Abstract
Background
Adjuvant chemotherapy (CT) and chemoradiotherapy (CRT) after surgery are necessary to reduce the risk of metastasis and recurrence for resectable gastric cancer (GC) patients. Adjuvant CT and CRT have been proven to significantly improve the prognosis for GC patients, when compared with surgery only. However, it is still unclear whether radiotherapy offers additional survival benefits to advanced gastric cancer (AGC) patients.
Methods
PubMed, Cochrane Library, and Embase databases were systematically searched for eligible studies that compared survival benefits between CRT and CT. The endpoints of this meta-analysis were measured as HR for OS or DFS and 95% CI using fixed- or random-effect models. Additionally, side effects, completed rate, and metastatic risk, were calculated as OR. Subgroup analyses according to clinicopathological factors were presented.
Results
A total of 28 eligible studies involving 20,220 patients were included in our study. Of these, 17 studies evaluated the survival benefits of additional radiotherapy on overall survival (OS) of gastric cancer patients, ten reported the impact of CRT on disease-free survival (DFS), and 26 studies showed long-term survival rate. The pooled results were significant (HR for OS 0.84, 95% CI 0.71–0.99; HR for DFS 0.76, 95% CI 0.66–0.89). The subgroup analysis showed that adjuvant CRT increased OS for patients without preoperative treatment; showed similar nausea/vomiting, but an increased risk of neutropenia; reduced the risk of locoregional recurrence; failed to improve OS for lymph node (LN)-positive GC patients; and significantly improved prognosis for R1-treated patients. Of note, DFS was improved in all the subgroups via decreasing the locoregional recurrence.
Conclusion
Compared with CT, adjuvant CRT can improve survival for advanced gastric cancer patients, with similar nausea/vomiting, but increased risk of neutropenia. Patients without preoperative treatment or with positive surgical margins should be strongly recommended to undergo CRT. Treatment regimens should be carefully decided by doctors based on patients’ tolerance, physical status, and reaction to treatment. Moreover, CRT improves the DFS for patients regardless of subgroups, because it significantly reduced the risk of locoregional recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although the incidence of gastric cancer has declined in recent years due to a reduction in Helicobacter pylori (HP) infection,1 it remains the third leading cause of cancer-related deaths in China.2,3 Most GC patients go to the hospital at an advanced stage because of the atypical symptoms and poor knowledge of GC, which leads to a poor prognosis for a majority of these patients, including those who have undergone radical resection. To reduce the rate of recurrence and improve survival in AGC patients, surgery combined with adjuvant treatments are imperative. In Eastern countries, D2 resection combined with chemotherapy is popular as the standard treatment, while D1 resection and adjuvant chemoradiotherapy is more common in Western countries. D2 resection improved survival by reducing the risk of recurrence and metastasis, but was associated with more serious operative complications and a higher mortality rate.4,5,6 Notably, D2 resection should be performed by experienced surgeons, as inadequate resection is common in developing areas. Thus, salvage treatments are necessary for patients because of their higher risk of lymphatic recurrence.4 When compared with surgery only, perioperative chemotherapy and adjuvant chemoradiotherapy bring benefits to AGC patients, as proven by the MAGIC and INT-0116 trials, respectively.7,8 Whether radiotherapy can offer additional benefits to postoperative AGC patients is still unclear.
Jabo and Han et al. demonstrated that patients who underwent adjuvant CRT had a better prognosis and milder side effects than those with CT.4,9 Some studies and meta-analyses revealed that additional radiotherapy could decrease locoregional recurrence, which was the main cause of poor prognosis for gastric cancer patients, but failed to elicit any superior survival benefits.10,11 Inconsistent conclusions given by these studies were potentially related to different baseline characteristics among the included patients. More than 50% of enrolled patients in the INT-0116 trial underwent a D0 resection, while only fewer than 10% of them received a D2 resection, which has been widely criticized.8 Additionally, more than half of all the patients enrolled in the ARTIST and CRITICS trials were TNM stage I and II, whose earlier tumor stage and lower recurrence rate potentially contributed to them being less likely to benefit from RT.12,13 Given the circumstances, a pooled analysis is necessary.
This updated meta-analysis aims to explore whether the addition of radiotherapy to adjuvant chemotherapy improves the prognosis of GC patients, and a subgroup analysis is provided to identify patients who will derive benefits from CRT.
Methods
Literature Search
In this meta-analysis, a systematic search was conducted using PubMed, Cochrane Library, and Embase databases up to December 2021. The following keywords were used to search the relevant studies: “gastric cancer or stomach neoplasms,” “chemotherapy,” “radiotherapy or chemoradiotherapy or radiochemotherapy.” We primarily collected studies through these combined keywords and then the references listed in the publications were screened to further identify relevant studies. Next, two investigators reviewed the titles and abstracts to evaluate the topic relevance. Finally, full texts of these potentially relevant studies were assessed and screened.
Eligibility Criteria and Quality Assessment
Studies meeting the following criteria were selected in this analysis: (1) patients undergoing gastrectomy and whose histological examination proved carcinoma of gastric lesions were eligible; (2) studies providing clinicopathological factors of patients; (3) studies that compared at least two treatment strategies, including adjuvant chemoradiotherapy and chemotherapy alone; (4) survival outcomes of grouped patients should be presented. Survival outcomes could be reported as long-term survival, or hazard ratio (HR) for overall survival (OS), relapse-free survival (RFS), or disease-free survival (DFS) and 95% confidence intervals (CI).
Studies concerning targeted therapy or immunotherapy or intraperitoneal chemotherapy were excluded, as were conference abstracts, reviews, case reports, studies without useable data, and studies published before 2010.
The Newcastle-Ottawa scale was used to assess the quality of studies,14 and studies were grouped according to their own scores: ≥ 8 were considered high quality; 7–8 were medium quality; less than 7 were low quality.
Statistical Analysis
The endpoints of this meta-analysis were measured as HR for OS or DFS and 95% CI. Considering that the definition of RFS was similar to that of DFS, RFS was regarded as DFS. Statistical heterogeneity for studies was evaluated using the Cochran Q test and I2 statistics, and significant heterogeneity was defined as I2 > 50% or P < 0.01. The pooled HRs and 95% CIs were measured by the fixed-effect or random-effect model according to the heterogeneity. If pooled results show significant heterogeneity, a random-effect model should be used. When it comes to side effects, completed rate, and recurrent risk, ORs calculated from each study were pooled and analyzed in the study. To detect a potential publication bias, we performed visual inspection of funnel plots and Egger’s test. Two-tailed P < 0.05 was considered significant bias, then sensitivity analysis would be used to find the potential outliers and improve the reliability of pooled results. All statistical analyses were performed by clinicians specialized in meta-analysis using Stata SE 15.0 software (Stata Corporation, College Station, TX, USA).
Results
Search Results and Study Characteristics
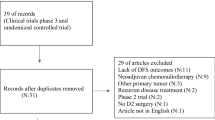
The literature search is shown as a flow diagram in Fig. 1. There were 3145 articles identified initially from various databases using search strategies, and 3076 studies were excluded via scanning the titles or abstracts, and the remaining 69 articles were further evaluated by the full-text view. Of these 69 studies, 21 studies were excluded because of their lack of original data, 5 articles were reviews, 7 were conference abstracts, 5 were secondary studies for existing randomized controlled trials (RCTs), and 3 studies were regarded as low quality. Eventually, a total of 28 studies were included in this meta-analysis, based on the eligibility criteria. Of these, 17 studies evaluated the additional benefit of adjuvant radiotherapy on overall survival (OS),2,4,9,11,12,15,16,17,18,19,20,21,22,23,24,25,26 10 studies offered disease-free survival (DFS), and 26 studies showed long-term survival rate.4,9,12,15,16,17,19,24,25,27
The characteristics of these included studies are presented in Table 1. All the included articles were retrospective cohort studies, and were published between 2010 and 2021, with a range of sample sizes from 61 to 5058. A total of 20,220 patients were included in this analysis. The detailed treatment strategies and sample sizes are summarized in Table 2. Twenty-three studies compared adjuvant CRT with postoperative CT,4,9,11,12,16,17,19,21,22,23,24,25,27,28,29,30,31,32,33,34,35,36,37 5 studies compared adjuvant CRT with perioperative CT,2,15,18,20,26 and chemotherapy regimens were varied among studies, mostly based on 5-FU, S-1, and oxaliplatin. For all studies, chemotherapy was regarded as the reference group, and measured HR for OS or DFS represented the impact of additional radiotherapy on survival outcomes. Survival outcomes of gastric cancer patients are summarized in Supplementary Table S1.
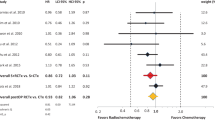
A total of 17 studies evaluated the survival benefits of additional radiotherapy on OS of gastric cancer patients, and the pooled results showed that, compared with chemotherapy alone, adjuvant chemoradiotherapy had better overall survival (HR 0.84, 95% CI 0.71–0.99, random-effect model, Fig. 2a). Ten studies reported the relationship between adjuvant CRT and DFS, compared with CT, and the pooled result remined significant (HR 0.76, 95% CI 0.66–0.89, random-effects model, Fig. 2b). In the subgroup analysis based on chemotherapy, adjuvant chemoradiotherapy increased OS and DFS for patients without preoperative treatment (CRT vs perioperative CT: HR for OS 1.00, 95% CI 0.69–1.47; CRT vs postoperative CT: HR for OS 0.79, 95% CI 0.71–0.99). Thus, CRT can improve survival for postoperative GC patients without preoperative treatment. The funnel plots were a little asymmetrical (Supplementary Figs. S1 and S2), but not significantly so (P = 0.485 and P = 0.671, respectively), which indicated that the pooled results were reliable.
(a) Hazard ratios for overall survival in the 17 eligible independent studies, grouped by preoperative chemotherapy; (b) hazard ratios for disease-free survival in the 10 eligible independent studies, grouped by preoperative chemotherapy. HR: hazard radio; CI: confidence interval; CRT: chemoradiotherapy; CT: chemotherapy
Among the included studies, neutropenia was the most frequent hematological toxicity, and nausea/vomiting was the commonest gastrointestinal adverse effect, and rates ranged from 50%/46% to 87%/93.2% among studies. Pooled results showed that the treatment-completed proportion of CRT among GC patients was not inferior to that of CT (OR 0.87, 95% CI 0.67–1.12, Fig. 3), with similar hematological side effects (OR 1.30, 95% CI 0.59–2.88, Fig. 4a), but increased risk of gastrointestinal ones (OR 1.43, 95% CI 1.01–2.04, Fig. 4b). Next, we further compared the risk of neutropenia and nausea/vomiting, which were the commonest hematological and gastrointestinal toxicities, respectively, between CRT and CT. Results revealed that CRT would increase the risk of neutropenia (OR 1.71, 95% CI 1.40–2.10, Fig. 4c), but presented a similar risk of nausea/vomiting as CT (OR 1.02, 95% CI 0.57–1.83, Fig. 4d). In conclusion, in addition to survival benefits, patients tolerated CRT treatment well, but potentially tended to suffer more serious gastrointestinal side effects and neutropenia.
To the best of our knowledge, poor prognosis of GC patients was caused by high risk of recurrence and metastasis. In this analysis, we found that CRT contributed to reducing the risk of metastasis and recurrence, especially locoregional recurrence (total metastasis: OR 0.64, 95% CI 0.43–0.94, Fig. 5a; locoregional recurrence: OR 0.61, 95% CI 0.44–0.83, Fig. 5b; peritoneal metastasis: OR 0.99, 95% CI 0.81–1.20, Fig. 5c; distant metastasis: OR 0.89, 95% CI 0.75–1.05, Fig. 5d).
According to the ARTIST trial, patients with node-positive disease and intestinal-type GC seemed to benefit more from CRT.12,27 Thus, we performed a subgroup analysis based on ARTIST to detect patients who mostly benefited from adjuvant CRT, and the results showed that additional radiotherapy could not improve OS for LN-positive GC patients (HR for OS 0.83, 95% CI 0.60–1.16, Fig. 6a), potentially resulting from a higher risk of lymphatic and distant metastasis; whereas CRT significantly increased DFS for patients with metastatic LNs, possibly due to its reduction of locoregional recurrence (HR for DFS 0.79, 95% CI 0.69–0.90, Fig. 6b). Lauren type is associated with prognosis of GC patients, and some research has revealed that CRT should be suggested for intestinal-type patients. Pooled results for DFS showed CRT provided benefits for both diffused-type and intestinal-type GC patients (among intestinal-type patients: HR for DFS 0.64, 95% CI 0.49–0.84, Fig. 6c; among diffuse-type patients: HR for DFS 0.82, 95% CI 0.69–0.98, Fig. 6d). OS was not analyzed because of lack of related information. Thus, CRT can improve prognosis for GC patients regardless of Lauren types, and intestinal-type GC patients receive more benefit from CRT than diffuse-type ones. More studies are needed in the future.
Pooled results indicated that patients treated with subtotal resection gained more benefit from CRT (HR for OS 0.60, 95% CI 0.45–0.81, Fig. 7c), while those treated with total resection benefited less from CRT (HR for OS 1.08, 95% CI 0.69–1.71, Fig. 7d). Notably, most distally located tumors were resected partially, not totally, and results demonstrated that CRT improved DFS for distal tumor-located gastric cancer patients (HR for DFS 0.68, 95% CI 0.53–0.87, not shown in this meta-analysis). It can be concluded that patients with tumors located in the distal stomach who are commonly treated with distal resection should be recommended for adjuvant CRT. D2 or R0 resection improved survival for advanced GC patients: this has been proved widely. Whether additional radiotherapy was necessary for patients undergoing D2 or R0 resection was still undetermined. In this meta-analysis, results indicated that CRT significantly improved OS for R1 patients, but not for R0 or D2 patients (R1 patients: HR 0.63, 95% CI 0.52–0.76, Fig. 7a; R0 patients: HR 0.86, 95% CI 0.72–1.03, Fig. 7b; D2 patients: HR 0.80, 95% CI 0.63–1.02, Supplementary Fig. S4a and S4b), possibly because those with radical resection or extensive lymphadenectomy had a relatively lower risk of locoregional recurrence. Of note, DFS was improved for all patients regardless of surgical methods (Supplementary Fig. S3). Consequently, adjuvant CRT should be recommended strongly for patients with positive surgical margins as a salvage treatment to reduce the risk of recurrence and metastasis. Operation selection should be considered for the decision of adjuvant treatment regimens.
To sum up, CRT is safe and well-tolerated by postoperative GC patients, providing survival benefits and reducing the risk of locoregional recurrence. Gastric cancer patients without preoperative treatment or with positive surgical margins should be recommended to undergo CRT.
Discussion
In this meta-analysis, we aimed to explore the necessity of additional radiotherapy for advanced gastric cancer patients. MAGIC and some other studies have confirmed that radical surgery combined with adjuvant chemotherapy improves the prognosis of GC patients.7,38 Furthermore, whether additional radiotherapy provides a survival benefit remains unclear. Different baseline characteristics and selection bias among studies may lead to inconsistent results. A meta-analysis including 8 studies published in 2019 revealed that adjuvant CRT improved DFS via reducing locoregional recurrence, but failed to improve the overall survival.10 This updated meta-analysis included 28 related studies and provided some detailed subgroup analysis to detect patients who will derive benefits from adjuvant CRT, achieving comprehensive and individual treatment for AGC. A total of 20,220 AGC patients from varied countries were included in this study. We found that compared with chemotherapy, adjuvant CRT improved survival for resectable gastric cancer patients, regardless of the OS or DFS. Subgroup analysis demonstrated that CRT significantly improved prognosis for patients without preoperative chemotherapy. Notably, patients who underwent R1 resection would benefit significantly from additional radiotherapy, regardless of preoperative treatment. For patients with positive lymph nodes and those who were treated with perioperative chemotherapy and R0/D2 resection, CRT and CT had similar oncological efficacy, with mild nausea/vomiting but higher risk of neutropenia. Moreover, the DFS was improved in all the subgroups via reducing the risk of metastasis and recurrence. Hence, CRT is an optional treatment strategy for GC patients, and further studies should be conducted on its benefits.
Additional radiotherapy can improve the DFS through reducing the risk of recurrence and metastasis, especially locoregional recurrence, which is consistent with previous studies.10,12,16,29 Studies detected that the risk of locoregional recurrence (LRR) increased with the N stage (5-year LRR for pN1-2 12.3%; pN3 23.4%), and among pN3 patients, LRR was approximately 15–30% in group 2 LNs, while up to 60% in group 3 LNs.16 Wang et al. demonstrated that radiotherapy decreased the recurrence of para-aortic a2 and b1 LNs, which were the most likely regions for locoregional recurrence.35 It can be concluded that LRR is closely related to lymphatic metastasis and tumor stage; thus, providing additional radiotherapy to patients with later N stages is optional, but its effects on group 2 and 3 LNs requires further exploration. Of note, in this study, for patients with positive lymph nodes, CRT had similar oncological efficacy as CT, which was controversial with the aforementioned conclusion, possibly because of the lack of N stage-based subgroup analysis. According to Ma et al., patients with earlier N stages could benefit more from CRT, while CRT failed to improve the prognosis of N3b, due to the fact that the poor survival of N3b patients mostly resulted from higher risk of distant metastasis, rather than locoregional recurrence, which CRT was unable to improve.22 In addition to the N stage, LN ratio (LNR) is also used to identify patients who potentially benefit from CRT. Some studies reported that CRT significantly improved the prognosis for patients with high LN burden (LNR > 25%), but for those whose LNR was more than 50%, CRT seldom offered a survival benefit.9,12,22 Given the lack of information, N stage- and LNR-based subgroup analysis was not performed in this study. Adequate LN assessment in the evaluation of CRT-treated patients is crucial and more studies are needed in the future.
Despite the fact that additional radiotherapy can alleviate the adverse effects related to chemotherapy via adjusting treating regimens, the completed rate of CRT is similar to that of CT, due to its higher risk of grade 3 or 4 gastrointestinal toxicities. In this study, pooled results revealed that patients in the CRT group were 43% more likely to suffer from serious gastrointestinal adverse effects as compared with patients in the CT group, even though most of the included studies just present a tendency.4,22,29,31,32 Gastrointestinal tissues are sensitive to radiotherapy, and easily get infected and edematous, leading to symptoms like diarrhea, vomiting, constipation, loss of appetite, and so on.39 Thus, careful and comprehensive evaluation before treatment is required for patients with a history of gastrointestinal diseases or gastrointestinal adverse reactions.
Neutropenia and thrombocytopenia are common manifestations of acute radiation injury and the leading causes of treatment interruption.40,41 According to this meta-analysis, additional radiotherapy would increase the risk of grade 3/4 neutropenia, compared with CT. It may be caused by myelosuppression related to marrow injury during radiotherapy and the administration of concurrent myelosuppressive chemotherapy drugs. It has been demonstrated in some studies that radiation-related marrow injury is associated with radiation dose and irradiated sites.42 Severe injury only occurs within irradiated marrow, whereas unirradiated marrow is unaffected. Hence, it can be speculated that accurate radiological localization and a reduced volume of irradiated marrow are effective ways to reduce marrow suppression. In addition, the duration required to repopulate the marrow cavity and restore active hematopoiesis would be prolonged with an increase in the radiation dose.42 Moreover, it has been proved that chemoradiotherapy increases the risk of myelosuppression, compared with chemotherapy or radiotherapy alone.40,43,44 In the treatment of GC patients, non-myelosuppressive chemotherapy combined with precise and controlled radiation should be performed by experienced clinicians to reduce neutropenia.
R1 resection, defined as a microscopic tumor-positive resection margin found in postoperative pathological examination, accounts for 2–22% of GC patients and predicts a poorer prognosis.45,46 For patients with R1 resection, a second surgery or adjuvant CRT are suggested. However, a more extensive resection is still under debate because of its higher risk of operative complications.47 Adjuvant CRT provides additional survival benefits via decreasing locoregional recurrence for R1-treated patients whose defects are caused by insufficient resection.12,22,29 In this study, we found that CRT could significantly improve the prognosis for R1-treated patients when compared with CT. However, only three studies were included in this subgroup analysis. Moreover, randomized controlled trials (RCTs) evaluating the impact of CRT for GC patients with positive margins are unlikely to be performed due to basic ethical considerations and treatment needs. Nevertheless, this study demonstrated that additional radiotherapy can considerably and safely improve the prognosis for R1 patients, but in our opinion, prevention of R1 resection is still the main treatment strategy.
Extensive lymphadenectomy is necessary for GC patients. D2 lymphadenectomy, requiring expert skills and widely practiced in Eastern countries, increases survival via reducing recurrence, but is associated with more surgical complications and higher mortality; whereas D1 resection, which is commonly practiced in Western countries, presents a better functional recovery and shorter hospitalization time, but has a higher risk of recurrence and metastasis.4,5,6 In this study, results showed that CRT could not provide additional survival benefits to patients who underwent D2 resections, possibly due to the fact that extensive lymphadenectomy decreased the risk of locoregional recurrence, and that patients’ poor physical status caused treatment delay or discontinuation. According to Wang, radiotherapy should be initiated in a timely way and a 4-month delay was associated with higher LRR and worse prognosis.35 Additionally, in our study, patients who underwent D2 lymphadenectomy presented negative margins as well, thus pooled results for D2 and R0 subgroups were consistent and insignificant. Moreover, Han et al. found that D1CRT is not inferior to D2CT (HR = 0.96, 95% CI = 0.88–1.14), whereas a poorer prognosis was observed when compared with D1CT patients (HR = 1.19, 95% CI 1.01–1.41),4 suggesting that adjuvant chemoradiotherapy provided additional survival benefits for those undergoing imperfect lymphatic resection. However, this study did not analyze the impact of CRT on patients with D1 resection because of limited information.
In clinical practice, surgical options are highly dependent on the location and size of tumors. Further, partial resection is commonly used for the treatment of patients with distal tumors, while total resection is commonly applied when treating patients with proximal and middle tumors. However, there is still a lack of a consensus on the surgical options applicable to CRT: subtotal or total resection? As per a report of Ma et al., CRT could provide more survival benefits for patients receiving subtotal resection, compared with CT (HR for OS 0.57, 95% CI 0.41–0.80); however, it achieved similar effects to CT for patients receiving total resection.22 In contrast, opposite conclusions that CRT can significantly improve OS of GC patients receiving total resection have been drawn by Mansouri et al. (CRT vs CT, 5-year OS for total resection: 64.5% vs 28%, P = 0.016; subtotal resection: 47.4% vs 39%, P = 0.277).32 As was suggested by Ejaz, there was no significant difference between surgical options among patients undergoing CRT.2 According to numerous studies, it should be noted that patients treated with total resection have a poorer prognosis than those with partial resection,24,25,28,36 which may be caused by more severe surgical complications, worse physical status, longer recovery, and delayed adjuvant treatment. In this study, it can be demonstrated that only patients treated with subtotal resection can attain better outcomes from CRT. Further, those patients with tumors located in the distal stomach who are commonly treated with distal resection can attain better outcomes from CRT, which has been revealed in this study. Also, according to the CRITICS trial and some recent studies, compared with chemotherapy, additional radiotherapy would not increase the risk of surgical complications, including anastomotic stenosis, obstruction, perforation, and leakage. As was indicated by CRITICS, patients undergoing perioperative chemotherapy were more likely to suffer from obstruction and perforation. Thus, CRT is a safe and effective therapy for postoperative patients.15 In summary, it can be speculated that those patients with tumors located in the distal stomach who have received subtotal resection are more suitable for CRT, due to their better status, less delay for CRT and, most importantly, higher risks of locoregional recurrence. Except for pathological factors, operation selection should be considered for the decision of adjuvant treatment regimens, and more studies are needed for further exploration.
In this study, results revealed that patients without preoperative treatment would benefit more from CRT, compared with CT. Perioperative treatment for advanced gastric cancer patients becomes more and more popular in Eastern countries according to the evidence from the MAGIC and FNCLCC/FFCD trials.7,48 Preoperative chemotherapy increases the rate of R0 curative resection via tumor shrinkage and downstaging49 and Schuhmacher et al. reported that neoadjuvant chemotherapy (NAC) resulted in a decrease in the number of positive lymph nodes compared with the surgery alone group (76.5% vs 61.4%; P = 0.018).50 The objective of adjuvant chemotherapy is to eradicate the remaining micrometastatic cancer cells and reduce tumor recurrence after curative resection. The current evidence suggested that the combination treatment of NAC plus AC was the optimal strategy for resectable gastric cancer.51 However, recent clinical trials reported that the proportion of NAC-pretreated patients who received adjuvant treatment following the curative resection was relatively small due to poor physical condition and compliance.7,48 In the light of the fact that additional radiotherapy potentially increases the severity of gastrointestinal toxicities and long recovery possibly delays the initiation of CRT and leads to treatment discontinuation, NAC-pretreated patients are less likely to benefit from CRT. Moreover, patients without preoperative chemotherapy show adequate tolerance to adjuvant treatment, and additional radiotherapy can alleviate toxicities caused by drugs to some extent via regulating doses and regimens. Therefore, CRT should be suggested for GC patients without preoperative treatment as a supplementary treatment after surgery to decrease recurrence and improve the prognosis. Patients with a favorable response to NAC are expected to obtain a better survival outcome from curative resection and subsequent adjuvant chemotherapy. Besides, additional radiotherapy is not required due to a lower risk of locoregional recurrence.
Notably, Stumpf et al. have confirmed that NAC-pretreated patients with a positive surgical margin can obtain favorable outcomes from RT,20 which is consistent with the results of this meta-analysis. Hence, once the positive surgical margin is proved in one patient by postoperative pathological examinations, adjuvant CRT should be recommended, regardless of the preoperative treatment of this patient. Therefore, for those patients with advanced diseases, positive surgical margin and intolerance to NAC, it is necessary to perform postoperative additional radiotherapy for them to decrease the rate of locoregional recurrence and prolong DFS. To the best of the authors’ knowledge, AGC patients with favorable physical status and without any acute signs or distal metastases shall undergo NAC, owing to the fact that it can exert positive impacts on R0 resection. In addition, radiotherapy can be considered as a supplementary therapy to NAC. Moreover, CRT is also an effective and safe therapeutic method for GC patients who are intolerant of or unwilling to accept NAC. The comprehensively personalized treatment regimens shall be formulated by experienced doctors for patients with gastric cancer.
Biomarkers, such as HER2 and ERCC1, are of great significance for chemotherapy selection. The overexpression of these biomarkers can predict better efficacy and longer survival. However, their effect on CRT was not confirmed by the studies of Park and Bamias, due to the possible fact that there is a relatively low proportion of HER2 positivity (5% and 7% in Park’s and Bamias’s studies, respectively) and that all patients received similar cisplatin-based chemotherapy, regardless of the expression of HER2.12,17 The effect of CRT on HER2-positive GC patients has not been clarified, and more studies are needed.
This meta-analysis is not free from limitations. Firstly, heterogeneity would be inevitably induced by diverse regimens and different baseline characteristics from the retrospective studies included in this meta-analysis; it was thus not possible to comprehensively detect the clinicopathological factors of patients who derived benefits from adjuvant CRT based on existing studies, but we have provided information and insights for future studies.
Conclusion
In summary, this study demonstrated that compared with CT, CRT can improve survival for resectable advanced gastric cancer patients, with similar nausea/vomiting, but increased risk of neutropenia. Patients without preoperative treatment or with positive margins should be recommended to undergo CRT. Treatment regimens should be carefully decided by doctors based on patients’ toleration, physical status, and reaction to treatment. Moreover, CRT improves the DFS for patients regardless of subgroups, because it significantly reduced local recurrence. More clinical trials are needed to further validate which patients may benefit more from CRT.
References
Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107(3):230–6.
Ejaz A, Spolverato G, Kim Y, et al. Impact of external-beam radiation therapy on outcomes among patients with resected gastric cancer: a multi-institutional analysis. Ann Surg Oncol. 2014;21(11):3412–21.
Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22.
Han J, Nie Z, Li P, et al. Comparison of treatment modalities for locally advanced gastric cancer: A propensity score matching analysis. J Cancer. 2020;11(15):4421–30.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49.
Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011;253(5):900–11.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med. 2006;355(1):11–20.
Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl J Med. 2001;345(10):725–30.
Jabo B, Selleck MJ, Morgan JW, et al. Role of lymph node ratio in selection of adjuvant treatment (chemotherapy vs. chemoradiation) in patients with resected gastric cancer. J Gastrointest Oncol. 2018;9(4):708–17.
Matuschek C, Haussmann J, Bölke E, et al. Adjuvant radiochemotherapy vs. chemotherapy alone in gastric cancer: a meta-analysis. Strahlenther Onkol. 2019;195(8):695–706.
Datta J, McMillan MT, Ecker BL, et al. Implications of lymph node staging on selection of adjuvant therapy for gastric cancer in the united states: a propensity score-matched analysis. Ann Surg. 2016;263(2):298–305.
Park SH, Sohn TS, Lee J, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015;33(28):3130–6.
Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–73.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–28.
Yu JI, Lim DH, Lee J, et al. Necessity of adjuvant concurrent chemo-radiotherapy in D2-resected LN-positive gastric cancer. Radiother Oncol. 2018;129(2):306–12.
Bamias A, Karina M, Papakostas P, et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol. 2010;65(6):1009–21.
Zaidi A, Khan A, Duval C, et al. Comparison of perioperative chemotherapy versus postoperative chemoradiotherapy for operable stomach cancer: a Western Canadian Province experience. Curr Oncol. 2021;28(2):1262–73.
Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104(3):361–6.
Stumpf PK, Amini A, Jones BL, et al. Adjuvant radiotherapy improves overall survival in patients with resected gastric adenocarcinoma: a National Cancer Data Base analysis. Cancer. 2017;123(17):3402–9.
Girardi DM, de Lima MA, Pereira GCB, et al. Chemoradiotherapy versus chemotherapy as adjuvant treatment for localized gastric cancer: a propensity score-matched analysis. BMC Cancer. 2018;18(1):378.
Ma GF, Zhang HG, Liu J, et al. Benefit of adjuvant chemoradiotherapy in patients with pathological stage III gastric cancer. Cancer Manag Res. 2019;11:6029–41.
Stiekema J, Trip AK, Jansen EP, et al. Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an R1 resection? Results from a Dutch cohort study. Ann Surg Oncol. 2015;22(2):581–8.
Zhou ML, Li GC, Yang W, et al. Adjuvant chemoradiotherapy versus adjuvant chemotherapy for R1 resected gastric cancer: a retrospective cohort study. Br J Radiol. 2018;91(1089):20180276.
Zhou ML, Yang W, Wang YQ, et al. Adjuvant chemoradiotherapy versus adjuvant chemotherapy for patients with N3 gastric cancer after D2/R0 resection: a retrospective study based on propensity score analyses. Cancer Manag Res. 2019;11:4855–70.
Fitzgerald TL, Efird JT, Bellamy N, et al. Perioperative chemotherapy versus postoperative chemoradiotherapy in patients with resectable gastric/gastroesophageal junction adenocarcinomas: a survival analysis of 5058 patients. Cancer. 2017;123(15):2909–17.
Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 Trial. Ann Oncol. 2021;32(3):368–74.
Fan M, Li G, Shen L, Zhang H, Liang L, Zhang Z. Identification of patients with lymph node metastasis from gastric cancer who may benefit from adjuvant chemoradiotherapy after D2 dissection—do N3 patients benefit from additional radiation? Br J Radiol. 2016;89(1059):20150758.
Kim TH, Park SR, Ryu KW, et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys. 2012;84(5):e585-592.
Kwon HC, Kim MC, Kim KH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol. 2010;6(4):278–85.
Li Q, Li G, Palmer JD, Zhang Z. Lymph node burden as a predictive factor for selective chemoradiotherapy in patients with locally advanced gastric cancer after a D2 dissection: a retrospective study. Am J Clin Oncol. 2017;40(4):375–80.
Mansouri H, Zemni I, Achouri L, et al. Chemoradiotherapy or chemotherapy as adjuvant treatment for resected gastric cancer: should we use selection criteria? Rep Pract Oncol Radiother. 2021;26(2):266–80.
Peng J, Wei Y, Zhou F, et al. D2-resected stage IIIc gastric cancer patients benefit from adjuvant chemoradiotherapy. Cancer Med. 2016;5(10):2773–80.
Turanli S, Atalay C, Berberoglu U, Gulben K. Adjuvant chemoradiation versus chemotherapy for stage III gastric cancer after surgery with curative intent. J Cancer Rese Ther. 2015;11(2):369–74.
Wang SB, Qi WX, Chen JY, et al. Identification of patients with locally advanced gastric cancer who may benefit from adjuvant chemoradiotherapy after D2 dissection: a propensity score matching analysis. Front Oncol. 2021;11:648978.
Yekedüz E, Doğan İ, Birgi SD, et al. Adjuvant treatment of gastric cancer in the D2 dissection era: a real-life experience from a multicenter retrospective cohort study. Euroasian J Hepato-Gastroenterol. 2021;11(2):51–8.
Yu C, Yu R, Zhu W, Song Y, Li T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol. 2012;138(2):255–9.
Miceli R, Tomasello G, Bregni G, Di Bartolomeo M, Pietrantonio F. Adjuvant chemotherapy for gastric cancer: current evidence and future challenges. World J Gastroenterol. 2014;20(16):4516–25.
Pan YB, Maeda Y, Wilson A, Glynne-Jones R, Vaizey CJ. Late gastrointestinal toxicity after radiotherapy for anal cancer: a systematic literature review. Acta Oncol. 2018;57(11):1427–37.
Prabhu RS, Cassidy RJ, Landry JC. Radiation therapy and neutropenia. Curr Probl Cancer. 2015;39(5):292–6.
Gershkevitsh E, Rosenberg I, Dearnaley DP, Trott KR. Bone marrow doses and leukaemia risk in radiotherapy of prostate cancer. Radiother Oncol. 1999;53(3):189–97.
Mac Manus M, Lamborn K, Khan W, Varghese A, Graef L, Knox S. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood. 1997;89(7):2303–10.
Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–8.
Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348(9034):1049-1054.
Cunningham SC, Kamangar F, Kim MP, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9(5):718–25.
Raziee HR, Cardoso R, Seevaratnam R, et al. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer. 2012;15(Suppl 1):S116-124.
Wang SY, Yeh CN, Lee HL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009;16(10):2738–43.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210–8.
Desiderio J, Chao J, Melstrom L, et al. The 30-year experience—a meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1–14.
Funding
This work was supported by the Natural Science Foundation of Liaoning province (No. 20180530026) and Shenyang Science and Technology Plan (No. 21-173-9-78).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no conflict of interest.
Ethical Approval and Informed Consent
No ethical approval or informed consent was required for this systematic review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Lu, H., Sun, Y., Zhu, Z. et al. Effect of Chemoradiotherapy on the Survival of Resectable Gastric Cancer Patients: A Systematic Review and Meta-Analysis. Ann Surg Oncol 29, 6962–6975 (2022). https://doi.org/10.1245/s10434-022-12005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12005-1