Abstract
Introduction
Liver steatosis (LS) has been increasingly described in preoperative imaging of patients undergoing pancreaticoduodenectomy (PD). The aim of this study was to assess the impact of preoperative LS on complications after PD and identify possible contributors to LS development in this specific cohort.
Methods
Pancreatic head adenocarcinoma (PDAC) patients scheduled for PD, with preoperative CT-imaging available were included in the study. LS was defined as mean liver density lower than 45 Hounsfield units. Patients showing preoperative LS were matched for patient age, gender, BMI, ASA score, neoadjuvant treatment, and vascular and multivisceral resections, based on propensity scores in a 1:2 ratio to patients with no LS. The primary outcome was postoperative complication severity at 90 days as measured by the comprehensive complication index (CCI)
Results
Overall, 247 patients were included in the study. Forty-three (17%) patients presented with LS at preoperative CT-scan. After matching, the LS group included 37 patients, whereas the non-LS group had 74 patients. LS patients had a higher mean (SD) CCI, 29.7 (24.5) versus 19.5 (22.5), p = 0.035, and a longer length of hospital stay, median [IQR] 12 [8–26] versus 8 [7–13] days, p = 0.006 compared with non-LS patients. On multivariate analysis, variables independently associated with CCI were: LS (16% increase, p = 0.048), male sex (19% increase, p = 0.030), ASA score ≥ 3 (26% increase, p = 0.002), fistula risk score (FRS) (28% increase for each point of FRS, p = 0.001) and vascular resection (20% increase, p = 0.019).
Conclusion
Preliminary evidence suggests that preoperative LS assessed by CT-scan influences complication severity in patients undergoing PD for PDAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pancreaticoduodenectomy (PD) is the surgical treatment of choice for pancreatic head adenocarcinoma (PDAC). PD is a challenging procedure that still carries a considerable risk of severe postoperative morbidity, mostly related to the occurrence of postoperative pancreatic fistulas (POPF).1,2 Several authors have proposed preoperative risk stratification models mostly focusing on patient comorbidities and physical function.3,4
Obesity, defined as an increased body mass index (BMI), represents a well-known risk factor for post-pancreatectomy complications including surgical site infections (SSIs) and POPF.5 In fact, the recently updated fistula risk score (FRS), namely the alternative-FRS (a-FRS), added increased BMI as a poor prognostic index.6 The association of obesity with postoperative morbidity is multifactorial, owing to: obesity-related comorbidities such as diabetes and cardiovascular disease,7 increased tissue trauma and blood loss during surgical dissection, and other postoperative factors including gastrointestinal hormone and endocrine dysregulation.
Liver steatosis (LS) is a common finding associated with obesity and the metabolic syndrome, and its incidence is rising in surgical oncology patients, also due to an increased use of preoperative multi-drug chemotherapy.8 There is convincing evidence showing that LS carries a higher risk of postoperative morbidity following hepatic resection,9,10 but this has been rarely studied in the context of pancreatic surgery.
During the last decade, preoperative computed tomography (CT) imaging has proven useful in predicting the occurrence of postoperative morbidity after PD; non dilated pancreatic duct, increased pancreatic parenchymal fat, and high intra-abdominal visceral adiposity have all been identified as preoperative radiological markers of POPF susceptibility.11,12,13,14,15,16 X-ray attenuation can also unveil the presence of LS, but only a single study described correlations between liver steatosis and short-term outcomes after PD.17 The aim of the present study was to determine the extent to which preoperative CT-assessed LS impacts on the overall complication burden following PD, and also identify factors contributing to LS development in this specific cohort.
Patients and Methods
Study Design
This single-center retrospective cohort study was conducted following the Strengthening for the Reporting of Observational Studies in Epidemiology Statement (STROBE) guidelines18 and in accordance with the Declaration of Helsinki. A formal ethical committee approval was waived due to the retrospective nature of the study, according to our institutional policy.
All adult patients (i.e., ≥ 18 years) who underwent PD for PDAC with curative intent at San Raffaele Hospital in Milan, Italy, from January 2016 to December 2020, were screened for inclusion in the study. Inclusion criteria were age, diagnosis of suspected PDAC of the pancreatic head, CT scan performed within 30 days before index surgery available for radiological review, and absence of distant metastasis at preoperative imaging. Exclusion criteria included intraoperative evidence of metastatic or locally unresectable disease, and surgery performed other than PD.
Radiological Workup
Preoperative imaging was retrieved from the digital storage system and provided for radiological review. All CT examinations were performed on 64-row multidetector CT scanners (scanner 1: SOMATOM Definition Flash Dual Source CT, Siemens Healthcare; scanner 2: BRILLIANCE, Philips medical system). CT protocol included administration of intravenous non-ionic iodine contrast medium [Iopromide, Ultravist 370 mg iodine/ml (Bayer HealthCare), 120 ml at a rate of 4 ml/s] and consisted of a multiphase acquisition (unenhanced, arterial and portal venous axial scans of the abdomen). In patients who underwent multiple preoperative CT scans within 30 days before index surgery, the most recent examination was used for review. CT scans were systematically reviewed by two independent senior radiologists to assess the presence of eventual LS by sampling three standardized regions of interest (liver segments V, VI, and VIII, respectively) (Fig. 1) on unenhanced scans. LS was defined as mean liver density lower than 45 Hounsfield units according to previous research.19
Axial unenhanced CT scans of the same patient with pancreatic adenocarcinoma before (a) and after (b, c and d) 3 months of neoadjuvant mFOLFIRINOX, demonstrating a dramatic change in the liver fat content, with appearance of severe steatosis (mean Hounsfield units: 8.1 vs baseline value of 58.8). Liver steatosis was assessed by sampling Hounsfield units of the regions of interest on liver segments VII (c) and segments V and VI (d), as indicated by the red dashed circles
Surgical Procedure and Perioperative Care
All procedures were carried out by experienced surgeons with a high volume in pancreatic surgery.20 Indications for surgery were discussed and approved for every patient in a PDAC dedicated multidisciplinary tumor board. The standard approach for PD was a pylorus-preserving procedure with standard lymphadenectomy. A two-layer, end-to-side, duct-to-mucosa, pancreatico-jejunal anastomosis, a single-layer interrupted suture end-to-side hepatico-jejunostomy, and a single-layer interrupted suture end-to-side duodeno-jejunostomy were carried out on the same jejunal loop. Two drains were usually placed, in proximity of the biliary and the pancreatic anastomoses. All patients were treated according to an enhanced recovery after surgery pathway as described in previous publications.21,22 An early drain removal (within POD 3) policy was followed according to drain fluid amylase value.
Outcome Measures
The primary outcome was 90-day postoperative complication severity measured by the comprehensive complication index (CCI),23 which is a validated measure based on the Dindo-Clavien classification,24 that considers both complication number and severity, generating a single score ranging from 0 (no complications) to 100 (death). Secondary outcomes included specific postoperative complications at 90 days after surgery and length of hospital stay (LOS).
Postoperative pancreatic fistula (POPF),25 delayed gastric emptying (DGE)26 and post-pancreatectomy hemorrhage (PPH)27 were defined and graded according to International Study Group of Pancreatic Surgery (ISGPS) classification. In particular, grades B and C POPF were considered as clinically relevant (CR-POPF). Surgical site infections (SSIs) were classified as superficial incisional, deep incisional, or organ–space according to the definition by the Center for Disease Control and Prevention.28
Clinical data were collected from the Division of Pancreatic Surgery prospectively maintained registry, which was queried for demographic data, perioperative information, and complications. For patients who underwent neoadjuvant treatment, chemotherapy regimen data were also collected.
Statistical Analysis
Continuous variables are presented as medians and interquartile range (IQR) and were compared using a Mann-Whitney U test. Categorical variables are reported as frequencies and percentages and were compared by Pearson chi-square test.
To mitigate the possible effect of confounding variables affecting the onset of LS and postoperative complications, a 2:1 propensity score matching was performed. Nearest-neighbor modality was adopted for matching, with a calliper of 0.05. Matching criteria included age, gender, BMI, ASA score, neoadjuvant treatment, and vascular and multivisceral resections. Multivariate linear regression analysis for log-transformed CCI was performed in the matched cohort, to assess the independent association of perioperative factors on postoperative complications.
A subgroup analysis including patients treated by neoadjuvant chemotherapy, whose CT scan imaging before starting chemotherapy was available in the institutional radiological storage system, was conducted to elucidate the effect of neoadjuvant chemotherapy on preoperative LS. Both pre-chemotherapy and preoperative CT-scan imaging underwent radiological processing to assess the presence of LS following the same methodology previously described. Radiologists were blinded to the type of neoadjuvant regimen and preoperative LS status.
A p-value < 0.05 was considered to be statistically significant. All the analyses were conducted using SPSS version 25 (SPSS, Inc., Chicago, Il).
Results
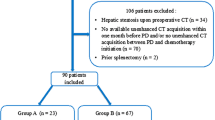
Figure 2 shows the study flow chart. Overall, 523 patients underwent PD for PDAC between January 2016 and December 2019. Of these, 247 patients with available imaging performed within 30 days before the index surgery were included in the study, and CT-scans underwent radiological review. Clinical characteristics of patients included and excluded from the study are summarized in Supplementary Table 1.
In the complete study cohort, 43 (17.4%) patients presented with LS at preoperative CT scan imaging while 204 patients (82.6%) did not show LS (Table 1). Compared with no LS patients, those with LS showed increased BMI (BMI ≥ 25 kg/m2 58.1% of patients with LS versus 33.8% in those without LS; p = 0.003) and treatment with neoadjuvant chemotherapy, with an LS incidence of 69.8% (n = 30) in the neoadjuvant group versus 30.2% (n = 13) in the upfront surgery group (p = 0.001). No significant between-group difference was found for pancreatic stump characteristics or intraoperative blood loss.
The multivariate analysis for preoperative predictors of LS is reported in Supplementary Table 2. In the final model, only BMI ≥ 25 kg/m2 (OR 2.794, 95%CI 1.33–5.87, p = 0.007) and previous treatment with neoadjuvant chemotherapy (OR 3.092, 95%CI 1.40–6.82, p = 0.005) were independent risk factors for preoperative radiological LS.
A log-rank analysis was performed in order to analyze the impact of preoperative LS on oncological outcomes (Supplementary Fig. 1). Median disease-free survival (DFS) was 20 months for patients without preoperative LS compared with 14 months of those with preoperative LS (p = 0.147).
Propensity Score Matching
After matching, the LS group included 37 patients while the non-steatosis group included 74 patients. Variables included in the propensity model included age, gender, BMI, ASA score, neoadjuvant treatment, and vascular and multivisceral resections. Absolute standardized differences in means before and after PSM and R-graphs for absolute standardized balance of different covariates before and after matching are reported in Supplementary Figs. 2 and 3. After matching, the two cohorts were homogeneous, as shown in Table 1.
Table 2 shows outcomes for LS and non-LS patients in the matched cohort. Patients with preoperative LS had a longer LOS [median 12 (8–26) vs. 8 (7–13), p = 0.006] and a higher mean CCI (29.7 ± 24.9 vs. 19.5 ± 22.5, p = 0.035) compared with the non-LS group. A higher rate of CR-POPF was observed in LS patients compared with those without LS (32.4% vs 18.9%); despite this trend, the result did not reach statistical significance (p = 0.113). Moreover, PPH and DGE occurred more frequently in LS patients (13.5% vs. 4.1%, p = 0.069 and 29.7% vs. 10.8%, p = 0.013, respectively). Conversely, no difference was observed in SSI (35.1% in LS group vs. 28.4% in non-LS group, p = 0.467) and overall infectious complications (43% in LS patients vs. 39.2% in non-LS ones, p = 0.682).
Regression Analysis for CCI
On univariate linear regression analysis for factors associated with CCI at 90 days after surgery (Table 3), only male gender, a low preoperative physical status (ASA > 2), vascular resection, FRS, and LS were retained in the final model. These results were confirmed with multivariate analysis. In particular, preoperative LS was as an independent risk factor, resulting in a 16% (95% CI 0.02–1.17, p = 0.048) increase in CCI, together with male gender (18.9% increase, 95% CI 0.06–1.20, p = 0.030), ASA score ≥ 3 (26.2% increase, 95% CI 0.33–1.50, p = 0.002), FRS (28.3% increase for each point of FRS, 95% CI 0.09–0.38, p = 0.001) and vascular resection (20% increase, 95% CI 0.14–1.50, p = 0.002).
Subgroup Analysis in Neoadjuvant Chemotherapy Patients
A subgroup analysis was performed in 47 patients treated by neoadjuvant chemotherapy (Table 4). In this cohort, only 5 patients (10.6%) presented with radiological LS before initiation of chemotherapy, while 18 patients (38.3%) showed radiological LS at the end of chemotherapy, before surgery (p = 0.042). In patients who underwent neoadjuvant chemotherapy, no factor was found to be associated with the development of LS. In particular, the rate of LS was not significantly different between patients treated with a chemotherapy regimen including oxaliplatin and/or irinotecan and those who were not (p = 0.435). Among 18 patients with LS, 12 (66.7%) were overweight (BMI > 25 kg/m2) versus 12 out of 29 (41.4%) patients in the non-LS group (p = 0.092).
Discussion
The present study performed in a consecutive cohort of PDAC patients with a high prevalence of preoperative neoadjuvant chemotherapy showed that liver steatosis, assessed by preoperative CT-scan, affects about 17% of patients scheduled for PD. The preoperative assessment of LS was particularly relevant since these patients experienced an increased rate of major complications including PPH and DGE, resulting in a higher complication burden as measured by CCI. Notably, obesity and neoadjuvant chemotherapy were the only patient factors significantly associated with LS.
The most common form of LS is non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, with an estimated global prevalence of 25%,29 which is expected to further increase during the next decade. In cancer patients, LS is not only related to obesity and other metabolic risk factors, but can also occur as a feature of chemotherapy drug-induced liver injury.30 The pathological mechanisms underlying the development of hepatotoxicity from cytotoxic drugs have been extensively studied. The pattern of chemotherapy-induced liver injury appears to be specific to the type of drugs used, as shown in a meta-analysis on chemotherapy-associated liver injury in patients with colorectal liver metastases.31 Specifically, 5-FU, taxol, and irinotecan are involved in the development of chemotherapy-associated steatohepatitis, a pattern similar to NAFLD.32 Platinum-based regimens instead lead to another form of liver damage, mainly caused by sinusoid injury and venous congestion. This issue has several implications for PDAC neoadjuvant treatments, as they are commonly based on different combinations of the above-mentioned antineoplastic drugs.
In the present series, neoadjuvant chemotherapy was one of the most important risk factors for developing LS at preoperative imaging. Overall, more than a quarter of patients (27%) treated with neoadjuvant chemotherapy presented with LS at the time of surgery, while less than 10% of patients scheduled for upfront surgery had LS. In a subgroup analysis of patients treated with neoadjuvant chemotherapy, our data revealed that most of them (70%) had no evidence of LS before chemotherapy, indicating that it developed during treatment. In a recent series, Flick and colleagues included 139 patients who underwent neoadjuvant treatment for PDAC and found a similar incidence of LS in their cohort (31%).17 It should be noted that most patients were treated with mFOLFIRINOX with a median of 2 months of chemotherapy, whereas in our study all patients received a long course chemotherapy (i.e., at least 4 months) and only half of them received mFOLFIRINOX or a platinum-based regimen. Interestingly, in our series we did not find a specific drug combination with increased likelihood of LS onset compared with others.
The impact of LS on postoperative complications has been investigated mainly in the setting of liver surgery. In a 2014–2018 ACS NSQIP database analysis on about 3000 patients receiving a major hepatic resection, Fagenson et al. found that fatty liver disease and metabolic syndrome increased the risk of severe morbidity, organ space SSIs, and pulmonary complications.10 This can be partially attributable to a more difficult intraoperative management of fatty liver parenchyma, which leads to increased blood loss and the need for perioperative blood transfusions.33 Additionally, it is very likely that LS patients also carry more comorbidities that are associated with higher postoperative morbidity.
In the present study, LS had a significant impact on postoperative outcomes, increasing complications severity as measured by CCI and LOS. The difference in CCI between the matched cohorts exceeded 10 points, which is considered to be clinically significant because it reflects the differential burden of at least one grade 1 complication in the Clavien-Dindo classification.23 Our results are partially consistent with a recent retrospective study by Flick et al., which found that LS resulted in a longer LOS after PD, but it did not significantly affect morbidity or mortality.17 In our series, LS was also associated with specific pancreatic surgery complications. In the matched cohort, although not statistically significant, patients with LS showed an almost twofold POPF rate compared with the non-LS group. It has been repeatedly shown that a soft, “fatty” pancreas is a risk factor for POPF development;34,35 whether any association between pancreatic texture and radiological liver steatosis exists remains debatable. Our results confirmed that soft pancreatic texture and small duct diameter (included in the FRS) are associated with increased CCI but we did not find a higher prevalence of soft texture pancreas among patients with radiological hepatic steatosis.36 Nonetheless, as LS is a highly specific macroscopic hallmark of subtle metabolic syndrome and sub-clinical systemic inflammation,37,38 which is believed to represent a major contributor to morbidity following PD, we can hypothesize that it may explain our finding of increased POPF occurrence in LS patients.
In our matched cohort analysis, PPH had a threefold incidence in patients with LS compared with non-hepatic steatosis patients. As most bleeding events after PD are related to POPF, it is very likely that a higher PPH rate in the LS group can be linked to more clinically relevant POPF in the LS group. Despite controlling for BMI, it is also likely that more patients in the LS group presented with increased visceral fat which is associated with an increased abdominal tissue fragility, also resulting from the presence of subtle inflammation. Additionally, Taipale and colleagues39 hypothesized that accumulated fatty acids and large droplets of triglycerides in liver cells trigger an inflammatory response that might result in coagulation imbalances (due to monocyte and platelet interactions). The significant association found between LS and postoperative DGE may represent the result of a generally more complicated postoperative course rather than a complication-specific association. Interestingly, despite what was previously reported in liver and colorectal surgery,40 LS was not associated with increased infectious complications after PD.
Taken together, our postoperative outcome data highlight the higher burden of complications affecting patients with LS compared with non-LS patients, and suggest that LS could represent a potential target for prehabilitation strategies prior to PD. Hepatic steatosis clearance, through a hypo-caloric, hyper-proteic diet, to promote liver shrinkage, has become standard practice before bariatric surgery.41 Living liver donors can also be managed preoperatively with a calorie-controlled diet, exercise, or drugs to improve hepatic parenchymal quality. However, conditioning cancer patients, who may have developed LS as a result of chemotherapy toxicity appears more challenging because of time restraints (i.e., risk of tumor progression while waiting for surgery) and few therapeutic options besides lifestyle and dietary interventions.42 Molecules such as liraglutide, pioglitazone, and ω-3 fatty acids have all been proposed for treating steatosis, but they require at least 4 months to obtain significant results.43 In a recent bi-institutional, surgeon-blinded, randomized prospective trial involving 60 overweight patients (BMI ≥ 25 kg/m2) undergoing liver resection for cancer, a preoperative 1-week hypocaloric low-fat diet (800 kcal/day; 20 g fat, 70 g protein) reduced intraoperative blood loss by almost half and the liver was deemed easier to mobilize and manipulate by operating surgeons.44 These outcomes appear less relevant for pancreatic surgery, as the liver is not the target organ for surgical dissection, and intraoperative blood loss appears unrelated to LS. However, a multimodal prehabilitation including physical and nutritional interventions may prove beneficial not only in decreasing LS preoperatively, but also in improving patient functional capacity and reducing systemic inflammation, which can have significant implications on the individual risk for postoperative complications.45
An exploratory analysis of our data suggests a possible association between LS and cancer survival outcomes. LS could potentially impact on tumor recurrence for many reasons. First, poor surgical outcomes and the higher rate of complications in LS patients could lead to a prolonged recovery, which could prevent or delay the delivery of adjuvant treatments. Second, LS could lead to lower tolerability to chemotherapy regimens used in adjuvant settings or in case of disease recurrence. Finally, the supposed pro-inflammatory status may be correlated with increased liver steatosis and visceral obesity, which is a potential risk factor for tumor recurrence. Further studies focused on this research hypothesis are needed.
The present study has several limitations, the main being its retrospective nature and the limited sample size, mostly secondary to a significant amount of missing preoperative CT scan imaging. However, the characteristics of included and excluded patients were similar. Moreover, no data were available regarding dose-reduction during chemotherapy or time from the end of treatment to surgery, which could have given extra information on the impact of neoadjuvant chemotherapy on the development of LS. Another potential limitation was the use of CT scan imaging to assess LS. CT is the gold standard imaging for PDAC staging and re-staging, but the standard for liver parenchymal composition is liver biopsy, which can provide both qualitative and quantitative analysis of liver parenchymal composition; however, it is an invasive procedure that cannot be considered as a possible screening exam. Conversely, CT-scan represents a safe, non-invasive tool for assessing LS before PD for PDAC. The high reproducibility of the technique and the adoption of propensity score matching to eliminate possible confounding factors on outcomes are among the strengths of this study.
Conclusions
In this retrospective single institution study, our results suggest that preoperative liver steatosis assessed by CT scan image analysis increases postoperative complication severity in patients undergoing pancreaticoduodenectomy for PDAC. The increasing indication for neoadjuvant chemotherapy in localized pancreatic cancer and the risk of developing chemotherapy related liver injury warrants the need for further larger prospective studies to confirm our results and investigate possible prehabilitation strategies to mitigate LS impact on postoperative outcomes.
References
Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: Where should we focus our efforts on improving outcomes? JAMA Surg. 2014. https://doi.org/10.1001/JAMASURG.2014.151.
Palumbo D, Tamburrino D, Partelli S, et al. Before sentinel bleeding: early prediction of postpancreatectomy hemorrhage (PPH) with a CT-based scoring system. Eur Radiol. 2021. https://doi.org/10.1007/S00330-021-07788-Y.
Uzunoglu FG, Reeh M, Vettorazzi E, et al. Preoperative Pancreatic Resection (PREPARE) score: a prospective multicenter-based morbidity risk score. Ann Surg. 2014. https://doi.org/10.1097/SLA.0000000000000946.
Junejo MA, Mason JM, Sheen AJ, et al. Cardiopulmonary exercise testing for preoperative risk assessment before pancreaticoduodenectomy for cancer. Ann Surg Oncol. 2014. https://doi.org/10.1245/S10434-014-3493-0.
Lovasik BP, Kron P, Clavien PA, Petrowsky H, Kooby DA. Pancreatectomy and body mass index: an international evaluation of cumulative postoperative complications using the comprehensive complications index. HPB (Oxford). 2019. https://doi.org/10.1016/J.HPB.2019.04.006.
Mungroop TH, Van Rijssen LB, Van Klaveren D, et al. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000002620.
Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010. https://doi.org/10.1245/S10434-009-0789-6.
Gangi A, Lu SC. Chemotherapy-associated liver injury in colorectal cancer. Therap Adv Gastroenterol. 2020. https://doi.org/10.1177/1756284820924194.
De Meijer VE, Kalish BT, Puder M, IJzermans JNM. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010. https://doi.org/10.1002/BJS.7194.
Fagenson AM, Pitt HA, Moten AS, Karhadkar SS, Di Carlo A, Lau KN. Fatty liver: The metabolic syndrome increases major hepatectomy mortality. Surgery. 2021. https://doi.org/10.1016/J.SURG.2020.11.021.
Hashimoto Y, Sclabas GM, Takahashi N, et al. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. J Gastrointest Surg. 2011. https://doi.org/10.1007/S11605-011-1687-3.
Ohgi K, Okamura Y, Sugiura T, et al. Pancreatic attenuation on computed tomography predicts pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford). 2020. https://doi.org/10.1016/J.HPB.2019.05.008.
Kirihara Y, Takahashi N, Hashimoto Y, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: The use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. 2013. https://doi.org/10.1097/SLA.0b013e31827827d0.
Jang M, Park HW, Huh J, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol. 2019. https://doi.org/10.1007/S00330-018-5790-7.
Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016. https://doi.org/10.1002/BJS.10063.
Xingjun G, Feng Z, Meiwen Y, et al. A score model based on pancreatic steatosis and fibrosis and pancreatic duct diameter to predict postoperative pancreatic fistula after pancreatoduodenectomy. BMC Surg. 2019. https://doi.org/10.1186/S12893-019-0534-4.
Flick KF, Al-Temimi MH, Maatman TK, et al. Hepatic steatosis after neoadjuvant chemotherapy for pancreatic cancer: incidence and implications for outcomes after pancreatoduodenectomy. J Gastrointest Surg. 2020. https://doi.org/10.1007/S11605-020-04723-2.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014. https://doi.org/10.1016/j.ijsu.2014.07.013.
Graffy PM, Pickhardt PJ. Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol. 2016. https://doi.org/10.1259/BJR.20151024.
Balzano G, Guarneri G, Pecorelli N, et al. Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg. 2020. https://doi.org/10.1002/bjs.11716.
Pecorelli N, Nobile S, Partelli S, et al. Enhanced recovery pathways in pancreatic surgery: State of the art. World J Gastroenterol. 2016. https://doi.org/10.3748/wjg.v22.i28.6456.
Braga M, Pecorelli N, Ariotti R, et al. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg. 2014. https://doi.org/10.1007/s00268-014-2653-5.
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013. https://doi.org/10.1097/SLA.0b013e318296c732.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surg (United States). 2017. https://doi.org/10.1016/j.surg.2016.11.014.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007. https://doi.org/10.1016/j.surg.2007.05.005.
Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007. https://doi.org/10.1016/j.surg.2007.02.001.
Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017. https://doi.org/10.1001/JAMASURG.2017.0904.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016. https://doi.org/10.1002/HEP.28431.
Dash A, Figler RA, Sanyal AJ, Wamhoff BR. Drug-induced steatohepatitis. Expert Opin Drug Metab Toxicol. 2017. https://doi.org/10.1080/17425255.2017.1246534.
Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012. https://doi.org/10.1245/S10434-012-2438-8.
White MA, Fong Y, Singh G. Chemotherapy-associated hepatotoxicities. Surg Clin North Am. 2016. https://doi.org/10.1016/J.SUC.2015.11.005.
McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007. https://doi.org/10.1097/01.SLA.0000251747.80025.B7.
Zhou L, Xiao W, Li C, Gao Y, Gong W, Lu G. Impact of fatty pancreas on postoperative pancreatic fistulae: a meta-analysis. Front Oncol. 2021. https://doi.org/10.3389/FONC.2021.622282.
Partelli S, Andreasi V, Schiavo Lena M, et al. The role of acinar content at pancreatic resection margin in the development of postoperative pancreatic fistula and acute pancreatitis after pancreaticoduodenectomy. Surgery. 2021. https://doi.org/10.1016/j.surg.2021.03.047.
Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007. https://doi.org/10.1097/SLA.0B013E31814A6906.
Den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004. https://doi.org/10.1161/01.ATV.0000116217.57583.6E.
Pickhardt PJ, Jee Y, O’Connor SD, Del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. Am J Roentgenol. 2012. https://doi.org/10.2214/AJR.11.7361.
Taipale T, Seppälä I, Raitoharju E, et al. Fatty liver is associated with blood pathways of inflammatory response, immune system activation and prothrombotic state in Young Finns Study. Sci Rep. 2018. https://doi.org/10.1038/S41598-018-28563-Y.
Kurmann A, Wanner B, Martens F, et al. Hepatic steatosis is associated with surgical-site infection after hepatic and colorectal surgery. Surgery. 2014. https://doi.org/10.1016/J.SURG.2014.02.020.
Romeijn MM, Kolen AM, Holthuijsen DDB, et al. Effectiveness of a low-calorie diet for liver volume reduction prior to bariatric surgery: a systematic review. Obes Surg. 2021. https://doi.org/10.1007/S11695-020-05070-6.
Doherty DT, Coe PO, Rimmer L, Lapsia S, Krige A, Subar DA. Hepatic steatosis in patients undergoing resection of colorectal liver metastases: a target for prehabilitation? A narrative review. Surg Oncol. 2019. https://doi.org/10.1016/J.SURONC.2019.07.007.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016. https://doi.org/10.1016/S0140-6736(15)00803-X.
Barth RJ, Mills JB, Suriawinata AA, et al. Short-term preoperative diet decreases bleeding after partial hepatectomy: results from a multi-institutional randomized controlled trial. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000002709.
Waterland JL, McCourt O, Edbrooke L, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta-analysis. Front Surg. 2021. https://doi.org/10.3389/FSURG.2021.628848.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987. https://doi.org/10.1016/0021-9681(87)90171-8.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973. https://doi.org/10.1002/bjs.1800600817.
Acknowledgments
The authors are grateful to Fondazione Umberto Veronesi for supporting Dr. Guarneri’s research fellowship. The authors are grateful to Dr. Alice Wong Licinio for her English language review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Giovanni Guarneri’s research fellowship, unrelated to this study, was funded by Fondazione Umberto Veronesi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors have no related conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2022_11946_MOESM2_ESM.tif
Supplementary Disease free survival (DFS) in patients with preoperative LS and in those without preoperative LS. Median DFS was 20 months for patients without preoperative LS compared with 14 months of those with preoperative LS (p=0.147) (TIF 157 KB)
Rights and permissions
About this article
Cite this article
Guarneri, G., Palumbo, D., Pecorelli, N. et al. The Impact of CT-Assessed Liver Steatosis on Postoperative Complications After Pancreaticoduodenectomy for Cancer. Ann Surg Oncol 29, 7063–7073 (2022). https://doi.org/10.1245/s10434-022-11946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11946-x





