Abstract
Background
Studies have shown that epidural analgesia (EDA) is associated with a decreased risk of pneumonia and anastomotic leakage after esophagectomy, and several guidelines strongly recommend EDA use after esophagectomy. However, the benefit of EDA use in minimally invasive esophagectomy (MIE) remains unclear.
Objective
The aim of this retrospective study was to compare the short-term outcomes between patients with and without EDA undergoing MIE for esophageal cancer.
Methods
Data of patients who underwent oncologic MIE (April 2014–March 2019) were extracted from a Japanese nationwide inpatient database. Stabilized inverse probability of treatment weighting (IPTW), propensity score matching, and instrumental variable analyses were performed to investigate the associations between EDA use and short-term outcomes, adjusting for potential confounders.
Results
Among 12,688 eligible patients, EDA was used in 9954 (78.5%) patients. In-hospital mortality, respiratory complications, and anastomotic leakage occurred in 230 (1.8%), 2139 (16.9%), and 1557 (12.3%) patients, respectively. In stabilized IPTW, EDA use was significantly associated with decreased in-hospital mortality (odds ratio [OR] 0.46 [95% confidence interval 0.34–0.61]), respiratory complications (OR 0.74 [0.66–0.84]), and anastomotic leakage (OR 0.77 [0.67–0.88]). EDA use was also associated with decreased prolonged mechanical ventilation, unplanned intubation, nonsteroidal anti-inflammatory drug use, acetaminophen use, postoperative length of stay, and total hospitalization costs and increased vasopressor use. One-to-three propensity score matching and instrumental variable analyses demonstrated equivalent results.
Conclusions
EDA use in oncologic MIE was associated with low in-hospital mortality as well as decreased respiratory complications, and anastomotic leakage, suggesting the potential advantage of EDA use in MIE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Subtotal esophagectomy, the mainstay of curative treatment for esophageal cancer, is one of the most complex and invasive gastrointestinal surgical procedures. The latest data from high-volume esophageal surgical centers in 14 countries showed that the proportion of mortality and morbidity remains high (4.5% and 59.0%, respectively).1 Minimally invasive esophagectomy (MIE) with small incisions and less pain is being increasingly performed worldwide2 and is associated with lower mortality and morbidity than traditional open esophagectomy (OE).3,4
Several guidelines strongly recommend the use of epidural analgesia (EDA) following esophagectomy because OE induces severe postoperative pain, which may cause worse short-term outcomes.5,6 In previous randomized controlled trials, EDA has demonstrated superiority over conventional analgesia in controlling pain,7,8,9,–10 reducing the time to first passage of flatus,10 and attenuating the systemic proinflammatory response after OE.8,9 Moreover, a recent propensity score (PS)-matched single-center cohort study involving 178 pairs of patients showed that EDA was associated with a lower occurrence of pneumonia and anastomotic leakage after esophagectomy compared with intravenous analgesia.11 However, most patients in this prior study underwent OE (77% of the matched cohort), and the advantages of using EDA in MIE remain unclear.11
Although MIE has become common partly because it induces less pain than OE, the anesthetic procedure for OE (i.e., EDA) is implemented in MIE without clear evidence. It is important to examine whether the use of EDA is beneficial for patients undergoing MIE because oncologic MIE is becoming increasingly more common. In the present study, we examined the association between EDA and short-term outcomes following MIE for esophageal cancer by PS and instrumental variable analyses using a Japanese nationwide inpatient database.
Methods
Database
This large-scale retrospective cohort study was performed using the Diagnosis Procedure Combination database in Japan.12 This inpatient database contains data from more than 1200 facilities nationwide and collects more than 8 million hospital administrative claims data and discharge abstracts annually.
The database includes the following information: sex; age; body mass index; smoking index; diagnosis and comorbidities at admission and complications after admission recorded using the International Classification of Diseases, Tenth Revision (ICD-10) codes; clinical cancer stage based on the Seventh Edition of the Union for International Cancer Control (UICC) Tumor, Node, Metastasis classification; preoperative chemotherapy/radiotherapy; interventional/surgical procedures according to the original Japanese codes; unique hospital identifier; length of stay; and total hospitalization costs. Previous validation studies have shown good sensitivity and specificity of the diagnoses and procedure records in the database and high validity of cancer diagnosis.13,14
Ethics
This study was approved by the Institutional Review Board of The University of Tokyo [approval number: 3501-(3)]. The requirement for informed consent was waived because of the anonymous nature of the data.
Study Protocol
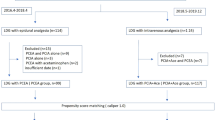
We investigated patients who underwent MIE (defined as thoracoscopic, mediastinoscopy-assisted, or robotic-assisted esophagectomy, regardless of whether the abdominal approach was open laparotomy or laparoscopy) with two-field (thoraco-abdominal) or three-field (cervico-thoraco-abdominal) lymph node dissection for esophageal cancer from 1 April 2014 to 31 March 2019. The Japanese original procedure codes for surgery were used to identify patients who underwent the procedures. Because the current database did not contain information on conversion to open thoracotomy during MIE, patients who underwent conversion to open thoracotomy were included in the analyses. In Japan, transhiatal esophagectomy (without upper mediastinal lymph node dissection) is not commonly performed as a curative surgery for esophageal cancer;15 therefore, we did not include patients who underwent laparoscopy-assisted transhiatal esophagectomy in this study. Patients who underwent two-stage reconstruction were also not included. The exclusion criteria were age <18 years, reconstruction using the intestine or vessel reconstruction, combined surgery for laryngeal/hypopharyngeal cancer, prescription of antiplatelet drugs (including aspirin, clopidogrel, cilostazol, prasugrel, ticagrelor, and ticlopidine) from the day of admission to the day of surgery, and missing data on body mass index. We excluded patients who were continuing antiplatelet drugs prior to surgery because Japanese anesthesiologists may avoid the use of EDA even in patients using aspirin alone. We did not exclude patients with stage IV cancer because esophageal cancer with supraclavicular lymph node metastasis is generally considered curable by surgery in Japan,16 although such metastasis is classified as stage IV with distant metastases in the UICC classification.
We compared patients who did not use postoperative continuous EDA (non-EDA group) and those who used postoperative continuous EDA from the day of surgery (EDA group).
The primary outcomes were in-hospital mortality, respiratory complications (J12-18, J80, J96, J690, J691, J958, J959), and anastomotic leakage (T813, long-term drainage tube placement [defined as placement for ≥3 weeks after surgery],3 and procedures for anastomotic leakage).
The secondary outcomes were 30-day mortality (defined as death occurring within 30 days of MIE during initial admission), major complications, use of mechanical ventilation for >2 days and >7 days (defined as mechanical ventilation use lasting >2 days and >7 days following surgery, respectively), unplanned intubation, vasopressor use (defined as continuous use of noradrenaline, dopamine, or dobutamine) within postoperative day (POD) 3, nonsteroidal anti-inflammatory drug (NSAID) use within POD 3, acetaminophen use within POD 3, postoperative length of stay, and total hospitalization costs. We defined major complications as respiratory complications, anastomotic leakage, pneumothorax, chylothorax, empyema, peritonitis, ileus, bowel obstruction, symptomatic hiatal/diaphragmatic hernia, pulmonary embolism, acute coronary syndrome, heart failure, stroke, acute kidney injury, sepsis, and other conditions resulting in unplanned intubation and/or death. The ICD-10 codes and procedures used to define each postoperative complication are shown in electronic supplementary Table 1.
We examined patient background factors, including sex, age, body mass index classification, smoking index, activities of daily living (Barthel index <95), comorbidities, clinical cancer stage, preoperative treatment (chemotherapy/radiotherapy), and field of lymph node dissection. Age was categorized into four groups: 18–64, 65–69, 70–74, and ≥75 years. The body mass index was categorized into five groups based on the criteria for Asia-Pacific populations by the World Health Organization17: <16.0 kg/m2, 16.0–18.4 kg/m2, 18.5–22.9 kg/m2, 23.0–27.4 kg/m2, and ≥27.5 kg/m2. The smoking index was categorized into five groups: 0–5, 6–20, 21–40, ≥41 pack-years, and missing. Comorbidities were scored using the Charlson comorbidity index based on ICD-10 codes18 and classified into three groups: 2, 3–4, and ≥5. The Charlson comorbidity index, a method of predicting mortality by classifying and weighting comorbidities, has been validated and widely used in nationwide studies.19 The clinical cancer stage was divided into three categories: 0–I, II–IV, and X/missing. In Japan, preoperative chemotherapy has been the first-line treatment for resectable esophageal cancer (clinical stage ≥II) since 2012, and preoperative chemoradiotherapy has been evaluated in a clinical trial.20 The field of lymph node dissection was either two fields (thoracic and abdominal approaches) or three fields (cervical, thoracic, and abdominal approaches; with or without supraclavicular lymph node dissection). In Japan, three-field lymph node dissection generally involves cervical anastomosis, whereas two-field lymph node dissection involves intrathoracic anastomosis.21
We also investigated hospital background factors (i.e., type of hospital, hospital volume, and hospital preference for early extubation) and fiscal year. The type of hospital was either nonteaching or teaching. Hospital volume was defined as the number of MIEs performed per year in each hospital and was categorized into four groups, with approximately equal numbers of patients in each group. Hospital preference for early extubation was examined similar to a previous study22 because some hospitals recently preferred early extubation to improve short-term outcomes.23,24 We defined the proportion of patients who were extubated on the day of surgery at each hospital as hospital preference for early extubation and categorized them into quartiles.
Statistical Analysis
We used stabilized inverse probability of treatment weighting (IPTW) to estimate the average treatment effect by creating a pseudo-dataset with PS.25,26 This analysis adjusts for measured potential confounding and preserves the sample size of the original data. The PS was estimated by a multivariable logistic regression model based on the background factors of patient, treatment, and hospital. We calculated the C-statistic using the area under the receiver operating characteristic curve to evaluate the ability of the model to predict EDA use after esophagectomy. To examine the balance in baseline covariates of patients between the two groups, absolute standardized differences were calculated, and a difference of <10% was considered acceptable.25 After generating stabilized IPTW cohorts, univariate regression analyses were performed to compare the outcomes between the non-EDA group and the EDA group: logistic regression analyses were performed for binary outcomes, and linear regression analyses were performed for continuous outcomes.
We performed four sensitivity analyses to confirm the result of the primary outcomes in the stabilized IPTW analysis. First, we conducted 1:3 PS matching.25 We used a nearest-neighbor matching algorithm within a caliper (≤0.2 of the pooled standard deviation of estimated logits) with replacement. Second, we performed a multivariable logistic regression analysis for the all-patient cohort. We fitted generalized estimating equations to adjust for within-hospital clustering, such as the patients’ characteristics or physicians’ practice patterns within the same hospital.27 The explanatory variables were the same as those in the regression analysis for estimating the PS. Third, we conducted an instrumental variable analysis, aiming to adjust for unmeasured background factors.28 We defined ‘facility annual EDA usage rates’ as the current instrumental variable. We used a two-stage residual inclusion method and robust standard errors.28 The explanatory variables were the same as above. An F-statistic of >10 indicates that the instrument is not weak.28 Finally, we performed subgroup-stabilized IPTW analyses stratified by hospital volume (<15.8 or ≥15.8 cases per year), and calculated the subgroup-specific PS for each subgroup analysis.
The t-test was used to compare continuous variables and the Chi-square test was used to compare categorical variables between the groups. Statistical significance was accepted at p < 0.05. All statistical analyses were conducted using STATA version 16 (StataCorp LLC, College Station, TX, USA).
Results
Overall, 13,203 patients who underwent MIE for esophageal cancer from April 2014 to March 2019 were identified. We excluded 515 patients who met the following exclusion criteria: reconstruction using the intestine or vessel reconstruction (n = 217), combined surgery for laryngeal/hypopharyngeal cancer (n = 71), prescription of antiplatelet drugs from the day of admission to the day of surgery (n = 199), and missing data on body mass index (n = 48). Thus, we analyzed 12,688 patients.
The patients’ mean age was 67 years, and 10,389 (81.9%) patients were male. EDA was used in 9954 (78.5%) patients. Table 1 shows the patients’ demographics and clinical characteristics before and after the stabilized IPTW, and electronic supplementary Table 2 shows the patients’ demographics and clinical characteristics after PS matching. The C-statistic was 0.67 in the PS models. In the all-patient cohort, EDA was unlikely to be used in third-quartile hospitals in hospital volume and teaching hospitals, and was likely to be used in hospitals that prefer early extubation. After the stabilized IPTW or PS matching, the patient distributions were well-balanced between the groups.
Table 2 shows the primary and secondary outcomes, and electronic supplementary Table 3 shows the detailed postoperative complications. In the all-patient cohort, the EDA group had lower mortality (in-hospital mortality, 1.4% vs. 3.3%), fewer occurrences of complications, a shorter postoperative length of stay, and lower total hospitalization costs than the non-EDA group.
In the stabilized IPTW analyses, the EDA group had significantly lower in-hospital mortality (1.4% vs. 3.1%; odds ratio [OR] 0.46 [95% confidence interval 0.34–0.61]), respiratory complications (16.2% vs. 20.6%; OR 0.74 [0.66–0.84]), and anastomotic leakage (11.7% vs. 14.7%; OR 0.77 [0.67–0.88]) than the non-EDA group. The EDA group also had lower 30-day mortality (OR 0.39 [0.22–0.70]), major complications (OR 0.79 [0.72–0.87]), use of mechanical ventilation for >2 days (OR 0.77 [0.67–0.88]) and >7 days (OR 0.78 [0.65–0.93]), NSAID use within POD 3 (OR 0.81 [0.74–0.89]), acetaminophen use within POD 3 (OR 0.80 [0.72–0.89]), and unplanned intubation (OR 0.74 [0.63–0.87]); a shorter duration of postoperative length of stay by 4.5 days (p < 0.001); and lower total hospitalization costs by $2303 (p < 0.001). Additionally, the EDA group had a higher proportion of vasopressor use within POD 3 (OR 1.10 [1.00–1.21]).
In the sensitivity analyses, 1:3 PS matching (n = 10,937), multivariable regression analyses, and instrumental variable analyses confirmed the relationship of EDA use with in-hospital mortality and the risk of respiratory complications and anastomotic leakage (Table 3). The F-statistic in the instrumental variable analysis was 2288, indicating that the facility annual EDA usage rates were strong instrumental variables for predicting whether a patient was in the EDA group or the non-EDA group.
Subgroup analyses showed that in-hospital mortality, respiratory complications, and anastomotic leakage were favorable in the EDA group regardless of hospital volume (Fig. 1).
Subgroup analyses of primary endpoints in stabilized inverse probability of treatment-weighted patients stratified by hospital volume. ORs of in-hospital mortality, respiratory complications, and anastomotic leakage associated with epidural analgesia use are shown. ORs were calculated with reference to patients in the non-epidural analgesia group. OR odds ratio, CI confidence interval
Discussion
In this nationwide database study, we examined the impact of EDA on short-term outcomes following MIE with two- or three-field lymph node dissection for esophageal cancer using two PS models and instrumental variable analyses. Compared with non-EDA use, EDA use was significantly associated with decreased in-hospital mortality as well as lower major complications, respiratory complications, and anastomotic leakage. To the best of our knowledge, this study is the first to analyze the association between EDA and short-term outcomes after MIE using a large-scale database.
With the rapid and widespread adoption of MIE for patients with esophageal cancer, it is meaningful to examine the impact of EDA use in MIE. Although the current guidelines from the Enhanced Recovery After Surgery Society strongly recommend the use of EDA following esophagectomy,6 the benefits of EDA use in MIE remain unknown. Because MIE is associated with less postoperative pain than OE,29 esophageal surgeons and anesthesiologists may not view EDA for MIE to be essential. Indeed, the use of EDA in MIE varied from 23 to 93% in previous multicenter studies30,31 and would have depended on the country and year of study.
Respiratory complications are the most common postoperative complications after esophagectomy,1,2 even after MIE.3,4 Prevention of respiratory complications after esophagectomy is particularly important because such complications are reportedly associated with perioperative mortality32 and poor overall and cancer-specific survival.32,33 One meta-analysis showed that EDA decreased the risk of respiratory complications, prolonged ventilation, and re-intubation following abdominal or thoracic surgery.34 In line with a previous study mainly focusing on OE,11 our results clearly showed that EDA use in MIE was associated with decreased respiratory complications. Additionally, the need for postoperative interventions presumably required for respiratory complications (i.e., use of mechanical ventilation for >2 days and >7 days, and unplanned intubation) was also decreased in the EDA group. Even in MIE with small incisions, good pain control by EDA would allow for early mobilization and rehabilitation, which can restore muscle and lung function, improve sputum evacuation,5,6 and consequently reduce respiratory complications and interventions.
Anastomotic leakage is the second most common complication after esophagectomy and worsens short-term outcomes.1,2,35 Whether EDA affects gastrointestinal anastomosis remains controversial.36,37 A previous study predominantly involving Sweet esophagectomy (partial esophagectomy with proximal gastrectomy) showed that EDA did not affect anastomotic leakage.38 Another study revealed that hypotensive episodes (defined as a drop in systolic blood pressure to ≤70% of baseline) due to bolus EDA may reduce gastric blood flow39 and increase the risk of anastomotic leakage after esophagectomy.40 However, our study demonstrated that EDA use in MIE was significantly associated with decreased anastomotic leakage, concordant with previous studies mainly in OE.11,41 Continuous EDA may improve the microcirculation of the gastric tube in the anastomotic area in the early postoperative period following esophagectomy.42 Indeed, EDA was reported to improve gastric (and transverse colon) mucosal blood flow after rectal cancer surgery.43 In the current study, EDA use was associated with increased vasopressor use. While EDA use may decrease blood pressure after esophagectomy,11 appropriate use of vasopressors with adequate fluid load may have helped to avoid anastomotic leakage due to EDA-induced hypotensive episodes.39 EDA use was also associated with decreased NSAID use. Previous studies have suggested that postoperative NSAID use may increase the risk of anastomotic leakage after gastrointestinal anastomoses, although its effect on anastomotic leakage after esophagectomy remains unclear.44
Notably, we showed that EDA was associated with low in-hospital mortality. The impact of EDA use on mortality would have been attributed to the two most frequent complications after esophagectomy, namely respiratory complications and anastomotic leakage, which are also major causes of death after esophagectomy.35 There are other possible mechanisms for this substantial reduction in postoperative mortality in the EDA group: reduction of the stress response;34 suppression of systemic inflammatory reactions;8,9 and improvement of coagulation, gastrointestinal, metabolic, and immune functions through suppression of central sympathetic stimulation.34 EDA is also considered to benefit cardiac outcomes.45 In the current study, EDA was associated with a low proportion of acute coronary syndrome (electronic supplementary Table 3), in line with previous studies.34,45 Additionally, EDA might have reduced the systemic analgesic requirements; the incidence of adverse effects, such as sedation, dizziness, respiratory depression, postoperative nausea, and vomiting caused by opioids;10 and the gastric mucosal, small bowel, and renal injury caused by NSAIDs.46
In our study, in-hospital mortality in patients undergoing MIE was only 1.8%, which is lower than that in previous nationwide studies from other countries.47,48 However, this low mortality is consistent with previous nationwide studies in Japan.3,4 Moreover, a previous report from the University of Pittsburgh, which acted as a benchmark of MIE,49 showed equivalent mortality, indicating the reliability of our dataset and study results.
The benefits of EDA in minimally invasive surgery are still under debate. To the best of our knowledge, no other study of any type of minimally invasive surgery has shown the association between EDA and favorable short-term outcomes other than pain control or the time to first passage of flatus.50,51 Recent studies of minimally invasive surgery in other fields such as colorectal surgery and lobectomy showed that EDA did not reduce postoperative complications36,52,53 and that it increased hospital stays36,53 and hospitalization costs;36 consequently, the use of EDA has been decreasing in these surgeries.36,53 This tendency was acceptable: EDA has been reported to provide less benefit in low-risk surgeries because of improvements in systemic analgesia during the past few decades.34 However, in MIE, which is associated with high mortality and morbidity even with its minimally invasive approach compared with other surgeries (such as colorectal surgery36 and lobectomy53), the use of EDA can improve the short-term postoperative outcomes. Therefore, EDA use in minimally invasive surgery might be recommended, especially in high-risk procedures such as thoraco-abdominal subtotal esophagectomy. To support this recommendation, further investigations may be required to uncover the pathological mechanisms of the substantial reduction in complications.
Several limitations of this study should be acknowledged. First, information on histopathology (squamous cell carcinoma or adenocarcinoma) was not available from the Diagnosis Procedure Combination database. In a previous report involving 336 institutions in Japan, squamous cell carcinoma accounted for 87.8% and adenocarcinoma for 6.3%.21 Three-field lymph node dissection was therefore the major technique used in the current study, unlike in previous Western studies. However, the surgical procedure was adjusted in the analysis, increasing the generalizability of the results. Second, the reasons for non-EDA use were unclear because of the limited available data in our database and the retrospective nature of the study; for example, some of these reasons might have been failure of EDA (e.g., technical failure of catheterization, catheter malpositioning or displacement), facility preference, coagulation abnormalities, and post-spine surgery. The proportion of failure of EDA for esophagectomy reportedly ranges from 17 to 21%,54 which is similar to the proportion of non-EDA use in the present study. Obesity might be associated with EDA failure,55 but we adjusted for body mass index in the current analyses. Facility preference was adjusted in the multivariable regression analysis with generalized estimating equations to adjust for within-hospital clustering. Moreover, the instrumental variable analysis could adjust for these unmeasured confounders. Third, postoperative pain management in the non-EDA group (e.g., intravenous patient-controlled analgesia, paravertebral block) was not available in our database. Additionally, we were unable to investigate pain scores and opioid use. This information warrants inclusion in further studies. Fourth, information on the abdominal approach was not included in the database. Although EDA is generally considered to be used more frequently in laparotomy than in laparoscopy, the EDA group had more favorable short-term outcomes than the non-EDA group. A previous study showed that following thoracoscopic esophagectomy, there were no significant differences in respiratory complications or anastomotic leakage between laparotomy and laparoscopy.56 Finally, the results regarding postoperative length of stay may not be generalizable to other countries because the median length of stay in our study was longer than that in previous studies in other countries.57 The length of stay in Japan is generally relatively long, presumably because of the health care system.58,59
Conclusions
The current study revealed that the use of EDA in MIE with two- or three-field lymph node dissection for esophageal cancer was associated with decreased in-hospital mortality, respiratory complications, and anastomotic leakage, regardless of hospital volume. EDA use was also associated with a shorter length of stay after surgery and lower total hospitalization costs. These findings support the use of EDA in MIE as well as in OE.
References
Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg. 2019;269:291–8.
Kuppusamy MK, Low DE. Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of >6000 patients using ECCG definitions and the online Esodata database. Ann Surg. 2022;275(3):515–25. https://doi.org/10.1097/SLA.0000000000004309.
Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a nationwide retrospective analysis. Ann Surg. 2021;274:324–30.
Yoshida N, Yamamoto H, Baba H, et al. Can minimally invasive esophagectomy replace open esophagectomy for esophageal cancer? Latest analysis of 24,233 esophagectomies from the Japanese National Clinical Database. Ann Surg. 2020;272:118–24.
Findlay JM, Gillies RS, Millo J, Sgromo B, Marshall RE, Maynard ND. Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg. 2014;259:413–31.
Low DE, Allum W, De Manzoni G, et al. Guidelines for perioperative care in esophagectomy: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2019;43:299–330.
Flisberg P, Törnebrandt K, Walther B, Lundberg J. Pain relief after esophagectomy: thoracic epidural analgesia is better than parenteral opioids. J Cardiothorac Vasc Anesth. 2001;15:282–7.
Fares KM, Mohamed SA, Hamza HM, Sayed DM, Hetta DF. Effect of thoracic epidural analgesia on pro-inflammatory cytokines in patients subjected to protective lung ventilation during Ivor Lewis esophagectomy. Pain Physician. 2014;17:305–15.
Wang J, Yin Y, Zhu Y, et al. Thoracic epidural anaesthesia and analgesia ameliorates surgery-induced stress response and postoperative pain in patients undergoing radical oesophagectomy. J Int Med Res. 2019;47:6160–70.
Li Y, Dong H, Tan S, Qian Y, Jin W. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: a single-center, randomized controlled trial. Medicine (Baltimore). 2019;98:e14362.
Li W, Li Y, Huang Q, Ye S, Rong T. Short and long-term outcomes of epidural or intravenous analgesia after esophagectomy: a propensity-matched cohort study. PloS One. 2016;11:e0154380.
Yasunaga H. Real world data in Japan: Chapter II The Diagnosis Procedure Combination Database. Ann Clin Epidemiol. 2019;1:76–9.
Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–82.
Shigemi D, Morishima T, Yamana H, Yasunaga Hideo, Miyashiro Isao. Validity of initial cancer diagnoses in the Diagnosis Procedure Combination data in Japan. Cancer Epidemiol. 2021;74:102016.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 2. Esophagus. 2019;16:25–43.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1–36.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Watanabe M, Tachimori Y, Oyama T, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24.
Savla JJ, Faerber JA, Huang YV, et al. 2-year outcomes after complete or staged procedure for tetralogy of Fallot in neonates. J Am Coll Cardiol. 2019;74:1570–9.
Imai T, Abe T, Uemura N, Yoshida K, Shimizu Y. Immediate extubation after esophagectomy with three-field lymphadenectomy enables early ambulation in patients with thoracic esophageal cancer. Esophagus. 2018;15:165–72.
Toh Y, Oki E, Minami K, Okamura T. Evaluation of the feasibility and safety of immediate extubation after esophagectomy with extended radical three-field lymph node dissection for thoracic esophageal cancers. Esophagus. 2009;6:167–72.
Yasunaga H. Introduction to applied statistics—Chapter 1 Propensity score analysis. Ann Clin Epidemiol. 2020;2:33-37.
Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–7.
Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75.
Aso S, Yasunaga H. Introduction to instrumental variable analysis. Ann Clin Epidemiol. 2020;2:69–74.
Berlth F, Plum PS, Chon SH, Gutschow CA, Bollschweiler E, Hölscher AH. Total minimally invasive esophagectomy for esophageal adenocarcinoma reduces postoperative pain and pneumonia compared to hybrid esophagectomy. Surg Endosc. 2018;32:4957–65.
Tankard KA, Brovman EY, Allen K, Urman RD. The effect of regional anesthesia on outcomes after minimally invasive Ivor Lewis esophagectomy. J Cardiothorac Vasc Anesth. 2020;34:3052–8.
Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18:1460–8.
Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg. 2017;265:1152–7.
Booka E, Takeuchi H, Suda K, et al. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open. 2018;2:276–84.
Pöpping DM, Elia N, Marret E, Remy C, Tramèr MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg. 2008;143:990-999; discussion 1000.
Busweiler LA, Henneman D, Dikken JL, et al. Failure-to-rescue in patients undergoing surgery for esophageal or gastric cancer. Eur J Surg Oncol. 2017;43:1962–9.
Halabi WJ, Kang CY, Nguyen VQ, et al. Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg. 2014;149:130–6.
Holte K, Kehlet H. Epidural analgesia and risk of anastomotic leakage. Reg Anesth Pain Med. 2001;26:111–7.
Wang W, Zhao G, Wu L, Dong Y, Zhang C, Sun L. Risk factors for anastomotic leakage following esophagectomy: Impact of thoracic epidural analgesia. J Surg Oncol. 2017;116:164–71.
Al-Rawi OY, Pennefather SH, Page RD, Dave I, Russell GN. The effect of thoracic epidural bupivacaine and an intravenous adrenaline infusion on gastric tube blood flow during esophagectomy. Anesth Analg. 2008;106:884-887, table of contents.
Fumagalli U, Melis A, Balazova J, Lascari V, Morenghi E, Rosati R. Intra-operative hypotensive episodes may be associated with post-operative esophageal anastomotic leak. Updates Surg. 2016;68:185–90.
Michelet P, D’Journo XB, Roch A, et al. Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest. 2005;128:3461–6.
Michelet P, Roch A, D’Journo XB, et al. Effect of thoracic epidural analgesia on gastric blood flow after oesophagectomy. Acta Anaesthesiol Scand. 2007;51:587–94.
Sala C, García-Granero E, Molina MJ, García JV, Lledo S. Effect of epidural anesthesia on colorectal anastomosis: a tonometric assessment. Dis Colon Rectum. 1997;40:958–61.
Jamjittrong S, Matsuda A, Matsumoto S, et al. Postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: systematic review and meta-analysis. Ann Gastroenterol Surg. 2020;4:64–75.
Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: a meta-analysis. Anesth Analg. 2001;93:853–8.
Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147.
Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2016;101:1281–1288; discussion 1288–1289.
Weksler B, Sullivan JL. Survival after esophagectomy: a propensity-matched study of different surgical approaches. Ann Thorac Surg. 2017;104:1138–46.
Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103.
Yoshioka M, Mori T, Kobayashi H, et al. The efficacy of epidural analgesia after video-assisted thoracoscopic surgery: a randomized control study. Annals Thorac Cardiovasc Surg. 2006;12:313–8.
Taqi A, Hong X, Mistraletti G, Stein B, Charlebois P, Carli F. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, nonaccelerated, perioperative care program. Surg Endosc. 2007;21:247–52.
Hanna MH, Jafari MD, Jafari F, et al. Randomized clinical trial of epidural compared with conventional analgesia after minimally invasive colorectal surgery. J Am Coll Surg. 2017;225:622–30.
Zeltsman M, Dozier J, Vaghjiani RG, et al. Decreasing use of epidural analgesia with increasing minimally invasive lobectomy: impact on postoperative morbidity. Lung Cancer. 2020;139:68–72.
Visser E, Marsman M, van Rossum PSN, et al. Postoperative pain management after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30:1–11.
Kula AO, Riess ML, Ellinas EH. Increasing body mass index predicts increasing difficulty, failure rate, and time to discovery of failure of epidural anesthesia in laboring patients. J Clin Anesth. 2017;37:154–8.
Nozaki I, Mizusawa J, Kato K, et al. Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg Endosc. 2018;32:651–65.
Giwa F, Salami A, Abioye A. Hospital esophagectomy volume and postoperative length of stay: A systematic review and meta-analysis. Am J Surg. 2018;215:155–62.
OECD. Length of hospital stay (indicator). 2022. https://doi.org/10.1787/8dda6b7a-en. Accessed 1 Jun 2022.
Konishi T, Fujiogi M, Michihata N, et al. Impact of body mass index on short-term outcomes after differentiated thyroid cancer surgery: a nationwide inpatient database study in Japan. Eur Thyroid J. 2022;11:e210081.
Acknowledgment
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 20AA2005) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Yuko Kitagawa has received grants from Chugai Pharmaceutical Co. Ltd, Taiho Pharmaceutical Co. Ltd, Yakult Honsha Co. Ltd, Asahi Kasei Pharma Corporation, Otsuka Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd, Tsumura & Co., Kaken Pharmaceutical Co. Ltd, Dainippon Sumitomo Pharma Co. Ltd, EA Pharma Co. Ltd, Eisai Co. Ltd, Otsuka Pharmaceutical Factory Inc., Medicon Inc., Kyowa Hakko Kirin Co. Ltd, Takeda Pharmaceutical Co. Ltd, Toyama Chemical Co. Ltd, Astellas Pharma Inc., Teijin Pharma Limited, Nihon Pharmaceutical Co. Ltd, and Nippon Covidien Inc., as well as lecture fees from Chugai Pharmaceutical Co. Ltd, Taiho Pharmaceutical Co. Ltd, Asahi Kasei Pharma Co. Ltd, Otsuka Pharmaceutical Factory Inc., Ono Pharmaceutical Co. Ltd, Shionogi & Co. Ltd, AstraZeneca K.K., Nippon Covidien Inc., Ethicon Inc., Bristol-Myers Squibb K.K., and Olympus Co. Outside the submitted work. Yuki Hirano, Hidehiro Kaneko, Takaaki Konishi, Hidetaka Itoh, Satoru Matsuda, Hirofumi Kawakubo, Kazuaki Uda, Hiroki Matsui, Kiyohide Fushimi, Hiroyuki Daiko, Osamu Itano, and Hideo Yasunaga have no disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hirano, Y., Kaneko, H., Konishi, T. et al. Short-Term Outcomes of Epidural Analgesia in Minimally Invasive Esophagectomy for Esophageal Cancer: Nationwide Inpatient Data Study in Japan. Ann Surg Oncol 29, 8225–8234 (2022). https://doi.org/10.1245/s10434-022-12346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12346-x