MS group were uncovered self-expandable metal stents (UCSEMS),17 full-covered SEMS (FCSEMS),15,16,20,21,22,23 partially covered SEMS (PCSEMS),14,19 and unspecified stents,13,18 whereas those in the PS group were 7 to 10 Fr in diameter and 5 to 9 cm in length.
Risk of Re-Intervention During Waiting Periods
The waiting period was 72.5 days (range, 8.2–136.9 days) in the MS group and 62.0 days (range, 10.5–113.4 days) in the PS group. During that time, the MS group was less likely to undergo intervention during the follow-up period (OR, 0.06; 95% CI 0.03–0.15; P < 0.001; I2 = 64.1 %; Fig. 2). The reasons for intervention were as follows: stent occlusion, stent migration, retrograde cholangitis, and unspecified conditions.
Risk of ERCP-Related Complications
Nine studies reported on ERCP-related complications. Overall, 582 patients (MS [n= 243] vs PS [n = 339]) were analyzed. The ERCP-related adverse event rate was significantly higher in the MS group than in the PS group during the follow-up period (OR, 2.22; 95% CI 1.13–4.36; P = 0.02; I2= 24.0 %; Fig. S1). Post-ERCP pancreatitis was observed in 10.7 % of the MS group, whereas it was observed in 4.7 % of the PS group (OR, 3.01; 95% CI 1.48–6.11; P = 0.002; I2= 0 %). The rate of cholecystitis after ERCP was 4.1 % in the MS group, whereas it was 1.5 % in the PS group (OR, 1.56; 95% CI 0.53–4.62, P = 0.42; I2= 0 %).
Postoperative Outcomes
Figure S2A–D shows a forest plot of the studies examining postoperative outcomes. Between the MS and PS groups, no significant differences in blood loss (MD, 43.0 ml 95% CI –202.1 to 288.2 ml; P = 0.73; I2 = 22.4 %) or operation time (MD, 18.0 min; 95% CI –29.4 to 65.5 min; P = 0.46; I2 = 64.3 %) were observed among 458 patients (MS [n = 205] vs PS [n = 253]; Fig. S2A and B). In addition, according to the 11 studies that included 571 patients (MS [n = 238] vs PS [n = 333]), no statistically significant difference between the two groups was found in the incidence of surgical complication (OR, 0.78; 95% CI 0.53–1.15; P = 0.21; I2 = 0 %; Fig. S2C). The surgical mortality rate was reported in six studies involving 315 patients (MS [n = 126] vs PS [n = 189]), and it was similar in the MS and PS groups (OR, 0.59; 95% CI 0.10–3.60; P = 0.57; I2 = 19.1 %; Fig. S2D).
Stent Patency
In the analysis of the stent patency rate 1, 2, and 3 months after insertion relative to stent type, the patients with PS had a lower rate of stent patency than those with MS (1 month: MS [94.0 %] vs PS [75.6 %], P = 0.006; 2 months: MS [91.1 %] vs PS [48.1 %], P < 0.001; 3 months: MS [87.0 %] vs PS [26.8 %], P < 0.001). Figure 3 shows a scatter plot of the surgical complication and cumulative stent patency rates 3 months after insertion. The cumulative stent patency rate varied from 70 % to 100 % in the MS group and from 30.0 % to 45.0 % in the PS group. K-mean clustering identified two subgroups of patients that corresponded to the stent type. This indicated that the distribution of stent patency and complication rates were distinct between the MS and PS groups.
Quality Assessment
The results of the quality assessment are presented in Table S2. Two clinical trials were graded as having a low risk of all biases, whereas other trials were referenced as having a moderate risk. In five studies, classification of intervention was described in detail. In addition, the funnel plots were symmetric and insignificant in the Harbord test (Figs. 4 and S3A–E).
Discussion
This meta-analysis assessed the superiority of MS over PS for potentially resectable PC in terms of pre- and postoperative outcomes. Deployment of MS was associated with a lower rate of re-intervention. The study showed no significant differences between the two groups in lengths of operation time, amounts of blood loss, surgical complication rates, or mortality rates. In contrast, MS insertion was correlated with an increased risk of post-ERCP complications (e.g., cholecystitis and pancreatitis).
Preoperative biliary drainage has become increasingly endorsed as a treatment choice for pancreatic head cancer since neoadjuvant chemotherapy has been widely accepted for patients with potentially resectable PC.2 Whereas several studies have examined the superiority of MS over PS in unresectable PC, the efficacy and safety of MS compared with PS as PBD for resectable and borderline resectable PC have not been well investigated, and few guideline recommendations have been available regarding this topic.5,9
To date, an increasing number of patients with PC have been treated with NAT. In NAT settings, the preoperative waiting period can be extended to 3 to 6 months according to recent clinical trials of NAT for resectable and borderline PC.32,33,34,35,36 Recent meta-analyses have demonstrated that patients with PC who received NAT and treatment with MS had a lower rate of re-intervention and NAT cessation without a greater incidence of postoperative outcomes than those treated with PS.37,38 However, these meta-analyses are limited to patients with NAT, making the application of this result to a broader population of patients with PC impossible. Furthermore, almost one–fourth of the patients treated with upfront surgery for PC underwent surgery 4 to 12 weeks after diagnosis.12,39 Therefore, PBD is important even for individuals who have undergone upfront surgery.
One advantage of using MS over PS is the longer period of stent patency due to the larger caliber of the lumen and its self-expansion force.40,41 In contrast, PS has generally been selected for PBD because the waiting period before surgery for resectable PC is relatively short.42 It is unclear whether the incidence of re-intervention needed after PS insertion is comparable with that after MS in the preoperative setting.
In the current study, the MS group was less likely to undergo re-intervention than the PS group. In addition, this study showed that the cumulative stent patency rates of the MS and PS groups differed significantly 1, 2, and 3 months after insertion. These results indicate that even in a limited period, biliary obstruction or cholangitis that needs re-intervention would often occur in patients treated with PS. Secured stent patency is more likely to be exhibited by MS for 3 or more months than by PS, assisting in MS deployment, especially in patients treated with NAT.43,44 Re-intervention would require repeated hospitalization and subsequently delay the planned curative resection, resulting in increased total cost and impaired survival.14,21,45 In summary, these results support the recommendation for MS insertion in the preoperative setting.
Previous investigators have demonstrated that the use of MS is a negative risk factor for post-ERCP pancreatitis or cholecystitis in patients with biliary obstruction due to unresectable periampullary malignancy.7,8 According to these previous studies, patients in whom an MS was inserted had more than five times higher rates of ERCP-related pancreatitis.7 The rate of cholecystitis after stent insertion was more than three times higher for patients treated with MS than for those treated with PS. However, whether the rate of cholecystitis and pancreatitis after MS insertion differs from that of PS for potentially resectable PC is poorly documented.8
In the current study, the ERCP-related adverse event rate was significantly higher in the MS group. This suggests that post-ERCP events should be meticulously observed and managed appropriately if adverse events occur after MS insertion because post-ERCP complication would prevent the receipt of NAC. Further studies are needed to investigate whether the increased ERCP-related adverse effects after MS insertion affect the cessation or delay of NAT administration and to identify which cases are more prone to post-ERCP complications.
Previously, there were concerns that MS insertion led to fibrotic changes in the surrounding bile duct due to stent-related inflammation, resulting in greater operative and postoperative complications and creating technical difficulties such as fibrotic adhesion of the portal vein and arteries, which may compromise R0 resection and interfere with biliary reconstruction.10,46,47 Although some patients who experienced ERCP-related pancreatitis or cholecystitis suffered from increased local inflammation, this meta-analysis demonstrated no significant difference in surgical outcomes (blood loss, operation time, surgical complications, and mortality). The fact that the two stent types had comparable effects on surgical outcomes could be attributable to the lower rates of obstructive cholangitis in patients treated with MS, which has a significant impact on surgical difficulties and mortality.48,49 The effect from a lower frequency of cholangitis would offset stent-related local inflammation. These results support the idea that MS deployment does not harm the subsequent pancreatoduodenectomy for resectable and borderline resectable PC. Previous studies have shown that postoperative complications precluded adjuvant chemotherapy for patients with PC, leading to poor survival. Therefore, MS deployment is an important therapeutic option for PC.50
Our study differed from previous meta-analysis on this topic for several reasons.37,38,42 First, in the meta-analysis by Crippa et al.,42 the patient population encompassed “periampullary cancer,” which includes not only pancreatic cancer but also distal cholangiocarcinoma and ampullary cancer. Although distal cholangiocarcinoma and ampullary cancer may share some anatomic similarities, they diverge from pancreatic cancer in terms of oncologic management. For instance, NAT is strongly recommended for pancreatic cancer, whereas no established, effective neoadjuvant treatment exists for distal cholangiocarcinoma and ampullary cancer.51,52 Therefore, we distinguish between pancreatic cancer and other periampullary cancers.
Second, Du et al.37 and Kumar et al.38 focused on patients with pancreatic cancer who underwent NAT. Although NAT is recommended for all pancreatic cancer cases, the rate of NAT administration was approximately 40 %.53 The underutilization of NAT was more pronounced among older patients treated at non-academic facilities.53 Given the low rate of NAT implementation, it may be more reflective of real-world practice to encompass both patients who receive NAT and those who do not. As such, we included the entire pancreatic cancer population. We contend that our study could contribute to expanding the existing evidence, thus strengthening the case for MS insertion efficacy for pancreatic cancer patients.
This meta-analysis had some limitations. First, most of the studies included in this analysis had relatively small samples. In addition, most of the included published papers were from Japan. This may have led to a decreased heterogeneity of the patient’ population, affecting the generalizability of this analysis.
Second, no comparison between full-covered and uncovered types of MS was performed in this analysis due to the scarcity of data. Therefore, further studies on this topic are needed.
Third, this study did not include a cost-effectiveness analysis between MS and PS. However, recent studies have demonstrated that patients treated with MS had a lower rate of cholangitis or occlusion that required hospitalization than patients treated with PS, and the total cost was equivalent with regard to stent type.14,21,45
Fourth, the patient population varied across studies, resulting in the heterogeneity of this meta-analysis. Moreover, this meta-analysis included nine retrospective studies, which might have been linked to selection bias, heterogeneity of study designs, disparities in data reporting, and unadjusted confounding factors.
Finally, data on stent latency were extracted from the graphs of the original studies. This method may have caused measurement bias.
In conclusion, use of MS was superior to use of PS postoperatively for resectable and borderline resectable PC regarding the incidence of re-intervention, stent-related adverse events, and short-term outcomes after surgery. The increased likelihood of avoiding re-intervention and obstructive cholangitis would facilitate neoadjuvant chemotherapy and prevent inflammation around the bile duct. As the optimal EBD for resectable or borderline resectable PC, MS is preferred over PS.
Data Availability
PROSPERO 423627.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Patel SH, Katz MHG, Ahmad SA. The Landmark Series: preoperative therapy for pancreatic cancer. Ann Surg Oncol. 2021;28:4104–29.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27.
van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140–9.
Weber A, Mittermeyer T, Wagenpfeil S, Schmid RM, Prinz C. Self-expanding metal stents versus polyethylene stents for palliative treatment in patients with advanced pancreatic cancer. Pancreas. 2009;38:e7–12.
Hasegawa S, Endo I, Kubota K. Plastic or self-expandable metal stent: which is the most suitable for patients with pancreatic head cancer in the upcoming era of neoadjuvant chemotherapy? a review. Dig Endosc. 2021;34(2):297–306.
Cote GA, Kumar N, Ansstas M, et al. Risk of post-ERCP pancreatitis with placement of self-expandable metallic stents. Gastrointest Endosc. 2010;72:748–54.
Cao J, Peng C, Ding X, et al. Risk factors for post-ERCP cholecystitis: a single-center retrospective study. BMC Gastroenterol. 2018;18:128.
Isayama H, Yasuda I, Ryozawa S, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310–5.
Vakil N. Expandable metal stents: principles and tissue responses. Gastrointest Endosc Clin North Am. 2011;21(351–7):vii.
Vakil N, Gross U, Bethge N. Human tissue responses to metal stents. Gastrointest Endosc Clin North Am. 1999;9:359–65.
Mirkin KA, Hollenbeak CS, Wong J. Time to surgery: a misguided quality metric in early-stage pancreatic cancer. J Gastrointest Surg. 2018;22:1365–75.
Decker C, Christein JD, Phadnis MA, Wilcox CM, Varadarajulu S. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endos. 2011;25:2364–7.
Kubota K, Sato T, Watanabe S, et al. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014;26:77–86.
Tol JA, van Hooft JE, Timmer R, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2016;65:1981–7.
Tsuboi T, Sasaki T, Serikawa M, et al. Preoperative biliary drainage in cases of borderline resectable pancreatic cancer treated with neoadjuvant chemotherapy and surgery. Gastroenterol Res Pract. 2016;2016:7968201.
Nakamura K, Sho M, Akahori T, et al. A comparison between plastic and metallic biliary stent placement in patients receiving preoperative neoadjuvant chemoradiotherapy for resectable pancreatic cancer. World J Surg. 2019;43:642–8.
Kuwatani M, Nakamura T, Hayashi T, et al. Clinical outcomes of biliary drainage during a neoadjuvant therapy for pancreatic cancer: metal versus plastic stents. Gut Liver. 2020;14:269–73.
Hasegawa S, Kubota K, Yagi S, et al. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci. 2021;28(7):617–24.
Kobayashi K, Kobara H, Kamada H, et al. Comparison of plastic stent versus metal stent in preoperative biliary drainage for pancreatic head cancer with neoadjuvant chemoradiotherapy. J Hepatobiliary Pancreat Sci. 2021;28(10):856–63.
Tamura T, Itonaga M, Ashida R, et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: a prospective randomized study. Dig Endosc. 2021;33(7):1170–8.
Mandai K, Tsuchiya T, Kawakami H, et al. Fully covered metal stents vs plastic stents for preoperative biliary drainage in patients with resectable pancreatic cancer without neoadjuvant chemotherapy: a multicenter, prospective, randomized controlled trial. J Hepatobiliary Pancreat Sci. 2021;29(11):1185–94.
Ichikawa H, Iwashita T, Iwasa Y, et al. Covered self-expandable metallic stent versus plastic stent for preoperative endoscopic biliary drainage in patients with pancreatic cancer: a multi-center retrospective cohort study. Scand J Gastroenterol. 2021;57(4):493–500.
Kataoka F, Inoue D, Watanabe M, et al. Efficacy of 6-mm diameter fully covered self-expandable metallic stents in preoperative biliary drainage for pancreatic ductal adenocarcinoma. DEN Open. 2022;2:e55.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. 2009;6:e1000100.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Systematic Rev. 2019;10:ED000142.
Irving JD, Adam A, Dick R, Dondelinger RF, Lunderquist A, Roche A. Gianturco expandable metallic biliary stents: results of a European clinical trial. Radiology. 1989;172:321–6.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020:962280219889080.
Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–52.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57.
Tachezy M, Gebauer F, Petersen C, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA: a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer. 2014;14:411.
Motoi F, Satoi S, Honda G, et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol. 2019;54(2):194–203.
Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020;272:481–6.
Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin. 2020;38:1763–73.
Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21:300.
Du J, Gao X, Zhang H, Wan Z, Yu H, Wang D. Stent selection in preoperative biliary drainage for patients with operable pancreatic cancer receiving neoadjuvant therapy: a meta-analysis and systematic review. Front Surg. 2022;9:875504.
Kumar N, Jena A, Sharma V, Shukla S, Shah J. Outcome of metal vs plastic stents for biliary obstruction in patients with pancreatic carcinoma undergoing neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2023;30(4):419–28.
Swords DS, Zhang C, Presson AP, Firpo MA, Mulvihill SJ, Scaife CL. Association of time-to-surgery with outcomes in clinical stage I-II pancreatic adenocarcinoma treated with upfront surgery. Surgery. 2018;163:753–60.
Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7.
Katsinelos P, Paikos D, Kountouras J, et al. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587–93.
Crippa S, Cirocchi R, Partelli S, et al. Systematic review and meta-analysis of metal versus plastic stents for preoperative biliary drainage in resectable periampullary or pancreatic head tumors. Eur J Surg Oncol. 2016;42:1278–85.
Ge PS, Hamerski CM, Watson RR, et al. Plastic biliary stent patency in patients with locally advanced pancreatic adenocarcinoma receiving downstaging chemotherapy. Gastrointest Endosc. 2015;81:360–6.
Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013;108:1713–22.
Gardner TB, Spangler CC, Byanova KL, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016;84:460–6.
Euscher ED, Marsh WL Jr, Lucas JG, Frankel WL. Histologic and immunohistochemical changes in the stented common bile duct. Appl Immunohistochem Mol Morphol. 2007;15:299–304.
Cavell LK, Allen PJ, Vinoya C, et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am J Gastroenterol. 2013;108:1168–73.
Darnell EP, Wang TJ, Lumish MA, et al. Preoperative cholangitis is an independent risk factor for mortality in patients after pancreatoduodenectomy for pancreatic cancer. Am J Surg. 2021;221:134–40.
Kosaka H, Satoi S, Kono Y, et al. Estimation of the degree of surgical difficulty anticipated for pancreatoduodenectomy: preoperative and intraoperative factors. J Hepatobiliary Pancreat Sci. 2022;29(11):1166–74.
Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–7.
National Comprehensive Cancer Network. Pancreatic Cancer, 2023. Retrieved7 September 2023 at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
National Comprehensive Cancer Network. Biliary Tract Cancer. 2023. Retrieved 7 September 2023 at https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.
Mellado S, Vega EA, Abudalou M, et al. Trends in preoperative chemotherapy utilization for proximal pancreatic cancer: are we making progress? J Gastrointest Surg. 2022;26:1–7.
Acknowledgment
We thank Editage (http://www.editage.com) for English-language editing and reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
FIG. S1
Forest plot of studies examining ERCP-related adverse events. ERCP, endoscopic retrograde cholangiopancreatography. FIG. S2 Forest plot of studies examining postoperative findings. A Blood loss. B Operation time. C Surgical complication. D Mortality. FIG. S3 Funnel plot for publication bias test of perioperative findings. A ERCP-related adverse effects. B Blood loss. C Operation time. D Surgical complications (P = 0.34, Harbord test). E Mortality. ERCP, endoscopic retrograde cholangiopancreatography (DOCX 422 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Endo, Y., Tanaka, M., Kitago, M. et al. Comparison Between Plastic and Metallic Biliary Stent Placement for Preoperative Patients with Pancreatic Head Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol 31, 1319–1327 (2024). https://doi.org/10.1245/s10434-023-14523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14523-y