Abstract
Background
As an alternative to traditional wire localization, an inducible magnetic seed system can be used to identify and remove nonpalpable breast lesions and axillary lymph nodes intraoperatively. We report the largest single-institution experience of magnetic seed placement for operative localization to date, including feasibility and short-term outcomes.
Methods
Patients who underwent placement of a magnetic seed in the breast or lymph node were identified from July 2017 to March 2019. Imaging findings, core needle biopsy, surgical pathology results, and type of surgery were collected. Outcomes included procedural complications, magnetic seed and biopsy clip retrieval rates, and need for additional surgery.
Results
A total of 842 magnetic seeds were placed by nine radiologists in 673 patients and retrieved by six surgeons at six operative locations. The majority of breast lesions were malignant (395/659, 59.9%); 136 seeds were placed for lymph node localization. The overall magnetic seed retrieval rate was 98.6%, whereas the biopsy clip retrieval rate was 90.9%. Only six patients (0.7%) experienced a complication from magnetic seed placement. Reexcision was performed in 15.2% of patients with breast cancer; 9.6% of benign/high risk lesions were upgraded to malignancy at surgical excision.
Conclusions
The magnetic seed technique is safe, effective, and accurate for localization of breast lesions and lymph nodes, and importantly uncouples surgery from the localization procedure. The high magnetic seed retrieval rate and low reexcision rate may reflect the accuracy of magnetic marker placement as a “second chance” localization procedure, especially in cases with biopsy clip migration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Wire localization is the most commonly used method for operative localization of nonpalpable breast lesions and has been considered the standard care for decades. However, this technique can limit the surgical approach, including incision placement. Also, the wire itself can migrate or become dislodged or transected.1,2 Wire placement must be performed the day of surgery and depends on coordination with radiology schedules, which limits operative efficiency and requires patients to be fasting for the localization procedure.3,4 The combination of fasting, anxiety, and multiple procedures on the day of surgery can result in a poor patient experience and complications, including vasovagal response during wire localization.
Alternative methods for localization have more recently been introduced and variably adopted. Radioactive seed localization (RSL) uses an iodine-125 containing seed and handheld gamma probe for detection. Available studies have yielded similar results to wire localization for volume of tissue excised, margin status, and rates of reoperation.6,7,8,9,10 However, mandatory isotope tracking and regulatory constraints have prevented widespread implementation of radioactive seed programs.5,11
Other wireless localization systems use radiofrequency or electromagnetic technology to detect reflectors or tags implanted within breast lesions. Results from a multi-institutional trial of the SAVI-SCOUT® (Cianna Medical, Inc.), which uses radar impulses, reported high success rates of reflector placement and retrieval, reexcision rates comparable to other localization methods, and positive feedback regarding workflow efficiency compared with wires.12 Notably, lesions deeper than 5 cm or obscured by hematoma may be more difficult to detect using this system, halogen lights in the operating room can interfere with the infrared technology, and electrocautery can disable the localization mechanism if dissection is too close to the marker.12,13
Magseed® (Endomagnetics, Inc.) is a 5- × 1-mm stainless steel marker placed under mammographic or ultrasound guidance from several months up to immediately before surgery.1,2,14 It is detected intraoperatively with a probe which generates a magnetic field to identify the magnetized seed. Using both auditory and visual feedback, the surgeon uses the probe to detect the magnetic seed location and thereby retrieve the lesion. This study reports the largest single-institution experience of magnetic seed placement for operative localization of nonpalpable breast lesions and axillary lymph nodes to date in the United States. Our goal was to demonstrate feasibility and short-term outcomes in our hospital system, which for this study included four dedicated breast centers and six operative sites.
Methods
Following Institutional Review Board approval, all patients who underwent placement of a magnetic seed in the breast or lymph node were identified from a prospectively maintained database of breast surgery patients treated at University Hospitals (UH) from July 2017 to March 2019. Before July 2017, wires were used as the standard localization method. The adoption of magnetic seeds was a practice shift for the breast team, with very few wire localizations performed after the initial implementation period. All magnetic seeds were placed by one of nine breast radiologists, and all patients underwent surgery with one of six breast surgeons. When possible, a titanium marker with gel covering was used as the biopsy clip so that the magnetic seed could be placed under ultrasound guidance. Once biopsy pathology returned and surgery was planned, the Magseed localization procedure often was scheduled the same day as surgical consultation to minimize multiple visits for the patient. A post-procedure mammogram was obtained to confirm accurate lesion localization with the magnetic seed; the seed was used as the target for intraoperative lesion excision, especially in cases with known biopsy clip migration. Intraoperative specimen x-ray confirmed retrieval of the lesion, magnetic seed, and biopsy clip. The combination of magnetic probe and pathology confirmation rather than x-ray was used in some instances to confirm removal of localized lymph nodes, especially when sent for frozen section. Core needle and surgical specimens were examined by UH pathologists, including review of outside specimens if indicated.
Clinical factors were collected, including patient age at diagnosis and procedure type. Breast surgery was classified as excisional biopsy, partial mastectomy, or mastectomy. Axillary surgery was categorized as sentinel lymph node biopsy (SLNB), axillary lymph node dissection (ALND), or both. Radiologic data included initial imaging modality (mammogram or ultrasound), findings warranting core needle biopsy (calcifications, asymmetry/distortion, mass, abnormal lymph node, other), and technique used for magnetic seed placement (stereotactic or ultrasound guidance). The number of biopsy clips and magnetic seeds placed and subsequently retrieved at surgery were recorded based on radiology report, operative report, pathology report, and intraoperative specimen imaging. Complications from magnetic marker placement were identified as hematoma, infection, vasovagal response, and other. Pathology information included diagnosis at core needle biopsy and after surgery. Breast results were classified as malignant, high risk, or benign. Lymph nodes were categorized as malignant or benign. For malignant breast lesions, the use of shave cavity margins (yes, no), margin status (negative, positive, DCIS > 2 mm, DCIS < 2 mm), and receipt of additional surgery (reexcision, mastectomy, or axillary surgery) were recorded.
Each breast and lymph node lesion in which a magnetic seed was placed was considered separately due to its unique clinicopathologic factors. Standard statistical analysis was performed using SAS Enterprise Guide, Version 7.1 (SAS Institute, Cary, NC).
Results
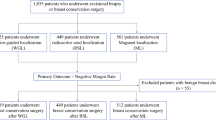
During a 20-month period, 842 magnetic markers were placed in 673 patients for intraoperative localization of breast lesions and axillary lymph nodes (Figs. 1 and 2). Median patient age at diagnosis was 58 (range 17–92) years. Patients with breast cancer were significantly older than those presenting with benign/high risk pathology (62 vs. 52 years, p < 0.001). A total of 131 patients underwent neoadjuvant chemotherapy, including 116 (88.5%) with positive lymph nodes at core needle biopsy.
For the 659 breast lesions, the most common radiologic find warranting core needle biopsy was a mass (N = 408, 61.9%), followed by calcifications (N = 164, 24.9%; Table 1). Among the breast lesions localized with magnetic seeds in this study, 39.6% (N = 261) were for benign/high-risk and 59.9% (N = 395) were for malignant preoperative core needle biopsy diagnosis (Fig. 1). For malignant breast lesions, the most common diagnosis was invasive ductal carcinoma (N = 235, 59.3%), followed by ductal carcinoma in situ (DCIS) (N = 72, 18.2%; Table 2). Of the 178 patients with high-risk results, 36.5% were atypical ductal hyperplasia and 15.7% were atypical lobular hyperplasia. For benign lesions, the most common diagnosis warranting excision was intraductal papilloma (N = 45, 54.2%). Three patients did not undergo core needle biopsy of the breast lesion before surgery. In two cases, the patient was planned to undergo excision of another breast lesion previously biopsied, and magnetic seeds were placed for intraoperative removal of suspicious calcifications or mass.
Partial mastectomy was performed for 375 (56.9%) of breast lesions, whereas excisional biopsy was completed in 267 (40.5%) cases. A small number of patients (N = 9) underwent mastectomy and were included due to placement of a magnetic marker for axillary lymph node localization.
Of the 261 breast lesions with benign/high risk pathology at core needle biopsy, the vast majority remained benign or high risk at surgical excision (Fig. 1). However, upgrade to malignancy was found in 25 cases (9.6%). The majority of these represented DCIS (12 cases, 48.0%) and invasive ductal carcinoma (6 cases, 24.0%).
Magnetic seed localization was used to identify 136 axillary lymph nodes targeted for surgical removal (Fig. 2). Two-thirds (65.4%) of these yielded malignant results at core needle biopsy. The single lymph node not biopsied before excision was enlarged by imaging characteristics in a patient with known malignant melanoma and excised at the time of surgery for the primary site. Eighty-nine (65.4%) patients underwent magnetic seed localized SLNB, 9 (6.6%) underwent planned ALND, and 38 (27.9%) were treated with SLNB and ALND. Of the initially malignant lymph nodes, 30% converted to negative pathology at surgical excision, representing a favorable response to neoadjuvant chemotherapy. Eight of the 46 lymph nodes benign at core needle biopsy were malignant at excision, yielding a false-negative rate of 17.4% for the core needle biopsy procedure.
For the 395 patients undergoing magnetic seed localized breast surgery for malignant lesions, additional and/or shave cavity margins were obtained in 272 (68.9%) of cases. Positive margins were found in 7.6% (30/395) of patients and DCIS < 2 mm from the margin in 9.6% (38/395). Reexcision was performed in 60 of 395 (15.2%) of patients with breast cancer. Only five patients (1.3%) underwent more than one reexcision, and seven (1.8%) converted to mastectomy. Seventeen patients returned to the operating room for additional axillary surgery; eight SLNB were completed for lesions upgraded to invasive malignancy, and nine ALND were performed following permanent section surgical pathology review and multidisciplinary breast team discussion. Of the 261 patients with benign/high risk pathology at core biopsy, 16 (6.5%) underwent additional surgery due to upgrade at surgical excision.
Of the 659 magnetic seeds used for intraoperative localization of breast lesions, the majority were placed via ultrasound guidance (N = 453, 68.7%), although one-third (N = 198, 30.0%) were placed stereotactically. Ultrasound was used to place nearly all magnetic markers for lymph node localization (N = 129, 94.8%). While most breast lesions were localized with one magnetic seed, a “bracketing” technique was used with two or more markers in 52 cases (8.0%; Fig. 3).
Clinical complications were experienced following magnetic seed placement in only six cases, yielding a complication rate of 0.7%. Of these, two were hematomas treated with compression, one was an infection treated with antibiotics, and one patient reported pain during deployment. In two cases, the magnetic marker was noted to have migrated on post-placement mammogram. A second magnetic seed or wire was inserted into the biopsied lesion during the same localization procedure and retrieved with the intended lesion at surgery.
The overall magnetic seed retrieval rate was 98.6%. The retrieval rate for breast lesions was slightly higher than for lymph nodes: 99.5% vs. 94.1% (Fig. 4). There were only three cases in which the magnetic marker was not contained within the breast specimen. In the first, the seed was visibly dislodged during dissection, and intraoperative ultrasound was used to localize the lesion. In the second (one of the complications noted above), the magnetic seed was not within the biopsied calcifications, so a wire was used for localization, and the seed was left in situ per patient preference. In the third case, the marker was likely inadvertently aspirated via suction during specimen removal.
The biopsy clip retrieval rate was somewhat lower for both breast and lymph node lesions, 93.1% and 80.1%, respectively (Fig. 4). This is largely attributed to documented clip migration or to gel clip dislodgement during dissection, and the use of the magnetic seed as a more accurate marker to ensure removal of the biopsied lesion intraoperatively (Fig. 5). Pathology review confirmed concordance of the core needle biopsy and surgical diagnosis. Biopsy-related changes were documented in the vast majority of these cases, indicating that the intended lesion was retrieved using the Magseed as the target rather than the biopsy clip. Seventeen of the 26 cases in which biopsy clips were not documented within localized lymph nodes were classified “unknown,” meaning that the clip was not mentioned in radiology, pathology, and surgery reports rather than actually not retrieved. In all of these cases, the intended lesion was retrieved using the seed for localization. No unplanned axillary dissections or returns to the operating room for reexcision were performed due to localization failure.
Discussion
We report the largest experience in the United States of 842 breast lesions and axillary lymph nodes localized and excised using a nonradioactive inducible magnetized seed system. Despite the expected learning curve for a novel technique by nine radiologists, six surgeons, and staff at six operative locations, 98.6% of magnetic seeds were retrieved within the specimen, demonstrating a high accuracy rate for localization.
Before implementing this practice change, our breast team carefully considered the costs and benefits of a magnetic seed program and several localization techniques were trialed. Magnetic seeds were preferred over radioactive seeds due to fewer regulatory issues, especially in a hospital system with multiple radiology and surgery sites. During the initial trial, radiologists noted the ease of magnetic seed placement compared with wires. Surgeons preferred this technique to facilitate cosmetic incision placement and direct dissection trajectory. The cost of the magnetic seeds and probe system was offset by the anticipated value of shorter procedure times for radiologists, increased OR efficiency for first-start cases, and the ability to schedule the localization procedure days to weeks in advance of surgery. Magnetic seeds also avoided technical problems encountered with other wireless techniques, including interference with OR lights and electrocautery, and limitations of lesion depth within the breast and axilla. Finally, the improvement in patient experience with a more streamlined and comfortable process, both before and the day of surgery that we noted with magnetic seeds instead of wires, was important to our breast team and helped ensure buy-in from institutional leadership.
Unlike wires, magnetic seeds can be placed days to months before surgery, allowing the localization procedure to be uncoupled from the morning of surgery. Our low magnetic marker placement complication rate (< 1%) may be attributable to the lack of patient restrictions for this separate procedure, as opposed to day-of-surgery wire placement, which occurs while patients are fasting, and may contribute to vasovagal reactions. Antecedent marker placement also facilitates first-start operative cases and allows independence of radiology and surgery schedules,1,2,4 improving the experience for both patients and providers. In addition, magnetic seed use allows breast surgery cases to be performed at ambulatory surgery centers without onsite radiologists, potentially decreasing personnel resource utilization and cost.
In our series, reexcision was performed in 15.2% of patients with breast cancer. This aligns with reexcision rates of 8.7–23% reported in studies using radioactive seeds and SAVI-SCOUT® and compares favorably with reexcision rates of 20–70% using traditional wire localization.4,6,12,15 Another potential benefit of seed localization is improved cosmetic outcomes, because this technique allows for incision placement remote from the lesion site and removal of smaller volumes of breast tissue.1,3
We also used magnetic seeds to localize and remove previously biopsied axillary lymph nodes, with a seed retrieval rate of 90.4%. The somewhat lower biopsy clip retrieval rate of 80.1% may reflect clip migration or dislodgement, in particular due to nodal response to neoadjuvant chemotherapy, which the majority of patients undergoing lymph node localization received. Although biopsy clips with gel casings were preferred to facilitate magnetic marker placement under ultrasound guidance due to their visibility, they can easily become dislodged during surgical dissection as the gel has less tissue traction than an uncovered clip. In fact, our early experience placing the magnetic seed within the gel surrounding the clip resulted in extrusion of both clip and seed on occasion and was quickly replaced by deploying the seed adjacent to the clip but firmly within the lesion. This recognized dislodgement issue decreased our biopsy clip retrieval rate, particularly because we included “unknown” clips (i.e., not documented) as “not retrieved” rather than excluding them from analysis. However, because the magnetic seed was intentionally used as the target to identify the lymph node or breast lesion rather than the biopsy clip, the lower clip retrieval rate did not result in a low lesion retrieval rate.
Interestingly, 17.4% of lymph nodes benign at core needle biopsy were malignant on surgical pathology. In addition to the known benefits of localizing initially positive lymph nodes to improve the accuracy of sentinel lymph node biopsy following neoadjuvant chemotherapy,16,17,18 the use of magnetic markers to remove initially negative lymph nodes in our study provided critical information that upstaged pathology and informed adjuvant treatment decisions. In light of this data, it is our current practice to localize and remove all previously biopsied lymph nodes, whether positive or negative at core needle biopsy.
Our study included a wide variety of benign, high risk, and malignant breast lesions with diverse presentations on imaging studies, demonstrating the versatility of the magnetized seed system. The participation of our entire breast radiology and breast surgery faculty at six geographically distinct sites, including breast centers, inpatient hospitals, and ambulatory surgery centers suggests generalizability of our results to other hospital systems. However, our study is a single-institution experience and subject to inherent limitations. Regarding reported complications, in the two cases in which magnetic seed migration occurred, a second magnetic marker or wire was placed during the same localization procedure, allowing surgery to proceed as planned. One drawback noted intraoperatively is occasional difficulty detecting the magnetic seed signal in very posterior lesions, particularly when breast tissue is extremely dense. This can usually be overcome by making the incision based on the known location of the lesion on preoperative mammogram, and subsequently the signal becomes audible with less tissue between the probe and the seed itself. Nonmetal instruments must be used to avoid interference with the magnetic probe. While this probe is different than the detection system for Technetium sulfur colloid radiotracer frequently used in SLNB, it does detect magnetic iron oxide nanoparticle tracer which can also be used for SLNB. However, the cost of a second probe system and special instruments should be considered when starting a magnetic seed program. Another limitation of magnetic marker localization is the significant artifact it produces on MRI; we recommend seed placement following MRI if used for initial breast cancer staging or surgical planning following neoadjuvant therapy.2
Conclusions
Magnetic seed localization is a safe, effective, and accurate method to identify and remove nonpalpable breast lesions and axillary lymph nodes. Advantages over wire localization include uncoupling surgery from the localization procedure, leading to fewer complications and improved workflow. The lack of regulatory issues with magnetic markers compared with radioactive seeds may be preferable in hospital systems with multiple radiology and surgery sites. While further studies are needed to document long-term outcomes, our results support the routine use of the magnetic seed localization system for benign and malignant breast disease. In our experience, the benefits of this wireless system—including operative efficiency, surgeon and radiologist approval, and patient satisfaction—outweigh the costs of implementation and add value for our breast team, our patients, and our hospital system.
References
Cheang E, Ha R, Thornton CM, Mango VL. Innovations in image-guided preoperative breast lesion localization. Br J Radiol. 2018;91:20170740.
Hayes MK. Update on preoperative breast localization. Radiol Clin North Am. 2017;55:591–603.
Jakub JW, Gray RJ, Degnim AC, Boughey JC, Gardner M, Cox CE. Current status of radioactive seed for localization of nonpalpable breast lesions. Am J Surg. 2010;199:522–8.
Zhang Y, Seely J, Cordiero E, et al. Radioactive seed localization versus wire-guided localization for nonpalpable breast cancer: a cost and operating room efficiency analysis. Ann Surg Oncol. 2017;24:3567–73.
Jakub J, Gray R. Starting radioactive seed localization program. Ann Surg Oncol. 2015;22:3197–202.
Murphy JO, Moo TA, King TA, et al. Radioactive seed localization compared to wire localization in breast-conserving surgery: initial 6-month experience. Ann Surg Oncol. 2013;20:4121–7.
Diego EJ, Soran A, McGuire KP, et al. Localizing high-risk lesions for excisional breast biopsy: a comparison between radioactive seed localization and wire localization. Ann Surg Oncol. 2014;21:3268–72.
Bloomquist EV, Ajkay N, Patil S, et al. A randomized prospective comparison of patient-assessed satisfaction and clinical outcomes with radioactive seed localization vs wire localization. Breast J. 2016; 22:151–7.
Gray RJ, Salud C, Nguyen K, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol. 2001;8:711–5.
Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multi-centered, randomized, controlled trial comparing radio-guided seed localization to standard wire localization for non-palpable, invasive, and in situ breast carcinomas. Ann Surg Oncol. 2011;18:3407–14.
Pouw B, De Wit-Van Der Veen L, Stokkel M, Loo C, Peeters M, Olmos R. Heading toward radioactive seed localization in nonpalpable breast cancer surgery? A meta-analysis. J Surg Oncol. 2015;111:185–91.
Cox CE, Russell S, Prowler V, et al. A prospective, single arm, multi-site, clinical evaluation of a nonradioactive surgical guidance technology for the location of nonpalpable breast lesions during excision. Ann Surg Oncol. 2016;23:3168–74.
Falcon S, Weinfurtner RJ, Mooney B, Niell BL. SAVI SCOUT® localization of breast lesions as a practical alternative to wires: outcomes and suggestions for trouble-shooting. Clin Imaging. 2018;52:280–6.
Price ER, Khoury AL, Esserman LJ, Joe BN, Alvarado MD. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. Am J Radiol. 2018;210:913–7.
Jacobs L. Positive margins: the challenge continues for breast surgeons. Ann Surg Oncol. 2008;15:1271–2.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy. Ann Surg. 2016;263:802–7.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261:378–82.
Caudle A, Yang W, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
This study was presented in poster format at the American Society of Breast Surgeons 20th Annual Meeting, April 30-May 5, 2019, Dallas, TX. The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miller, M.E., Patil, N., Li, P. et al. Hospital System Adoption of Magnetic Seeds for Wireless Breast and Lymph Node Localization. Ann Surg Oncol 28, 3223–3229 (2021). https://doi.org/10.1245/s10434-020-09311-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09311-x