Abstract
Background
The purpose of the current study is to evaluate the efficacy and complications of concurrent chemoradiotherapy (CCRT) for the treatment of gastric cancer patients after D1/D2 surgery.
Methods
Sixty-eight untreated gastric cancer patients (T3/T4 and/or N+) were enrolled. After surgery, they were randomized into two groups: the CCRT group and the single chemotherapy group. Radiotherapy patients were treated according to the Intergroup 0116 guidelines. The chemotherapy consisted of continuously administered 5-fluorouracil (5-FU) and tetrahydrofolic acid (LV). The CCRT began 28 days after the first cycle of chemotherapy, and chemotherapy was given within the first four and last three days during the CCRT period, at a radiation dosage of 45 Gy/25 f, i.e., 1.8 Gy 5 times per week. Two cycles of the same chemotherapy were administrated 1 month after the radiotherapy. Five cycles of 5-FU and LV were applied to CG.
Results
One-, two-, and three-year survival rates were 85.9, 73.4, and 67.7%, respectively, in the CCRT group and 68.0, 50.0, and 44.1%, in the single chemotherapy group (P < 0.05). The corresponding disease-free survival rates were 73.5, 64.7, and 55.8% in the CCRT group and 61.8, 38.2, and 29.4% in the single chemotherapy group (P < 0.05). The major side effects were gastrointestinal reactions and neutrocytopenia. In both the CCRT and single chemotherapy groups, the incidence of these side effects was 73.5% (25/34) and 44.1% (15/34) (P < 0.05) for Grade I and Grade II anorexia, 82.35% (28/34) and 73.5% (25/34) (P > 0.05) for nausea and vomiting, and 70.6% (24/34) and 44.1% (15/34) (P < 0.05) for neutrocytopenia, respectively. The other indices showed no significant differences.
Conclusions
Our findings indicate that CCRT can increase the one-, two-, and three-year total survival rates, as well as the disease-free survival rates of gastric cancer patients (T3/T4 and/or N+) who have been initially treated with surgery. The major adverse reactions were Grade I and Grade II nausea and vomiting, as well as myelosuppression. CCRT is well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past, radiotherapy was generally used in alleviating the symptoms induced by cancers. With the development of radioactive sources, radiology, and treatment methods, radiotherapy is becoming a more integral treatment approach. Postoperative radiotherapy is becoming more important in the treatment of gastric cancer. A milestone study in the treatment of gastric cancer is a phase III trial conducted by Macdonald et al. (2001) (Intergroup0116, INT0116). Based on this study in 2004, the National Comprehensive Cancer Network (NCCN) in the USA adopted concurrent chemoradiotherapy (CCRT) as the standard treatment for gastric cancer patients (T3/T4 and/or N+) who underwent surgery prior to CCRT treatment. However, this recommendation has not been totally accepted in China, mainly because of the small number of patients who met D1/D2 surgery standards in the INT0116 trial, especially because only 10% of patients met the D2 clearance standards (Macdonald et al. 2001). Sixty-eight gastric cancer patients (T3/T4 and/or N+) were enrolled in the present study. They underwent standard D1/D2 clearance surgery from March 2006 to July 2007. A randomized, controlled design was applied to divide the patients into the CCRT and single chemotherapy groups; the former received treatment (as described in INT0116), which consisted of intensity-modulated radiotherapy, and the latter were treated with five cycles of 5-fluorouracil (5-FU) and tetrahydrofolic acid (LV). The current study aims to investigate the significance of postsurgical CCRT for local advanced gastric cancer patients in China.
Materials and methods
General data
The inclusion criteria were as follows: (1) the subjects must agree to participate in the study and sign an informed consent form; (2) men or women who were 18–70 years old; (3) the presence of gastric cancer with a pathological stage T3/T4 and/or N+ gastric adenocarcinoma, as proven through histology; (4) previously untreated and with no prior history of cancer, chemotherapy, or radiotherapy; and (5) laboratory tests at baseline are as follows: hemoglobin (Hb) ≥ 110 g/L, WBC ≥ 3.5 × 109/L, platelet ≥ 100 × 109/L, hepatic and renal function <1.25 times normal upper limit, and blood glucose in normal range (Table 1). This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of the First People’s Hospital in Huai’an (Permit Number: 20060108006). Written informed consent was obtained from all participants.
Treatment method
All the patients received therapy 3–4 weeks after surgery. In the CCRT group, intensity-modulated radiotherapy was applied, and the radiation scope was determined based on the intraoperative situation and the silver-clip labels, as well as the NCCN guidelines. The target areas consisted of the tumor bed, the stroma, and the draining lymph nodes. The therapeutic machine was a Siemens ONCOR Lineal Accelerator, and CMS treatment planning system was used. The radiation limits of sensitive tissues were as follows: 60% < 30 Gy for the liver, <45 Gy for the spinal cord, an average dosage of <10 Gy and the volume treated with 20 Gy < 20% for the kidneys, and 1/3 < 50 Gy for heart. The dosage for the lungs and the left ventricle was reduced as much as possible. The dosage for the target area was 45 Gy/28. All patients underwent chemotherapy that consisted of 425 mg/m2 5-FU and 25 mg/m2 LV for one cycle prior to the concurrent radiotherapy. Chemotherapy was also given within the first 4 days and last 3 days during the chemoradiotherapy period (400 mg/m2 5-FU and 25 mg/m2 LV) and after chemoradiotherapy (two cycles of 425 mg/m2 5-FU and 25 mg/m2 LV). In the single chemotherapy group, 425 mg/m2 5-FU and 25 mg/m2 LV were given for five cycles.
Evaluation of toxic reaction
Acute toxic reactions were evaluated using the criteria described by the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Statistical analysis
Survival time was defined as the duration from definitive diagnosis until death. SPSS 13.0 software was used for data management. The data were compared using a χ2 test. Survival analysis was performed using the Kaplan–Meier method using a log-rank test. P < 0.05 was considered statistically significant.
Results
Treatment state
All patients in the CCRT group completed the treatment plan except for four who discontinued treatment after 2–5 days because of severe adverse reactions during radiotherapy. All patients in the single chemotherapy group continued treatment.
Survival state
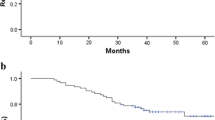
All patients completed the 3-year follow-up period. One-, two-, and three-year survival rates were, 85.9, 73.4, and 67.7% in the CCRT group and 68.0, 50.0, and 44.1% in the single chemotherapy group (χ2 = 4.367, P = 0.037). The corresponding disease-free survival rates were 73.5, 64.7, and 55.8% in the CCRT group and 61.8, 38.2, and 29.4% in the single chemotherapy group (χ2 = 5.297, P = 0.021) (Figs. 1, 2).
Adverse reactions
The major adverse reactions were gastrointestinal reactions and neutrocytopenia. In both the CCRT and the single chemotherapy groups, the incidences were 73.5% (25/34) and 44.1% (15/34) (P < 0.05) for Grade I and Grade II anorexia, 82.35% (28/34) and 73.5% (25/34) (P > 0.05) for nausea and vomiting, and 70.6% (24/34) and 44.1% (15/34) (P < 0.05) for neutrocytopenia, respectively (Table 2).
Discussion
Gastric cancer has the second highest morbidity and mortality rates worldwide. In America, approximately 36,830 new patients are diagnosed with upper gastrointestinal cancer and 25,200 cases died from the disease in 2006 (Jemal et al. 2006). Surgery is the primary treatment for gastric cancer. However, most patients are initially diagnosed with middle and advanced stage cancer, and a single surgery has poor efficacy mainly because of the high local relapse rate after surgery (Roukos and Kappas 2005). As a result, CCRT is, as of 2004, the standard therapy for gastric cancer patients treated with surgery by NCCN, following the results of the phase III clinical trial (INT0116) by Macdonald et al. (Macdonald et al. 2001). Consequently, the proportion of gastric cancer patients who receive CCRT after surgery increased from 6.5 to 13.3% (P < 0.0001) in the USA based on the results of an authoritative survey (Kozak and Moody 2008). A similar trend was also observed in Canada where the proportion increased from 14.6 to 30.4% (Coburn et al. 2008) (P < 0.001). Considering the regional differences in medical stages and the variations in the scope of surgical clearance, this treatment model has become the internationally accepted approach for patients with D0 or positive postoperatively at the surgical margins. However, improvement of the survival rate among patients with D1/D2 clearance standards is controversial. Therefore, a consensus has not been reached on this treatment, especially in China. Consequently, data were collected from 68 gastric cancer patients (T3/T4 and/or N+) who were treated with standard D1/D2 surgery with R0 surgical margins. A randomized controlled design was applied to clarify the significance of CCRT in China.
After treatment, their one-, two-, and three-year survival rates were 85.9, 73.4, and 67.7% in the CCRT group and 68.0, 50.0, and 44.1% in the single chemotherapy group, respectively (P < 0.05). The corresponding disease-free survival rates were, 73.5, 64.7, and 55.8% in the CCRT group and 61.8, 38.2, and 29.4% in the single chemotherapy group, respectively (P < 0.05). We found that more than 50% of patients relapse locally or regionally (Roukos and Kappas 2005; Coburn et al. 2008), and the 5-year overall survival rate does not exceed 40.0%, even after R0 resection surgery therapy. The relapse rate reached up to 60% for tumors, regional lymph nodes, and the stump and stroma of patients of T4/Tx and/or N+, whereas their 5-year survival rate was only around 25% (Hartgrink and van de Velde 2005). Consequently, radiotherapy-supplemented surgery is a reasonable option for reducing the local or regional relapse rate. Korean scholars not only carried out a phase II clinical trial (Lim et al. 2004) similar to INT0116 (all patients received D2 surgery) but also performed a multicentric retrospective research (Kim et al. 2005). In the postoperative CCRT group and the single operation group, the relapse rates in the radiotherapy field were 14.9 and 21.7%, the 5-year relapse-free survival rates were 54.5 and 47.9%, and the 5-year overall survival rates were 57.1 and 51.0%, respectively. The meta-analysis by Francesco (Fiorica et al. 2007) also indicated that the combination of chemotherapy and radiotherapy after surgery greatly reduces the 5-year mortality rate of patients with gastric cancer compared with surgery alone. Although the toxic side effects also increased significantly, the treatment-related mortality did not exhibit a similar trend. There are some similarities and differences between our conclusion and those above, i.e., the current study compared CCRT and postoperative chemotherapy and utilized a randomized controlled study design that specifically highlights the value of radiotherapy.
The use of a large radiation scope after gastric cancer surgery enables the more accurate tracking of damage to sensitive tissue during radiotherapy. Several groups reported (Jansen et al. 2007) that if 20% of one kidney is radiated with more than 20 Gy during radiotherapy, 11 and 52% of patients suffer from renal insufficiency within half a year and within 1 year after radiotherapy, respectively. For the radiotherapy method, the present study utilized intensity-modulated radiotherapy to limit the dose received by the kidneys to an average of less than 10 Gy and a volume of 20 Gy below 20%. Milano et al. (2006) treated seven gastric cancer patients with IMRT and compared the IMRT plan, conventional anterior and posterior field radiation plan, and three-dimensional conformal radiotherapy. IMRT was found to have an advantageous dose distribution in the target area and the protection of high-risk organs. In the current study, the indices relating to liver and kidney injuries in the CCRT group are not significantly different from the single chemotherapy group, which is consistent with the aforementioned conclusion. In the CCRT and single chemotherapy groups, the incidences were 73.5% (25/34) and 44.1% (15/34) (P < 0.05) for Grade I and II anorexia, 82.35% (28/34) and 73.5% (25/34) (P > 0.05) for nausea and vomiting, and 70.6% (24/34) and 44.1% (15/34) (P < 0.05) for neutrocytopenia, respectively. Apparently, Grade I and II anorexia, as well as neutrocytopenia, is significantly more common in the CCRT group than in the single chemotherapy group. These effects were mainly observed in the Grade I and II, which had no effect on the treatment. Four patients (11.8%, 4/34) in the CCRT group discontinued treatment because of adverse reactions; the longest duration of treatment discontinuation was 5 days, and no patient died from the treatment.
The chemotherapy scheme using 5-FU and LV was used in the current study, and it is still recommended by the NCCN guidelines. Even if new chemotherapeutic agents are developed, the combination of capecitabine and oxaliplatin with radiotherapy is used internationally. These new chemotherapy schemes are expected to provide promising results.
In conclusion, CCRT is preferable for gastric cancer patients (T3/T4 and/or N+) who have undergone standard D1 or D2 surgery. For the advanced intensity-modulated radiotherapy in the present paper, the side effects can be tolerated and the dose received by sensitive tissue such as the kidneys can be controlled.
References
Coburn NG, Guller U, Baxter NN, Kiss A, Ringash J, Swallow CJ, Law CH (2008) Adjuvant therapy for resected gastric cancer–rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys 70:1073–1080
Fiorica F, Cartei F, Enea M, Licata A, Cabibbo G, Carau B, Liboni A, Ursino S, Camma C (2007) The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev 33:729–740
Hartgrink HH, van de Velde CJ (2005) Status of extended lymph node dissection: locoregional control is the only way to survive gastric cancer. J Surg Oncol 90:153–165
Jansen EP, Saunders MP, Boot H, Oppedijk V, Dubbelman R, Porritt B, Cats A, Stroom J, Valdes OR, Bartelink H, Verheij M (2007) Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 67:781–785
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics. CA Cancer J Clin 56:106–130
Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO, Kim WS, Jung CW, Park YS, Im YH, Sohn TS, Noh JH, Heo JS, Kim YI, Park CK, Park K (2005) An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 63:1279–1285
Kozak KR, Moody JS (2008) The survival impact of the intergroup 0116 trial on patients with gastric cancer. Int J Radiat Oncol Biol Phys 72:517–521
Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, Kim S, Noh JH, Joh JW, Choi SH, Sohn TS, HeoJS Park CH, Park JO, Lee JE, Park YJ, Nam HR, Park W, Ahn YC, Huh SJ (2004) Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist’s view. Br J Cancer 91:11–17
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Milano MT, Garofalo MC, Chmura SJ, Farrey K, Rash C, Heimann R, Jani AB (2006) Intensity-modulated radiation therapy in the treatment of gastric cancer: early clinical outcome and dosimetric comparison with conventional techniques. Br J Radiol 79:497–503
Roukos DH, Kappas AM (2005) Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol 2:98–107
Acknowledgments
We would like to thank the staff in the department of Gastroenterology, CT Medical Imaging, Medical Record, and Tumor Radiotherapy for their assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, C., Yu, R., Zhu, W. et al. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol 138, 255–259 (2012). https://doi.org/10.1007/s00432-011-1085-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1085-y