Abstract
Background
Selected patients with colorectal cancer peritoneal metastases (CRPM) could be offered a curative-intent strategy based on complete cytoreductive surgery (CRS), potentially combined with hyperthermic intraperitoneal chemotherapy (HIPEC) and perioperative systemic chemotherapy. The impact of different neoadjuvant systemic chemotherapy (NACT) regimens remains unclear due to a lack of comparative data.
Methods
Consecutive CRPM patients from a monocentric database who were treated with complete CRS after single-line NACT were included in this study. Chemotherapy regimens were tailored as a doublet drug (FOLFOX/FOLFIRI) with/without targeted therapy (anti-epidermal growth factor receptor/bevacizumab) and triplet-drug combination (FOLFIRINOX). Morphological response (MR) was assessed using the Response Evaluation Criteria in Solid Tumors criteria, and pathological response (PR) was assessed using the Peritoneal Regression Grading Score (PRGS). Long-term oncologic outcomes were compared.
Results
The cohort comprised 388 patients, including 127, 202, and 59 patients in the doublet, doublet + targeted, and triplet groups, respectively. MR rates were higher in the triplet (68.0%) and doublet + targeted groups (64.2%) when compared with the doublet group (42.4%, p = 0.003). Complete and major PRs were observed in 13.6% and 32.0% of patients, respectively. Higher MR rates were observed after doublet + targeted or triplet regimens, while no difference was observed for PR rates. In multivariate analysis, FOLFIRINOX was independently associated with better overall survival (hazard ratio 0.49, 95% confidence interval 0.25–0.96; p = 0.037). FOLFIRINOX also resulted in a higher rate of severe postoperative complications.
Conclusions
In this retrospective study, a FOLFIRINOX regimen as NACT seemed to result in better long-term outcomes for CRPM patients after complete CRS/HIPEC, although with higher morbidity. Prospective studies are needed, including groups without NACT and those with FOLFIRINOX + bevacizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Half of colorectal cancer patients develop metastatic disease, with the main sites being the liver, lung, and peritoneum.1 Effective systemic chemotherapy, together with advances in surgical techniques and perioperative management, have allowed the development of curative-intent strategies.2,3 These comprehensive treatments led to significant improvement in prognosis, with response to chemotherapy and achievement of complete resection as determinant prognostic factors.4 In selected patients, a cure could even be expected.5,6 Neoadjuvant systemic chemotherapy (NACT) has three main objectives: assessing chemosensitivity, controlling metastatic spread, and reducing metastatic burden to increase the chance of a complete resection.4,7 These objectives allow for better patient selection. The drawbacks of NACT could be increased postoperative morbidity, development of chemoresistance, and the loss of a resectability window.
Several protocols exist, based on the combination of leucovorin and 5-fluorouracil with oxaliplatin and/or irinotecan, defining the doublet (FOLFOX, FOLFIRI) and triplet (FOLFIRINOX) drug regimens. Depending on RAS and BRAF mutational status, the addition of targeted therapies has been proposed to increase the response rates (anti-epidermal growth factor receptor antibody in RAS wild-type patients or an anti-angiogenic antibody, typically bevacizumab). Differences in long-term outcomes were reported in colorectal metastatic patients, with an advantage for the triplet combination with anti-angiogenic agents at the cost of increased morbidity.8,9,10,11 However, comparisons were not usually performed in patients selected for a surgical curative-intent strategy, and rarely focused on colorectal peritoneal metastases (CRPM).
When compared with other metastatic sites, CRPM are less sensitive to systemic chemotherapy, determining the worst prognosis.1 A complete cytoreductive surgery (CRS) combined with perioperative systemic chemotherapy improved the median survival to 41 months,12 which compared favorably with the 16–24 months obtained without resection.1,13 In expert centers, CRS is typically followed by hyperthermic intraperitoneal chemotherapy (HIPEC), with contrasted outcomes.12,13 Morphological response (MR) and pathological response (PR) to NACT were reported to be major prognostic factors,14 however new NACT regimens have not been compared in CRPM patients treated with curative intent. We present a retrospective comparative analysis of MR and PR with the main NACT protocols, along with the inherent postoperative results and long-term outcomes.
Methods
Patient Selection
Consecutive patients treated for CRPM at Lyon Sud University Hospital between January 2005 and June 2018 were screened for inclusion. Eligibility criteria were pathologically proven CRPM, treated with one line of NACT, and complete CRS defined by a completeness of cytoreduction (CC) score of 0 or 1, corresponding to residual disease of < 2.5 mm.15 Non-resectable patients, those with a CC score > 1, or those without preoperative chemotherapy were excluded. Appendiceal PM patients were also excluded. All data were extracted from a prospectively maintained database (BIG-RENAPE–NCT02823860). Data regarding race and ethnicity were not recorded. The study was performed in accordance with the precepts of the Helsinki declaration and was approved by the Lyon Human Investigation Committee. All patients signed informed consent at the time of their initial management to authorize the use of their data.
Treatment Strategy
The main treatment strategy decisions were taken according to national guidelines, based on comprehensive work-up during multidisciplinary team (MDT) meetings. In particular, extension of the peritoneal disease and the presence of extraperitoneal metastases (EPM) were precisely determined using a standardized imaging strategy. The contrast-enhanced thoraco-abdomino-pelvic computed tomodensitometry (CT) scan was completed by liver magnetic resonance imaging (MRI) in the case of doubtful liver lesions, and by positron emission tomography (PET) scan in the case of EPM. A peritoneal MRI was systematically performed a few days before CRS to map peritoneal lesions, and also, when necessary, to explore abdominopelvic EPM (for example, parietal). In case of doubtful resectability of PM, a staging laparoscopy was added to the work-up.
During the study period, the treatment strategy of CRPM patients was based on perioperative systemic chemotherapy and CRS/HIPEC.
Neoadjuvant Chemotherapy
The choice of chemotherapy regimen was based on ongoing national guidelines reported in the Thesaurus National de Cancerologie Digestive (TNCD), updated every 12–18 months.16 The FOLFOX and FOLFIRI protocols defined the doublet regimen and FOLFIRINOX defined the triplet regimen. Depending on RAS and BRAF mutational status, targeted therapies could be added. Three groups were compared—doublet, doublet + targeted, and triplet. In the triplet group, patients did not receive targeted therapies. Schematically, the choice of protocol depended on the current policy of the department (with the search for consistency at the center level), the expected level of response, and the patient’s history.
Upfront resectable patients were intended to receive four cycles of NACT before being resected, while patients in whom tumor burden shrinking was needed received as many cycles as needed. NACT could also be prolonged for different reasons, i.e. scheduling constraints in the theater, tolerance issues, and/or for prehabilitation purposes. Therefore, a cut-off of six cycles (one cycle lasting 2 weeks) was used to define short- and long-lasting NACT.
Postoperative chemotherapy was based on the doublet regimen corresponding to the preoperative regimen, without targeted therapy.
Morphological Response Evaluation
Every four cycles of NACT, patients were re-assessed with thoraco-abdomino-pelvic CT scan to define the MR according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.17 Regarding CRPM, only well-defined nodular peritoneal nodules ≥10 mm were considered as target lesions, otherwise CRPM were considered as non-target lesions.18 This MR was stated during the MDT meeting, based on expert radiologist interpretation.
Pathological Response Evaluation
The PR was based on the percentage of viable tumor cells with respect to the nodule area, independent of chemotherapy-related tissue injury, fibrosis, or necrosis.14,19 The Peritoneal Regression Grading Score (PRGS) was used to rate the pathologic response: no residual cancer cells in all specimens (complete response – PRGS 1), 1–49% residual cancer cells (major response – PRGS 2), ≥50% residual cancer cells (minor response – PRGS 3), and no response (PRGS 4) (5,10). For patients with multiple specimens, mean values were used to define the PR, including at least one sample for each resection.14,19
Surgery
Chemotherapy was discontinued at least 4 weeks before CRS (6 weeks for targeted therapies). Patients underwent a midline laparotomy under general anesthesia, with comprehensive exploration leading to calculation of the Peritoneal Carcinomatosis Index.20 A complete CRS was performed if deemed reasonable, combining peritonectomies and organ resections.21,22 Before the inclusion period of PRODIGE7, patients received HIPEC following a complete CRS.12 After PRODIGE7, HIPEC was administered according to national guidelines.23 When indicated, HIPEC was performed according to the ‘closed abdomen’ technique as previously described.24 Depending on the treatment period, cytotoxic agents were mitomycin C, oxaliplatin, irinotecan, or cisplatin, alone or in combination.25 The 90-day postoperative mortality and morbidity were recorded according to the Dindo–Clavien classification, with grade 3 or 4 used to define severe complications.26
Follow-Up
Patients were offered perioperative systemic chemotherapy, with the aim of achieving 12 cycles in total, avoiding targeted therapies postoperatively, and shifting to the doublet regimen for patients using the triplet regimen in the neoadjuvant setting.
Patients were followed-up every 3 months for 2 years, then every 6 months for 3 years, and then annually. Clinical examination, tumor markers and thoraco-abdomino-pelvic CT scan were performed. In case of recurrence, a second-line treatment was proposed.
Statistical Analysis
Student’s t-test was used for continuous variables, and parametric and McNemar tests were used for categorical variables, as a non-parametric test. The Fisher’s exact test was used for comparisons within groups. The Mann–Whitney U test was used as a non-parametric test for comparisons between independent variables without Gaussian distribution and equality of variance assumption.
Univariate and multivariate survival analyses with Cox model regression were conducted. Missing data were dealt with imputation using the mice package (v3.13.0; van Buuren and Groothuis-Oudshoorn, 2011). Imputation was conducted for the multivariate regression model and the selected covariates. The overall survival (OS) rate was evaluated depending on preoperative chemotherapy schemes used, as well as MR and PR.
Survivals were calculated from the date of CRPM diagnosis. Survival rates were first estimated using the Kaplan–Meier method and compared using the log-rank test. However, given the non-randomized nature of this comparative study, multivariate analyses were used as primary analyses. The hazard ratios (HRs) were estimated with 95% confidence intervals (CIs) through Cox regression multivariate models. Assumption of hazard proportionality through time was confirmed for the selected model. The best regression model was chosen using a combination of the literature-based prognostic factors and based on the best Akaike information criterion (AIC) in a stepwise algorithm, using the ‘stats’ R package. Survival rates were estimated using the Kaplan–Meier method and compared using the log-rank test. Analysis was performed using RStudio Software (RStudio: Integrated Development for R. PBC, Boston, MA, USA; 2020). A two-sided p-value <0.05 indicated statistical significance.
Results
Descriptive Data
Over the study period, 427 consecutive CRPM patients were treated in curative intent with 499 procedures. The selection criteria defined a study population of 388 patients, including 127 (33%), 202 (52%), and 59 (15%) patients in the doublet, doublet + targeted, and triplet groups, respectively (see electronic supplementary material Fig. 1). Analysis of the number of patients included in each group per year showed that, notably, 63% of the FOLFIRINOX patients were included during the last 5 years (2014–2018), as highlighted in the histogram shown in Fig. 1.
Baseline characteristics are depicted in Table 1. Patients throughout the three groups were comparable for age, sex, primary tumor location, and EPM. When compared with the doublet and doublet + targeted groups, the triplet group highlighted two significant differences: a higher proportion of synchronous presentation (64% and 44% vs. 81%, respectively) and less NACT cycles (fewer than six in 75% and 73% vs. 93%, respectively). A similar proportion of patients received adjuvant chemotherapy. The differences in mutational status, notably the significantly higher incidence of KRAS mutation in the doublet group (67% vs. 47% and 54%, respectively) and the higher proportion of BRAF mutation in the triplet group (22% vs. 7% and 13%, respectively) was hardly interpretable considering the high proportion of missing data (29% and 44%, respectively). Of note, when adding RAS and RAF mutations, the triplet group included 57% of mutated patients compared with 42% and 44% in the doublet and doublet + targeted groups, respectively.
The proportion of CC0 resections and HIPEC were broadly comparable. The median intraoperative Peritoneal Cancer Index (PCI) was 6 (6.7), consistent throughout the groups; however, the triplet group exhibited a higher proportion of patients with PCI >20 (17% vs. 4% and 6%, respectively). Eleven (2.8%) patients died postoperatively and the postoperative major morbidity rate was 51.2%. Patients in the triplet group presented a higher rate of severe complications (61% vs. 43% and 53%, respectively).
Morphological and Pathological Responses
MR and PR according to NACT protocols are depicted in Table 2, with no data available for 53/388 (14%) and 160/388 (41%) patients, respectively. Regarding MR, 58.8% of patients were considered as responders and 35% showed stable disease. Comparison between regimens showed a higher MR rate after doublet chemotherapy with targeted therapies (64.2%) or triplet therapy (68.0%) than in the doublet group (42.4%) [p = 0.003].
Regarding PRs, PRGS 1 and PRGS 2 were observed in 31 (13.6%) and 73 (32.0%) patients, respectively. The comparison between NACT groups did not show significant differences, with 43–48% of patients presenting a PRGS 1 or 2. The 31 patients with complete response exhibited a median PCI of 4 (4.1). Twelve of these patients (39%) were treated with the doublet regimen, 17 (55%) were treated with doublet + targeted drugs, and 2 (6%) were treated with the triplet regimen.
Survival Analysis
After a median follow-up of 24.7 months (interquartile range [IQR] 30.2), the median OS of the study population was 51.9 months (95% CI 42.8–77.2). The median follow-up was 25.3 months (IQR 30.4), 25.3 months (IQR 30.8), and 19.2 months (IQR 24.1) for the doublet, doublet + targeted, and triplet groups, respectively.
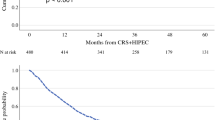
Survival outcomes according to NACT regimens are presented in Fig. 2. The median OS was 45.0 months for the doublet + targeted group, and not reached for the doublet and triplet groups, with respective 5-year OS rates of 42.2%, 50.3%, and 63.3% (log-rank test, p = 0.21).
The 5-year PFS was 17.1%, 23.1%, and 25.7% in the doublet + targeted, doublet, and triplet groups, respectively (log-rank test, p = 0.18), corresponding to a median PFS of 12.4, 14.4, and 14.4 months, respectively.
Long-term outcomes according to PR and MR are reported in Figs. 3 and 4, respectively. The difference in 5-year OS between patients with MR (46.9%) and patients with progression (33.4%) was not significant (p = 0.45), while the advantage was significant for 5-year PFS, which was null for patients in progression and nearly 20% in responding patients (log-rank test, p = 0.048).
PR was predictive of both OS and PFS. In particular, the 5-year OS was 96.9%, 48.2%, 31.8%, and 26.9% in the PRGS 1–4 groups, respectively (p < 0.001), while the 5-year PFS was 58.9%, 21.3%, 15.4% and 7.2% in PRGS 1, 2, 3 and 4 patients, respectively (p < 0.001).
Multivariate Analysis
Univariate and multivariate analysis for OS and PFS are presented in Table 3. Univariate Cox regression showed that PCI, extraperitoneal and retroperitoneal metastases (RPLNM), major postoperative complications, and PR were negative OS predictors. In multivariate analysis, the triplet regimen was an independent prognostic factor of OS (HR 0.49, 95% CI 0.25–0.96; p = 0.037), as well as PCI, with HR increasing with PCI subgroups (p < 0.001), RPLNM (HR 2.65, 95% CI 1.34–5.24, p = 0.008) and PR (p < 0.01).
The doublet + targeted group, long-lasting NACT, PCI level, EPM, and pathologic response were associated with PFS in univariate analysis. In multivariate analysis, PCI level, EPM (HR 1.54, 95% CI 1.19–1.99; p = 0.001) and pathologic response were independently associated with PFS.
Discussion
This study comparing NACT regimens for CRPM patients undergoing complete resection confirmed the strong prognostic impact of PR and argues in favor of the triplet regimen in this setting, with FOLFIRINOX being an independent factor of better OS (HR 0.49, p = 0.037). This survival advantage was obtained despite a higher rate of patients with PCI >20 and a higher rate of RAS/RAF-mutated patients, factors of poor prognosis.27
Perioperative systemic chemotherapy management remains debated for CRPM patients. If it seems demonstrated that systemic chemotherapy is associated with better long-term outcomes when compared with no perioperative systemic treatment, the best timing is still in question.28 Moreover, NACT regimens were rarely compared in retrospective series with heterogenous data, as highlighted by a systematic review.29 Our team proposed a retrospective analysis of 115 patients focused on PR in 2014. The results were consistent with the present study, however the triplet regimen was not used at that time and 20% of patients had two or more lines preoperatively.14 Passot et al. reported complete and major response rates of 9.7% and 20.2%, while the present study reported rates of 14% and 32%. The regimen associated with the best OS was FOLFOX + bevacizumab.14 In both studies, PR was strongly associated with OS, whereas MR was not. Another retrospective study comparing NACT protocols found bevacizumab to be an independent factor of improved OS.7
Interestingly, in the present analysis, the addition of targeted therapy improved the radiological response when compared with the doublet group (p = 0.003) and also increased the complete PR rate; however, when including PRGS 1 and 2, the difference was attenuated (43%, 48%, and 43% of the doublet, doublet + targeted, and triplet regimens, respectively). Finally, only FOLFIRINOX was an independent factor of better OS (HR 0.49, p = 0.037), while no regimen gave significant survival advantage in Kaplan–Meier analysis, with the best OS (63.3%) and progression-free survival (PFS; 25.7%) being observed in the FOLFIRINOX group.
In a retrospective study of CRPM patients, preoperative bevacizumab was reported to induce higher rates of severe postoperative complications (34% vs. 19 %, p = 0.020), despite an appropriately-timed discontinuation before CRS/HIPEC.30 In this current study, the highest severe morbidity was observed in the triplet group.28 The phase II part of the CAIRO6 randomized trial compared postoperative outcomes after NACT (37 patients, all but one with bevacizumab) with upfront CRS/HIPEC (42 patients). No significant difference was found in severe morbidity (22% vs. 33% of patients; HR 0.65, 95% CI 0.31–1.37; p = 0.25).31
Several large randomized controlled trials compared chemotherapy regimens for non-resectable metastatic colorectal patients.8,9,10,11 Cremolini et al. gathered these outcomes in an individual patient data meta-analysis of 1697 patients, confirming that FOLFOXIRI + bevacizumab provided longer median OS than doublets + bevacizumab (28.9 vs. 24.5 months; HR 0.81, 95% CI 0.72–0.91; p < 0.001) and higher R0 resection rate (16.4% vs. 11.8%; p = 0.007), at a cost of significantly increased adverse events (severe neutropenia and diarrhea).10 Thus, the current stake of systemic chemotherapy regimen choice in the colorectal metastatic patient population seems to aim to increase response and conversion rates while limiting toxicities.32,33,34 Results are rapidly evolving, with a trend toward more individualized treatment strategies, based on molecular stratification, favoring the use of targeted therapies in addition to the doublet or triplet regimen.10,11 In these trials, no independent analysis was performed regarding CRPM patients, and little was known about surgical treatment, precluding clear conclusions being drawn on the choice of NACT regimen in the setting of peritoneal metastases. In the present study, no patients were treated with the quadruplet regimen and the best long-term outcomes were associated with the triplet regimen as NACT, at a cost of increased severe complications but with no difference in the proportion of patients receiving adjuvant chemotherapy, questioning the opportunity of more accurately evaluating the role of targeted therapies in NACT regimens in the future.
However, with high rates of missing data, the complete and major response rates were 13.6% and 32.0% in this analysis, while in liver metastases, rates of 10% and 50% were reported.35,36 This difference could reflect the lower chemosensitivity of CRPM.1,37,38 Intra-nodule inefficient microcirculation39,40 and high interstitial pressure41,42 prevent the drug from accessing the tumor cells.43,44 The pivotal role of PR should put into perspective the outcomes of recent RCTs evaluating oxaliplatin/HIPEC.12,45,46 While PRODIGE7 reported excellent outcomes after complete CRS, performed in expert centers with perioperative chemotherapy, the addition of short-course high-dose oxaliplatin/HIPEC failed to demonstrate benefit.12 Among the factors that could have interfered with that outcome, the question of oxaliplatin chemoresistance induced by NACT was raised.47,48 Prabhu et al. assessed chemosensitivity through cell cultures of CRPM patients and found that oxaliplatin/NACT led to a significantly higher rate of chemoresistance to oxaliplatin at CRS.49
While pathologic response was confirmed to be a strong predictor of oncologic outcomes in the analysis by Prabhu et al., the morphologic response was not. One of the explanations could be that the RECIST criteria are not well adapted for peritoneal lesions.50 Indeed, most peritoneal lesions are thickening of the peritoneum, whose modifications with NACT do not translate into significant response. Therefore, some responding patients were ranked as having stable disease. Some authors proposed a modified RECIST score system for pleural mesothelioma, prone to be extended to peritoneal metastases to encompass that pitfall.51 Conversely, pathologic response was studied with a four-tie score, the PRGS, more clearly defining the response level and resulting in a strong prognostic factor.
Beyond the pitfalls inherent to the retrospective nature of our series (long inclusion period with a majority of patients in the group having the best results included in the second part of the study period, significant proportion of missing data for pathologic response analysis), selection bias was induced by including only patients ultimately having undergone a complete CRS. Ideally, an intention-to-treat analysis of this CRPM population would have required the inclusion of all patients referred for evaluation at our center. This group of unresected CRPM patients is heterogeneous, made up of inoperable patients, those with initial disease not amenable to secondary CRS, and those with disease progression during NACT. Unfortunately, a large proportion of these patients is managed externally and their follow-up is not recorded in the database, preventing a comprehensive analysis. On the other hand, focusing on patients ultimately resected allowed us to compare treatments in a highly selected population, at a single-center level, leading therefore to a population slightly more homogeneous. Another pitfall of that analysis is the absence of a ‘control’ group, made up of patients with no preoperative systemic chemotherapy, although this approach is gaining interest for the management of CRPM patients52 and also puts these results into perspective. Finally, the fact that NACT regimen choice was not the result of randomization but rather of the evolution of national guidelines translated in the center’s policy, was also a major bias. The duration of NACT was also variable, influenced by medical and logistic parameters, which could have interfered with the level of response. These are the limits of that retrospective analysis which was however the first to compare NACT regimens in that setting.
Conclusions
Long-term oncologic results of CRPM patients treated with CRS/HIPEC and NACT analyzed in this retrospective monocentric study seemed to favor the FOLFIRINOX regimen, despite an increased rate of postoperative severe complications. Among others, pathologic response was confirmed as a determinant prognostic factor.
References
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19. https://doi.org/10.1016/s1470-2045(16)30500-9.
Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24(24):4011–9. https://doi.org/10.1200/jco.2006.07.1142.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. https://doi.org/10.1016/s0140-6736(08)60455-9.
Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–8. https://doi.org/10.1200/jco.2009.23.9285.
Goere D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–71. https://doi.org/10.1097/sla.0b013e31827e9289.
Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–80. https://doi.org/10.1200/jco.2007.11.0833.
Ceelen W, Nieuwenhove YV, Putte DV, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21(9):3023–8. https://doi.org/10.1245/s10434-014-3713-7.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. https://doi.org/10.1056/nejmoa032691.
Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–18. https://doi.org/10.1056/nejmoa1403108.
Cremolini C, Antoniotti C, Stein A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020;38(28):JCO2001225. https://doi.org/10.1200/jco.20.01225.
Rossini D, Antoniotti C, Lonardi S, et al. Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: the Phase III TRIPLETE study by GONO. J Clin Oncol. 2022;40(25):2878–88. https://doi.org/10.1200/jco.22.00839.
Quenet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–66. https://doi.org/10.1016/s1470-2045(20)30599-4.
Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–5. https://doi.org/10.1200/jco.2008.19.7160.
Passot G, You B, Boschetti G, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21(8):2608–14. https://doi.org/10.1245/s10434-014-3647-0.
Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am. 2003;12(3):649–71. https://doi.org/10.1016/s1055-3207(03)00037-1.
Phelip JM, Tougeron D, Léonard D, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Updated July 2022. https://www.snfge.org/content/4-cancer-colorectal-metastatique. Accessed Dec 2022.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Dohan A, Hoeffel C, Soyer P, et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg. 2017;104(9):1244–9. https://doi.org/10.1002/bjs.10527.
Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 2016;1(2):99–107. https://doi.org/10.1515/pp-2016-0011.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: PH Sugarbaker, editor. Peritoneal carcinomatosis: principles of management, vol 82, Kluwer Academic Publishers; 1996. p. 359–74. https://doi.org/10.1007/978-1-4613-1247-5_23.
Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. https://doi.org/10.1097/00000658-199501000-00004.
García-Fadrique A, Estevan RE, Ortí LS. Quality standards for surgery of colorectal peritoneal metastasis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2022;29(1):188–202. https://doi.org/10.1245/s10434-021-10642-6.
Abboud K, André T, Brunel M, et al. Accord d’experts français pour la prise en charge des métastases péritonéales de cancers colo-rectaux. J Chir Visc. 2019;156(5):412–5. https://doi.org/10.1016/j.jchirv.2019.05.007.
Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863–9. https://doi.org/10.1245/aso.2003.01.018.
Passot G, Vaudoyer D, Villeneuve L, et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113(7):796–803. https://doi.org/10.1002/jso.24248.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Schneider MA, Eden J, Pache B, et al. Mutations of RAS/RAF proto-oncogenes impair survival after cytoreductive surgery and HIPEC for peritoneal metastasis of colorectal origin. Ann Surg. 2018;268(5):845–53. https://doi.org/10.1097/sla.0000000000002899.
Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant systemic chemotherapy vs active surveillance following up-front resection of isolated synchronous colorectal peritoneal metastases. JAMA Oncol. 2020;6(8):e202701. https://doi.org/10.1001/jamaoncol.2020.2701.
Rovers KP, Simkens GA, Punt CJ, van Dieren S, Tanis PJ, de Hingh IH. Perioperative systemic therapy for resectable colorectal peritoneal metastases: sufficient evidence for its widespread use? A critical systematic review. Crit Rev Oncol Hematol. 2017;114:53–62. https://doi.org/10.1016/j.critrevonc.2017.03.028.
Eveno C, Passot G, Goere D, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21(6):1792–800. https://doi.org/10.1245/s10434-013-3442-3.
Rovers KP, Bakkers C, Nienhuijs SW, et al. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases. JAMA Surg. 2021. https://doi.org/10.1001/jamasurg.2021.1642.
Fornaro L, Lonardi S, Masi G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013;24(8):2062–7. https://doi.org/10.1093/annonc/mdt165.
Cremolini C, Antoniotti C, Lonardi S, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 2018;4(4):529. https://doi.org/10.1001/jamaoncol.2017.5314.
Ychou M, Rivoire M, Thezenas S, et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer. 2022;126(9):1264–70. https://doi.org/10.1038/s41416-021-01644-y.
Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299–304. https://doi.org/10.1093/annonc/mdl386.
Blazer DG, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(33):5344–51. https://doi.org/10.1200/jco.2008.17.5299.
Köhne CH, Cunningham D, Costanzo FD, et al. Clinical determinants of survival in patients with 5-fluorouracil- based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13(2):308–17. https://doi.org/10.1093/annonc/mdf034.
Assersohn L, Norman A, Cunningham D, Benepal T, Ross PJ, Oates J. Influence of metastatic site as an additional predictor for response and outcome in advanced colorectal carcinoma. Br J Cancer. 1999;79(11/12):1800–5. https://doi.org/10.1038/sj.bjc.6690287.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–92. https://doi.org/10.1038/nrc1893.
Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36(3):321–31. https://doi.org/10.1055/s-0030-1253454.
Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13. https://doi.org/10.1038/nrc1456.
Steuperaert M, Labate GFD, Debbaut C, et al. Mathematical modeling of intraperitoneal drug delivery: simulation of drug distribution in a single tumor nodule. Drug Deliv. 2017;24(1):491–501. https://doi.org/10.1080/10717544.2016.1269848.
Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7(2):108–15. https://doi.org/10.1038/nrclinonc.2009.217.
Löke DR, Helderman RFCPA, Franken NAP, et al. Simulating drug penetration during hyperthermic intraperitoneal chemotherapy. Drug Deliv. 2021;28(1):145–61. https://doi.org/10.1080/10717544.2020.1862364.
Goere D, Glehen O, Quenet F, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21(9):1147–54. https://doi.org/10.1016/s1470-2045(20)30322-3.
Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–70. https://doi.org/10.1016/s2468-1253(19)30239-0.
Ceelen W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: the end of the road? Eur J Surg Oncol. 2019;45(3):400–2. https://doi.org/10.1016/j.ejso.2018.10.542.
Nagourney RA, Evans S, Tran PH, Nagourney AJ, Sugarbaker PH. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur J Surg Oncol. 2021;47(4):738–42. https://doi.org/10.1016/j.ejso.2020.09.017.
Prabhu A, Brandl A, Wakama S, et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: proof-of-concept study. BJS Open. 2021. https://doi.org/10.1093/bjsopen/zraa075.
Kepenekian V, Bhatt A, Péron J, et al. Advances in the management of peritoneal malignancies. Nat Rev Clin Oncol. 2022;19(11):698–718. https://doi.org/10.1038/s41571-022-00675-5.
Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–60. https://doi.org/10.1093/annonc/mdh059.
Ljunggren M, Nordenvall C, Palmer G. Direct surgery with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastases. Eur J Surg Oncol. 2021;47(11):2865–72. https://doi.org/10.1016/j.ejso.2021.05.046.
Author information
Authors and Affiliations
Contributions
A total of 11 authors made contributions to this manuscript as a result of multidisciplinary team work requiring multiple areas of expertise. Study design: OG, GP, FF, VK and JP. Data collection: IB, LV, FF, AK. Data analysis: FF, AK, JP, LV, GP, PR, BY, NB, OG, VK. Statistics: AK, JP. Clinicians specialized in digestive peritoneal surface malignancies (medical oncologist, pathologist, radiologist and surgeons): JP, BY, NB, PR, GP, OG and VK. Manuscript writing and editing: FF, AK, JP, IB, LV, GP, PR, BY, NB, OG, VK.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Study flowchart. CRS cytoreductive surgery, HIPEC hyperthermic intraperitoneal chemotherapy, CRC colorectal cancer, NACT neoadjuvant chemotherapy (TIFF 13335 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fanget, F., Kefleyesus, A., Peron, J. et al. Comparison of Neoadjuvant Systemic Chemotherapy Protocols for the Curative-Intent Management of Peritoneal Metastases from Colorectal Cancer, Regarding Morphological Response, Pathological Response, and Long-Term Outcomes: A Retrospective Study. Ann Surg Oncol 30, 3304–3315 (2023). https://doi.org/10.1245/s10434-023-13150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13150-x