Abstract
Background
For breast-conserving surgery (BCS), several alternatives to wire localization (WL) have been developed. The newest, electromagnetic seed localization (ESL), provides three-dimensional navigation using the electrosurgical tool. This study assessed operative times, specimen volumes, margin positivity, and re-excision rates for ESL and WL.
Methods
Patients who had ESL-guided breast-conserving surgery between August 2020 and August 2021 were reviewed and matched one-to-one with patients who had WL based on surgeon, procedure type, and pathology. Variables were compared between ESL and WL using Wilcoxon rank-sum and Fisher’s exact tests.
Results
The study matched 97 patients who underwent excisional biopsy (n = 20) or partial mastectomy with (n = 53) or without (n = 24) sentinel lymph node biopsy (SLNB) using ESL. The median operative time for ESL versus WL for lumpectomy was 66 versus 69 min with SLNB (p = 0.76) and 40 versus 34.5 min without SLNB (p = 0.17). The median specimen volume was 36 cm3 using ESL versus 55 cm3 using WL (p = 0.001). For the patients with measurable tumor volume, excess tissue was greater using WL versus ESL (median, 73.2 vs. 52.5 cm3; p = 0.017). The margins were positive for 10 (10 %) of the 97 ESL patients and 18 (19 %) of the 97 WL patients (p = 0.17). In the ESL group, 6 (6 %) of the 97 patients had a subsequent re-excision compared with 13 (13 %) of the 97 WL patients (p = 0.15).
Conclusions
Despite similar operative times, ESL is superior to WL, as evidenced by decreased specimen volume and excess tissue excised. Although the difference was not statistically significant, ESL resulted in fewer positive margins and re-excisions than WL. Further studies are needed to confirm that ESL is the most advantageous of the two methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Breast cancer is the most common cancer diagnosed in women in the United States and the most common cancer diagnosed globally as of 2021.1 With the implementation of screening protocols for both average and high-risk women, many cancers are being found at an earlier stage, making them amenable to breast-conserving surgery (BCS). This has become increasingly popular since BCS was proven to have a survival equivalent to mastectomy when combined with radiation,2,3 which together constitute breast-conserving therapy (BCT). Many of these newly diagnosed breast cancers are not palpable and therefore require preoperative localization before BCS.
Wire localization (WL), the first preoperative radiographic localization technique developed nearly 50 years ago, remains the standard technique used currently.4 Since that time, WL has seen many changes, currently with a variety of wires available and placement possible under mammographic, tomographic, sonographic, or magnetic resonance guidance.
Wire localization has several advantages. It is cost-effective compared with non-wire options,5 is not radioactive, and can use multiple wires for multifocal or extensive disease by bracketing the area in question. However, multiple disadvantages have been demonstrated with this technology as well. The wire may migrate or become fractured before and during surgery,5,6 may cause significant discomfort or vasovagal symptoms in some patients,7 and must be placed on the same day as surgery because one end sticks out of the patient, which can lead to delays in operation start time.
During the past two decades, several non-wire localization alternatives have been developed including radioactive seed localization (RSL), magnetic seed localization (MSL), radiofrequency identification (RFID)-guided localization, and reflector-guided localization (RGL).
A recently developed technique, wireless electromagnetic seed localization (ESL) (EnVisio Navigation System, Elucent Technologies, Eden Prarie, MN, USA) uses a percutaneously placed detection marker (SmartClip [SC]) to provide real-time, three-dimensional navigation during surgery (Elucent Technologies, Eden Prarie, MN, USA). The system comprises a console, display, patient pad, and foot pedal as well as a sterile navigator and calibration disk. Intraoperatively, the patient pad connected to the device is placed on the thoracic region of the operating room table, and the navigation probe, which is smaller than the electrosurgical instrument, is attached to and calibrated on the electrosurgical tool using a calibration disk. This allows distance to be measured and displayed in three dimensions between the tip of the electrocautery device (not the affixed probe) and the marker for ease of dissection. The probe communicates with the activated SC, which emits a high-frequency signal transmitted between the surgical bed pad and navigation tool. Similar to Global Positioning System (GPS) navigation, this provides a continuous relative location between the clip and electrocautery pen so the surgeon needs to focus only on the location of the electrocautery for dissection to the SC without having to move the cautery out of the way of a separate detection probe because the two are attached. This differs from other wireless localization technologies that often require an optical direct sight line and show only distance to the tip of the detector, necessitating movement of the detector to determine directionality.8 These distances then are pictured on a tablet display screen with constant measurements of depth, distance, and superior/inferior, lateral/medial, and superficial/deep distances provided within millimeters of accuracy (Figs. 1 and 2).
The SCs can either be placed into a breast primary or used to localize a lymph node for targeted axillary dissection after neoadjuvant chemotherapy. Up to three differentiated SCs can be used for multifocal disease or for bracketing.8 To date, this technologyis the only one to provide both distance and three-dimensional directionality because the other technologies provide distance to the marker alone.
Although several studies have compared non-WL techniques with WL, to our knowledge, no data exist in the United States evaluating the newer ESL technology. This study was performed to assess the impact of this new localization method on operative times, specimen volumes, margin positivity, and margin re-excision rates compared with WL.
Methods
This study was evaluated and approved by the Fox Chase Cancer Center Institutional Review Board. Patients undergoing ESL-guided BCS between August 2020 and August 2021 by five breast surgeons at a single institution were reviewed. The inclusion criteria encompassed males or females older than 18 years who underwent lumpectomy for invasive or in situ breast cancer, excisional biopsy for benign pathology, or lumpectomy with sentinel lymph node biopsy/axillary dissection. All operative procedures were performed between the specified dates using preoperative localization with ESL and at least one SC or WL. Patients were excluded if they did not undergo BCS, if they underwent BCS in combination with reconstruction or mastectomy, and if their medical records did not contain critical variables or could not be matched based on a minimum of surgeon and procedure.
Qualifying patients then were matched 1:1 to patients who underwent BCS with WL between 2006 and 2021. Wire localization rarely was performed after the implementation of ESL (August 2020) and was recommended only when ESL was considered not feasible. This was based either on the body habitus of the patient because there is a theoretical 35-cm anteroposterior maximum thoracic distance in which the technology is guaranteed to work or on a need to localize more than three lesions because up to three individual SCs can be placed in the ipsilateral breast/axilla. The matching was based on surgeon, procedure type with stratification for patients who had no nodal procedures, and pathologic stage or benign pathology. When more than one match was identified, selection was randomized. If pathologic overall stage was not available, matches were based on pathologic T stage. For benign pathology, matches were determined by International Classification of Diseases (ICD)-10 benign diagnosis codes grouped to include atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, radial scar, intraductal papilloma, fibroadenoma, and benign phyllodes tumor.
Data were collected and stored in a password-protected RedCap database. Operative times were determined by a medical records review to determine the time from incision to closure via the anesthesia record. The main segment volumes (cm3), defined as the total primary lumpectomy resection specimen encompassing the tumor, were calculated using specimen dimensions provided in the pathology report. When available, discrete tumor volume (cm3) was also determined using pathology documentation.
Data were collected to account for additional cavity shave margins, taken at the time of surgery based on the surgeon’s practices and discretion. For some surgeons, the preference was routine shave margins, whereas for others, shave margins were directed by findings based on intraoperative imaging. Total specimen size was calculated as lumpectomy volume plus shave margin volumes. Excess tissue excised was calculated as total specimen size minus tumor volume. Positive margin specifics and re-excisions required at a separate operation were recorded from the medical record.
Main-segment margins were used to determine margin positivity because it was thought that this best represented the accuracy of the localization technology, and additional shave margins were based on surgeon discretion. Re-excisions were performed in accordance with indications as outlined in the Society of Surgical Oncology/American Society of Clinical Oncology/American Society for Therapeutic Radiation Oncology (SSO/ASCO/ASTRO) consensus statements, in which pure ductal carcinoma in situ (DCIS) lesions necessitated re-excision for margins closer than 2 mm, and those with an invasive component larger than 1 mm necessitated re-excision for positive margins unless the margin was on skin or the chest wall.9,10
Intraoperative x-ray was standardly used by all surgeons throughout the cohort to confirm the presence of the SC or wire(s) and biopsy clip(s) as of 2007 and thereafter. Before 2007, specimens were sent to radiology for imaging and then were available for review through the Picture Archiving and Communication System (PACS) for intraoperative decision-making.
Continuous variables (operative times, specimen size, excess volume excised) and categorical variables (positive margin rates, re-excision rates) were compared between the patients undergoing BCS with ESL and those who had BCS with WL using Wilcoxon rank-sum tests and Fisher’s exact tests, respectively.
Results
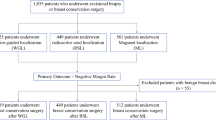
Between August 2020 and August 2021, 179 patients were identified, 97 of whom met the inclusion criteria and were matched with a WL patient who had surgery between 2006 and 2021 (Fig. 3). The surgeries performed included 53 ESL–WL matched pairs who underwent partial mastectomy with sentinel lymph node biopsy (SLNB), 24 pairs who underwent partial mastectomy without SLNB, and 20 pairs who had an excisionnal biopsy using ESL. Five of the ESL patients had two or more SCs placed, whereas eight WL patients had more than one needle placed for multiple lesions or for bracketing.
The matched-set sample included 190 females and 4 males. The median age was 64 (interquartile range [IQR], 57–71 years) in the ESL group and 61 years (IQR, 53–69 years) in the WL group (p = 0.15). The median body mass index (BMI) was 27.6 kg/m2 (IQR, 24.2–34.7 kg/m2) in the ESL group compared with 30.5 kg/m2 (IQR, 25.2–34.3 kg/m2) in the WL group (p = 0.34).
Race was not collected because use of ESL was implemented for patients across all races and ethnicities as of August of 2020 and therefore not thought to be germane to the procedures or outcomes being evaluated (partial mastectomy operative case times, specimens resected, margins, and re-excisions). Benign pathology was seen in 25 pairs, whereas 72 pairs underwent surgery for malignant pathology. Most malignant lesions were pathologic stage 0 (ESL [n = 18] vs. WL [n = 16]) or stage 1A (ESL [n = 44] vs. WL [n = 53]) (Table 1). In the WL cohort, 13 localizations were performed after implementation of ESL (12 with 1 wire, 1 with >1 wire; median age, 67 years; median BMI, 30.73 kg/m2; final pathology, benign in 4 patients and malignant in 9 patients [stage 0 [n = 3]; stage 1A [n = 6]).
The median operative time for ESL versus WL for lumpectomy was 66 versus 69 min with SLNB (p = 0.76) and 40 versus 34.5 min without SLNB (p = 0.17). The median specimen volume was 36 cm3 with ESL versus 55 cm3 with WL (p = 0.001). For those with measurable tumor/benign lesion volume (ESL [n = 79] vs. WL [n = 83]), the median volume with ESL versus WL was 0.39 cm3 versus 0.77 cm3 (p = 0.07). When the ESL patients who had a single SC (n = 92) were compared with the WL patients who had a single wire (n = 89), the median specimen volume was 34.3 cm3 (mean, 51.2 cm3) for ESL versus 52.5 cm3 (mean, 73.8 cm3) for WL (p = 0.003). The excess tissue excised was greater with WL than with ESL (median, 73.2 vs. 52.5 cm3; p = 0.017; Table 2).
Additional shave margins were taken in 63 ESL patients and 55 WL patients. Only one of the five surgeons included in the study took routine shave margins at the time of surgery as part of their practice, accounting for 32 ESL patients and 32 WL patients. The remaining shave margins were presumably based on intraoperative findings, imaging, or both. The main-segment margins were positive in 10 (10 %) of the 97 ESL patients compared with 18 (19 %) of the 97 WL patients (p = 0.17). In the ESL group, 6 (6 %) of the 97 patients had margin re-excision at a separate procedure compared with 13 (13 %) of the 97 patients in the WL group (p = 0.15).
Of those with positive main-segment margins in the ESL group (n = 10), six had a single positive margin (anterior [n = 2], inferior [n = 1], lateral [n = 1], medial [n = 2]) and four had multiple positive margins (inferior/posterior [n = 1], inferior/superior [n = 2], superior/medial [n = 1]). The final pathology for these patients included invasive ductal carcinoma (n = 5), invasive lobular carcinoma (n = 2), and ductal carcinoma in situ (n = 3).
Of the WL group with positive main-segment margins (n = 18), 11 had a single positive margin (anterior [n = 3], lateral [n = 2], medial [n = 2], posterior [n = 2], superior [n = 2]), whereas 7 had multiple positive margins (anterior/lateral [n = 1], anterior/posterior [n = 1], inferior/lateral/posterior [n = 1], medial/posterior [n = 1], superior/medial [n = 2], superior/posterior [n = 1]). The final pathologies included invasive ductal carcinoma (n = 7) and ductal carcinoma in situ (n = 11) (Table 3).
In the ESL group, 6 of the 10 patients with positive main-segment margins proceeded with margin re-excision. Four of these patients did not undergo re-excision due to no further tissue at that margin (n = 1), a negative peripheral shave margin (n = 1), or patient choice (n = 2). In the WL group of positive main-segment patients, 12 underwent margin re-excision. Of the six patients who did not, four had no further tissue at the positive margin, one had a negative peripheral margin, and one was unknown. One patient in the WL group underwent margin re-excision after negative main-segment margins due to positive peripheral margins.
Discussion
Satisfactory preoperative localization is paramount to performance of safe and effective BCS for non-palpable breast lesions. The ideal preoperative localization method optimizes patient comfort, surgical efficiency, and localization accuracy; provides the potential to achieve adequate margins while removing the least tissue possible; and is cost and time efficient. This study showed ESL to be superior to WL, as evidenced by a smaller overall specimen and excision of less excess tissue volume while having a comparable operative time.
Wire localization changed the landscape of breast-conserving surgery for non-palpable lesions. Although proven to be cost effective and safe, this technique has been associated with patient discomfort, scheduling conflicts, operative delays, and wire migration or fracturing.5,6 Several novel non-wire localization technologies have been developed to mitigate these disadvantages including radioactive seed localization, magnetic seed localization, and reflector-guided localization, with radioactive seed localization waning because of regulatory requirements.
The general advantages of non-wire techniques include removal of less tissue, improved workflow, and better psychological effects of a visible wire.11 Some disadvantages of these techniques involve migration of the seed and limitations of placement (e.g., magnetic seeds are not MRI compatible; some seeds are limited to shallow depths of placement; and some seeds cannot be repositioned once placed). In addition, several studies have evaluated intraoperative ultrasound-guided excision as an alternative to wire localization, but this approach is limited to lesions visible by ultrasound and is user dependent.
As with other non-wire techniques, workflow for SC placement differs from that of WL. The SC may be placed on any date before the operative procedure via 15G needle deployment under ultrasound or mammographic guidance and is Food and Drug Administration (FDA)- approved to stay in the breast. At our institution, the SC typically is placed the same day as preadmission testing to eliminate the need for multiple visits. Because the SC is placed a day before the operative date, ESL cases can be performed as the first case. This is in contrast to performance of WL the morning of surgery, which prohibits performance of WL cases at the start of the day. Although placement of the SC on a prior day requires one additional visit, it removes the stress and potential delays of same-day placement as well as logistical issues related to external wire discomfort for the patient.
One of the most dramatic findings from this study was the difference in specimen volumes and excess tissue volumes between ESL and WL. The median specimen volume was 55 cm3 with WL versus 36 cm3 with ESL (p = 0.001), and the excess tissue excised was greater with WL than with ESL (median, 73.2 vs. 52.5 cm3; p = 0.017). Studies comparing RSL and RFL with WL have shown differing results, with Chagpar et al.12 finding no significant difference in specimen volume between the three methods and Srour et al.13 finding that WL did result in a greater volume of tissue excised when multiple markers or wires were used.
As technologies improve, more focus is being placed on aesthetic outcomes and oncoplastic techniques, making the amount of excess tissue excised increasingly important to consider. By removing less tissue while maintaining safe oncologic results, patients will have fewer tissue deficits, require fewer oncoplastic rearrangements, and have smaller noticeable discrepancies between breasts. Like other non-wire options, the SC-based, three-dimensional navigation also allows for variability with more aesthetically placed incisions, whereas location of the wire in WL often dictates incision choice. Additionally, the ESL display indicates distances in three dimensions (anterior-posterior, medial-lateral, and superior-inferior) between the SC and the detector, providing localization information that is not available via WL or other current technologies.
Although not statistically significant, ESL resulted in a lower rate of positive margins and fewer margin re-excisions than WL. This is consistent with other non-wire localization technologies, including RFID and RSL, which have been shown to have better or similar rates of positive margins and re-excisions, although the difference is not statistically significant.12,13,14,15 However, in a recent randomized control trial, Taylor et al.16 reported statistically lower re-excision rates with RSL versus WL, although the difference in positive margin rates was not statistically significant. Our data showed a difference between the number of positive main-segment margins and patients having re-excisions in both ESL and WL patients. This is explained by the presence of negative shave margins in the same patient, no further tissue to be excised at said margin due to dissection down to the chest wall or skin, or patient preference not to proceed with further surgery.
The study also showed no difference in operative times between patients undergoing ESL versus WL for lumpectomy/excisional biopsy, irrespective of whether or not they had a sentinel node biopsy. This was somewhat unexpected because one advantage of the ESL technology is that it allows for a single tool and three-dimensional localization not afforded with wire localization. Although unexpected, this is consistent with other non-wire localization tools, which have been shown to have operative times similar to those for WL.13,14 This could be explained by the learning curve required for a new technology at a single institution that has classically used WL as well as the presence of training fellows and residents participating in surgical cases.
Although ESL provides distinct benefits over wire localization, it has some disadvantages, similar to those of other non-wire technologies. There is potential for seed migration, although this is exceedingly rare and was not encountered during the study period. Although the bloom on MRI from the SC is not insignificant, it remains smaller than that caused by ferromagnetic seeds, making placement after post-neoadjuvant MRI imaging advisable. Additionally, although cost was not specifically analyzed, we believe ESL is more costly than wire localization and requires an investment in specialized equipment.
This study had several limitations. First, the study was retrospective and performed at a single institution. Additionally, the sample size was limited to meet strict matching and inclusion criteria. Moreover, even though this was comparable to the standards set by prior studies evaluating other non-wire localization techniques, the cohort matching process here was added to further limit confounders. Attempts were made to avoid the impact of confounding variables by matching patients one-to-one based on surgeon, procedure, and pathology.
Another potential limitation was the short period during the cohort (<1 year in 2006), occurring before the implementation of intraoperative imaging to allow immediate specimen review by the surgeon for margin assessment. During this time, specimens were sent to radiology for imaging, which was immediately made available for review through PACS. This provided access for review by the surgeon intraoperatively, but this slight difference in practice during a period shorter than 1 year may have slightly, and in a non-correctible manner, confounded the variable regarding margin positivity and re-excision for those few WL patients during that period (1 patient). Additionally, cost analysis was not performed and would have been a valuable addition to the data presented.
Conclusion
As shown in this study, ESL is superior to WL because it enables excision in a similar time but minimizes excess tissue resected without compromising margin status, which has the potential to improve cosmesis. Although the difference was not statistically significant, ESL resulted in lower rates of positive margins and margin re-excisions than WL. The ESL technology allows for single-tool, three-dimensional localization, with the patient convenience of preoperative placement. Further assessment of ESL versus other wire and non-wire localization technologies should be performed to refine the determination of which localization technology is most advantageous in breast-conserving surgery.
References
U.S. Breast Cancer Statistics | Breastcancer.Org. BreastCancer.org. 2021. https://www.breastcancer.org/symptoms/understand_bc/statistics.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA Surg. 2015;150:778–86.
Tayeh S, Wazir U, Mokbel K. The evolving role of radiofrequency-guided localization in breast surgery: a systematic review. Cancers. 2021;13:4996.
Law W, Hong N, Ravi A, et al. Budget impact analysis of preoperative radioactive seed localization. Ann Surg Oncol. 2021;28:1370–8.
Mayo R, Kalambo M, Parikh J. Preoperative localization of breast lesions: current techniques. Clin Imaging. 2019;56:1–8.
Helvie MA, Ikeda DM, Adler DD. Localization and needle aspiration of breast lesions: complications in 370 cases. Am J Roentgenol. 1991;157:711–4.
Elucent Medical (2022). "Technology: Physicians. Learn how physicians are using EnVisio™ Surgical Navigation to improve outcomes for a variety of patient cases." Accessed July 25, 2022, from https://elucentmedical.com/physicians/.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of surgical oncology-American society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88:553–64. https://doi.org/10.1016/j.ijrobp.2013.11.012.
Morrow M, Van Zee KJ, Solin LJ, et al. Society of surgical oncology-American society for radiation oncology-American society of clinical oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23:3801–10.
Normal C, Lafaurie G, Uhercik M, et al. Novel wire-free techniques for localization of impalpable breast lesions-a review of current options. Breast J. 2021;27:141–8.
Chagpar A, Garcia-Cantu C, Howard-McNatt M, et al. Does localization technique matter for non-palpable breast cancers? Am Surg. 2021;88:1–6.
Srour M, Kim S, Amersi F, Giuliano A, Chung A. Comparison of multiple wire, radioactive seed, and Savi Scout radar localization for management of surgical breast disease. Ann Surg Oncol. 2021;28:2212–8.
Lee M, Sanaiha Y, Kusske A, et al. A comparison of two non-radioactive alternatives to wire for the localization of non-palpable breast cancers. Breast Cancer Res Treat. 2020;182:299–303.
Garzotto R, Comoretto R, Michieletto S, et al. Preoperative non-palpable breast lesion localization, innovative techniques, and clinic outcomes in surgical practice: a systematic review and meta-analysis. Breast. 2021;58:93–105.
Taylor DB, Bourke AG, Westcott EJ, et al. Surgical outcomes after radioactive 1251 seed versus hookwire localization of non-palpable breast cancer: a multicentre randomized clinical trial. Br J Surg. 2021;108:40–8.
Acknowledgment
This work was supported by the National Cancer Institute of the National Institutes of Health under award no. P30CA006927 for analysis of the data via support of our biostatistics facility, and by generous private donor support from the Marlyn Fein Chapter of the Fox Chase Cancer Center Board of Associates for analysis and interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There were no conflicts of interest on performing and writing the study. Dr. Bleicher became a consultant for Elucent after the study was performed and written.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in part at the 23rd Annual Meeting of the American Society of Breast Surgeons, 6–10 April 2022, Las Vegas Nevada.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jordan, R.M., Rivera-Sanchez, L., Kelley, K. et al. The Impact of an Electromagnetic Seed Localization Device Versus Wire Localization on Breast-Conserving Surgery: A Matched-Pair Analysis. Ann Surg Oncol 30, 4111–4119 (2023). https://doi.org/10.1245/s10434-023-13366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13366-x