Abstract
Purpose
To determine whether pancreatic steatosis (PS) is associated with the risk of postoperative pancreatic fistula (POPF) after radical gastrectomy, and if so, to investigate whether pre-assessment by diagnostic imaging can mitigate the risk.
Methods
The clinical records of 276 patients with cStage I gastric cancer who underwent laparoscopic gastrectomy with D1 + lymphadenectomy between 2012 and 2015 were reviewed. In the first phase up to July 2013 (n = 138), PS was classified from computed tomography (CT) findings into type S (superficial fat deposition) or type D (diffuse fatty replacement) and examined for association with POPF. In the second phase (n = 138), the preoperative CT assessment of PS was routinized. Separate samples from pancreatoduodenectomy consistent with each type were histologically examined.
Results
In the first phase, the incidence of POPF was significantly higher in group S, but not in group D, compared with normal pancreas (16.3% and 9.1% vs. 3.6%, respectively; P = 0.03). The drain amylase level was lowest in group D, reflecting exocrine insufficiency. Histologically, the loose connective-tissue space between the fat infiltrating the pancreas and the peripancreatic fat containing the lymph nodes was unclear in type D but conserved in type S. In the second phase, surgery was performed with more intention on accurately tracing the dissection plane and significantly lowered incidence of POPF in Group S (16.3% to 2.1%; P = 0.047).
Conclusion
Peripancreatic lymphadenectomy is more challenging and likely to cause POPF in patients with PS. However, the risk may be reduced using appropriate dissection techniques based on the CT pre-assessment findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative pancreatic fistula (POPF) is a frequent and serious complication after radical gastrectomy [1,2,3]. One of the mechanisms suggested for POPF formation is intraperitoneal lipolysis due to leakage of exocrine enzymes associated with pancreatic parenchymal injury [4]. As causative external or technical factors, direct compression of the pancreas or thermal damage to it associated with the use of electrosurgical devices such as electrocautery and ultrasonic instruments may exacerbate pancreatic juice leakage [5,6,7,8,9]. As a causative internal factor, visceral obesity is reported to be associated with an increased risk of developing POPF, probably due to difficulties in dissecting the peripancreatic lymph nodes (LNs) [10, 11].
Excess visceral fat causes lipid infiltration of organs such as the liver (known as non-alcoholic steatohepatitis or fatty liver disease), striated muscle, heart, and pancreas [12, 13]. Recent evidence suggests that pancreatic steatosis (PS), a general term for pancreatic fat accumulation, plays a role in type 2 diabetes, pancreatic exocrine dysfunction, acute pancreatitis, and pancreatic cancer [14,15,16]. In pancreaticoduodenectomy, PS is considered a more reliable risk factor for leakage from the pancreatojejunostomy that causes POPF than in the normal pancreas [17, 18]. However, to date, there is no clear evidence that PS is involved in the development of POPF even in gastrectomy that is not accompanied by anastomosis with the pancreas.
In gastric cancer surgery, lymphadenectomy around the pancreas is important to spare the organ from the mesogastrium [19, 20]. Its safety is assured by sharp dissection of the loose connective-tissue space that interfaces between the pancreas and peripancreatic fat (PPF) that contains the regional LNs of the stomach [21]. Several reports have demonstrated that magnified images obtained by laparoscopy enable safe LN dissection while avoiding pancreatic damage [22, 23]. Therefore, it can be hypothesized that the obscuration of the dissection plane associated with PS can result in damage to the pancreatic parenchyma, leading to POPF.
The aim of this study was to determine whether PS is associated with the risk of POPF following radical gastrectomy, and if so, to investigate whether the risk can be mitigated by pre-assessment using diagnostic computed tomography (CT) imaging.
Materials and methods
Patients
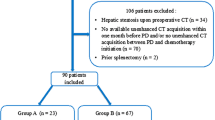
In this retrospective study, demographic and perioperative characteristics were collected for analysis from the perioperative records of 276 patients with clinically suspected stage I gastric cancer who underwent laparoscopic distal or total gastrectomy with D1 or D1 + lymphadenectomy between January 2012 and January 2015 at Toranomon Hospital in Tokyo, Japan. Patients with advanced cancer or who received neoadjuvant chemotherapy were excluded to avoid the effects of complicated lymph node dissection on the development of POPF. Table 1 shows the characteristics of patients in this series, their oncological background, type of operation performed, and postoperative morbidity as determined by the Clavien-Dindo classification [24]. The detailed technique of the lymphadenectomy performed has been reported elsewhere [22, 23, 25,26,27].
In the first phase of this study, 138 patients who underwent surgery before July 2013 were analyzed to examine whether PS is associated with the risk of POPF. In the second phase from August 2013 to July 2015, we routinely evaluated PS preoperatively on CT images for all patients (n = 138). All surgeries were performed by one of two surgeons (HS and SH) certified according to the endoscopic surgical skill qualification guidelines of the Japan Society for Endoscopic Surgery. This study was approved by the Ethics Committee of Toranomon Hospital.
Classification of steatosis from CT images
Preoperative images obtained from contrast-enhanced CT were used to evaluate PS in each patient. As a control, we used images of normal pancreas, which has an even and dense parenchyma with a smooth surface (Fig. 1a). PS was classified into two types according to the degree and distribution of low attenuation corresponding to the fat components [28,29,30,31]: type S denoting superficial fat deposition with an irregular but dense parenchymal surface (Fig. 1b); and type D denoting diffuse fatty replacement with low attenuation areas unevenly distributed throughout the parenchyma (Fig. 1c). The classification was determined by two surgeons blinded to the presence/absence of subsequent POPF.
Preoperative computed tomography images of histology of the pancreas. a Normal pancreas showing an even and dense parenchyma with a smooth surface. b Pancreatic steatosis (PS) classified as type S showing superficial fat deposition with an irregular but dense parenchymal surface. c PS classified as type D showing diffuse fatty replacement with unevenly distributed low attenuation areas throughout the parenchyma
Definition of POPF
POPF was assessed in accordance with the International Study Group on Pancreatic Surgery (ISGPS) definition and grading of postoperative pancreatic fistula [32]. Patients who needed persistent drainage for more than 3 weeks or repositioning of the operatively placed drains through interventional, image-guided means were graded as B POPF; those who required reoperation, who had organ failure, or who died were graded as C POPF. Patients with biochemical leak (concentration of amylase in abdominal drainage fluid more than 3 times the upper limit of the institutional normal serum value) were excluded from the analysis of POPF incidence because it has no clinical importance.
Histologic analysis
Distribution of fat accumulation in the pancreas and consistency of the fascial interface with PPF were histologically investigated using separate samples from patients with cancer of the pancreatic head after pancreatoduodenectomy. Each of three surgical specimens that were determined to be consistent with normal pancreas, PS type S, or PS type D based on the above-mentioned CT criteria was fixed using a standard mixture containing 10% formalin, embedded in 10% gelatin, sliced into 4-mm-thick sagittal sections, and then mounted on slides for hematoxylin and eosin staining.
Statistical analysis
To compare between the groups, we used Welch’s t-test or Wilcoxon’s rank-sum test for continuous variables expressed as means and standard deviations (SD) or medians and ranges, respectively, and we used Fisher’s exact test for categorical variables. Statistical significance was set at P < 0.05. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 24 (IBM Corp., Armonk, NY).
Results
Incidence of POPF in the first phase
In the first phase of this study, the clinical records were analyzed to examine whether PS is associated with the risk of POPF. Mean age was 63.8 ± 10.7 years and mean BMI was 23.0 ± 3.8. Among the 138 patients in this phase, 106 underwent distal gastrectomy and 32 underwent total gastrectomy. Table 2 shows their baseline characteristics. Eighty-four patients had normal pancreas (group N) and 54 had PS, 43 with type S (group S) and 11 with type D (group D). There was no significant difference in sex distribution between the groups. Patients in group S were significantly older than patients in group N (P < 0.01). Mean BMI was significantly higher in groups S and D than in group N (P = 0.04 and P = 0.02, respectively). Mean operating time was significantly longer in group S than in group N (P = 0.01). Intraoperative blood loss did not differ significantly between the groups. On postoperative day 1, the drain amylase level was significantly lower in group D than in group N. The incidence of POPF with ISGPS grade B/C was significantly higher in group S (7/43, 16.3%; P = 0.03), but not significantly so in group D, compared with group N. There was no significant difference in the number of LNs harvested between the groups.
Differences in histological features
To investigate why the risk of POPF differed depending on the presence and type of PS, we compared histological features with the separate samples obtained from patients with cancer of the pancreatic head after pancreatoduodenectomy. Figure 2 shows macroscopic and microscopic findings of representative cases. In normal pancreas, the contour of the parenchyma is smooth and a clear boundary is apparent with PPF, which differs in color from the pancreas (Fig. 2a). Microscopically, pancreatic exocrine acinar cells form a lobular structure, in which branches of the pancreatic duct and blood vessels are evident. They are encapsulated as a whole and interface with the loose connective-tissue space seen between the pancreas and PPF that contains the LNs (Fig. 2b).
Representative macroscopic and microscopic findings of samples obtained from pancreatoduodenectomy determined to be consistent with normal pancreas, pancreatic steatosis (PS) type S, and PS type D. a Sagittal section of the normal pancreas. The contour of the parenchyma is smooth and a clear boundary with peripancreatic fat (PPF) is evident (white arrowheads). b Tissue section of the inset in a. Pancreatic exocrine acinar cells form a dense lobular structure, and a loose connective-tissue space between PPF containing lymph nodes (LNs) is evident (black arrowheads). P, pancreatic duct; V, vein. c Sagittal section of the pancreas categorized as PS type S. The contour of parenchyma is rough and irregular. A thick fat layer is deposited on the surface of the parenchyma and infiltrates along the interstices of the lobules (*), but a clear boundary with the PPF is conserved (white arrowheads). d Tissue section of the inset in c. Loose connective-tissue space with PPF remains visible (black arrowheads). Branches of the pancreatic duct and blood vessels are buried in the fat deposit. A, artery. e Sagittal section of the pancreas categorized as PS type D. A marble pattern of the pancreatic parenchyma, whose stroma is filled by infiltrating fat replacement, is evident. Note, the boundary with the PPF is unclear. f Tissue section of the inset in e. The fat deposit on the pancreatic surface is not encapsulated, and the loose connective-tissue interface between the PPF is obscure
In PS type S, the contour of the parenchyma is rough and irregular. A thick fat layer deposited on the surface of the parenchyma infiltrates along the interstices of the lobules like a fjord. However, a clear boundary is still apparent between the fat deposit and the PPF (Fig. 2c). Microscopic findings reveal that, like normal pancreas, the loose connective-tissue space between the pancreas and PPF remains. Branches of the pancreatic duct and blood vessels are buried in the fat deposit encapsulated with the loose connective-tissue, but they are not evident within the PPF (Fig. 2d).
In type D PS, the pancreatic parenchyma, whose stroma is filled with infiltrating fatty replacement, shows a marbled pattern macroscopically (Fig. 2e). Unlike the other two histological types, however, the boundary with the PPF is unclear. Microscopically, the fat deposit on the pancreatic surface is not encapsulated, and the loose connective-tissue space between the pancreas and PPF is no longer apparent (Fig. 2f). Figure 3 shows schematic diagrams of the histological features of the pancreas and PPF in the normal pancreas and two PS types.
Schematic diagrams showing the histological features of the pancreas and peripancreatic fat (PPF) in the normal pancreas (N) and two pancreatic steatosis (PS) types. Broken line in PS type D indicates the unclear loose connective-tissue interface, which is apparent in N and is still conserved in PS type S (arrowheads). LN, lymph node; P, pancreatic parenchyma
Histology-based LN dissection in PS type S
The histological evidence obtained in the first phase informed our surgical approach, making us more cautious of the loose connective-tissue space during peripancreatic LN dissection. Figure 4 shows laparoscopic images of infrapyloric lymphadenectomy in patients with PS type S. Applying optimal countertraction to the PPF to be removed expands the loose connective-tissue space widely between the pancreatic surface and PPF to enable dissection (Fig. 4a). As a result, the superficial fat deposit can be separated from the pancreas as a different component and left on the remnant side (see Fig. 4b for a close-up view). Desired dissectible planes also become apparent on the lateral side of the right gastroepiploic vein (Fig. 4c and d). After completion of lymphadenectomy, the dissection planes of both the removed PPF and the spared pancreas are coated with glossy connective tissue (Fig. 4e). Exposing the parenchyma is avoided by optimal dissection, namely, by not removing the fat deposit deeply from the pancreatic surface (see Fig. 4f for a close-up view).
Operative images during infrapyloric lymphadenectomy performed with more focus on tracing the dissectible planes in a patient with pancreatic steatosis type S. a Dissecting the medial side of the anterior surface of the pancreas head. The loose connective-tissue space between the pancreas and peripancreatic fat (PPF) is expanded (arrow). RGEV, right gastroepiploic vein. b A close-up view of the dissection plane in a. Note that the fat deposits infiltrating along the interstices of the lobules remain (*), while the PPF is dissected by tracing the loose connective-tissue space (arrow). c Dissecting the lateral side of the pancreas head. Dissection forceps inserted into the loose connective-tissue space reveal the surface of the pancreas (P) at the confluence of the anterior superior pancreatoduodenal vein (ASPDV). Broken arrow indicates the upcoming excision margin of the PPF. d Dissecting the PPF upwards after dividing the RGEV. The loose connective-tissue space between the pancreas and PPF is further expanded (arrows). e After infrapyloric lymphadenectomy is completed, the dissection plane of the removed PPF is coated with glossy connective tissue (arrowheads). RGEA, right gastroepiploic artery. f A close-up view of the inset in e. The dissection plane of the pancreas is also coated with glossy connective tissue (arrowheads). Note that exposing the parenchyma was avoided by optimal dissection, where the fat deposit was not removed deeply from the pancreatic surface
Incidence of POPF in the second phase
In the second phase of the study, we routinely evaluated PS preoperatively on CT images for all patients. Table 3 shows the characteristics and POPF incidence of the 138 patients who underwent surgery during this second phase. Seventy-two patients had normal pancreas, 47 had PS type S, and 19 had PS type D; the proportions for the three groups were almost the same as in the first phase. Again, mean BMI was significantly higher in groups S and D than in group N (both P < 0.01). Despite performing the surgery more carefully, total operating time in group S was not much longer overall. The drain amylase level on postoperative day 3 tended to be lower in group D than in group N, but not significantly so. Only one patient in group S experienced POPF, indicating incidence was significantly decreased to 2.1% in this second phase (P = 0.047 compared with the first phase). Number of LNs harvested did not differ significantly between the groups, and the number harvested in group S was not lower than that in the first phase (P = 0.14).
Discussion
In the first phase of this study, we found an increased risk of POPF with PS present preoperatively. When PS was broadly classified into two histological types on preoperative CT images, POPF was significantly more common in patients with superficial fat deposit (type S), but not with diffuse fatty replacement (type D), compared with patients with normal pancreas. In the second phase, when the preoperative CT assessment of PS was routinized, surgery was performed with more intention on accurately tracing the dissection plane and significantly lowered the incidence of POPF in patients with PS type S. Thus, PS may be a largely avoidable risk factor for POPF after radical gastrectomy.
Pathological fatty replacement has been referred to variously as pancreatic lipomatosis, fatty infiltration, and fatty pancreas, among other terms [14,15,16]. PS is a general term that can be used for all forms of pancreatic fat accumulation [14]. In the liver, hepatocytes accumulate fat in intracellular lipid droplets; in the pancreas, lipids are mainly stored in adipocytes, which infiltrate the parenchyma [33]. Aging and overweight, especially visceral obesity, are most associated with this condition [14,15,16, 34, 35]. One of the mechanisms suggested is the failure of lipid regulation to prevent lipid overload and fat apoptosis in certain organs in sarcopenia caused by aging and metabolic syndrome [36]. In both our study phases as well, patients in groups S and D were older and had a higher BMI than those in group N. PS is most often found using modern imaging technology such as ultrasonography [37] and magnetic resonance imaging [17]. With CT, focal PS can be seen as the separation of parenchymal lobules, with intermixed areas of lower attenuation corresponding to the fat components [28], and the individual lobules become more apparent as PS progresses [29]. Fatty infiltration of the pancreas is generally a diffuse process, but it may be unevenly distributed in the pancreas, usually most prominently in the anterior aspect of the head of the pancreas [30, 31]. Thus, the presence of PS confers some risk for infrapyloric LN dissection in gastric cancer surgery.
In the first phase of this study, 31.1% of patients had PS type S, 16.3% of whom experienced grade 2 or higher POPF. This high incidence might have resulted from our attempts to remove fat deposits deeply from the pancreatic surface, thereby exposing the parenchyma to thermal damage from the energy device and causing pancreatic enzymes to leak. The unavoidable need for coagulation hemostasis at the parenchymal surface may also have increased the damage. As reported previously, lipolysis and proteolysis resulting from leaked pancreatic enzymes are thought to form abscesses at the surgical site and exacerbate pancreatitis and systemic inflammation [38, 39]. With the introduction of laparoscopic gastrectomy, surgeons have aimed for complete removal of peripancreatic adipose tissue to achieve more accurate LN dissection under the magnified image provided by the endoscope. However, evidence from a recent Japanese nationwide survey has revealed a somewhat higher incidence of POPF in laparoscopic gastrectomy than in open surgery [40]. At the very least, when performing prophylactic LN dissection, we need to keep in mind the optimal dissection planes on the surface of the pancreas.
In the embryonic stage, the primordium of the pancreas arises from the duodenal wall [41], grows between the layers of the mesoduodenum, and eventually extends into the dorsal mesogastrium [20, 42]. During this process, areolar interfaces consisting of connective tissue, referred to as the investing fascia [21], are formed to encapsulate the parenchyma and give the organ shape. During surgery, the investing fascia provides our desired dissection plane, allowing us to isolate the PPF containing the LNs with a connective tissue coating [19, 22]. In the present study, we found that this fascial interface is histologically conserved in PS type S and that the risk of POPF could be mitigated by sharp dissection of the loose connective-tissue space seen in the magnified laparoscopic image. In principle, lymphatic channels are not present in the fat components infiltrating the pancreas, so their removal should not contribute to improving the accuracy of LN dissection [43]. Indeed, the median number of retrieved LNs in our second phase was not lower than that in our first phase. Thus, maintaining the dissectible plane between the pancreas and PPF will be important for safe and ontologically sufficient LN dissection, especially in obese patients.
Endocrine and exocrine function of the pancreas may be associated with fat accumulation in islet and acinar cells [44]. At present, there is insufficient evidence to conclude that PS causes pancreatic b-cell dysfunction leading to impaired insulin secretion in the context of insulin resistance [14, 45], although several reports have suggested that PS is present in the prediabetic phase and the amount of pancreatic fat increases prior to the onset of type 2 diabetes mellitus [46, 47]. In contrast, it is widely accepted that PS correlates with a reduction in pancreatic exocrine enzymes [14, 29], such as elastase-1 [48] and amylase [49]. The proposed mechanism involves the negative paracrine effect of pancreatic adipocytes on acinar cells, which serves to reduce the exocrine function [14]. These reports are consistent with our finding that the drain amylase levels in group D were consistently lower than those in the other groups. This may be one of the reasons why the risk of PS type D in the development of POPF was consistently low throughout our study period, despite histologically obscure dissectible planes.
This study has some limitations. First, the prediction of PS type S from preoperative CT images is qualitative and it is therefore difficult to definitively distinguish type S from type D. Due to the various stages of PS, the obscurity of the areolar interface observed in type D may not be even, depending on whether the fatty sparing is mild or severe. However, regardless of the type, it would be possible to determine a safe resection margin by understanding the histology of PS, predicting the pattern of fat deposition by preoperative CT in each patient, and performing surgery. Second, the learning curve effect cannot be completely excluded. The occurrence of postoperative complications can be affected by the skills and experience of the surgeons, assistants, or both. Although all of the gastrectomies analyzed in this study were performed by qualified surgeons, the drawback of comparing outcomes across different time periods is that these confounding factors are not adjusted for. Therefore, it may be difficult to conclude that the lower incidence of POPF was the result of performing lymphadenectomy focused on dissectible planes alone. However, among various complex factors, PS is related to patient background such as visceral obesity and age, and it is one of the risks that can be predicted from CT images before surgery. A report on human error states that nearly 30% of surgical complications are caused by misrecognition during the operation [50]. For patients who have been diagnosed with PS, it is important that surgery is performed with awareness and caution so as not to damage the pancreatic parenchyma.
Conclusion
Peripancreatic lymphadenectomy is more challenging and likely to cause POPF in patients with PS. There is a higher risk of POPF with superficial fat deposits than with diffuse fatty replacement with exocrine insufficiency. However, the risk may be reduced by using appropriate dissection techniques that are selected based on the preoperative CT findings, which themselves can enhance surgical cognition during peripancreatic LN dissection.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the medical data including participants’ personal data.
Code availability
Not applicable.
References
Kung CH, Lindblad M, Nilsson M, Rouvelas I, Kumagai K, Lundell L, Tsai JA (2014) Postoperative pancreatic fistula formation according to ISGPF criteria after D2 gastrectomy in Western patients. Gastric Cancer 17:571–577. https://doi.org/10.1007/s10120-013-0307-1
Degiuli M, Sasako M, Ponti A, Italian Gastric Cancer Study Group (2010) Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 97:643–649. https://doi.org/10.1002/bjs.6936
Yu HW, Jung DH, Son SY, Lee CM, Lee JH, Ahn SH, Park DJ, Kim HH (2013) Risk factors of postoperative pancreatic fistula in curative gastric cancer surgery. J Gastric Cancer 13:179–184. https://doi.org/10.5230/jgc.2013.13.3.179
Uchida Y, Masui T, Nakano K, Yago A, Sato A, Nagai K, Anazawa T, Takaori K, Tabata Y, Uemoto S (2019) Clinical and experimental studies of intraperitoneal lipolysis and the development of clinically relevant pancreatic fistula after pancreatic surgery. Br J Surg 106:616–625. https://doi.org/10.1002/bjs.11075
Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagi K, Ida S, Okumura Y, Sano T, Yamaguchi T (2017) “Pancreas-compressionless gastrectomy”: a novel laparoscopic approach for suprapancreatic lymph node dissection. Ann Surg Oncol 24:3331–3337. https://doi.org/10.1245/s10434-017-5974-4
Fujita T, Ohta M, Ozaki Y, Takahashi Y, Miyazaki S, Harada T, Iino I, Kikuchi H, Hiramatsu Y, Kamiya K, Konno H (2015) Collateral thermal damage to the pancreas by ultrasonic instruments during lymph node dissection in laparoscopic gastrectomy. Asian J Endosc Surg 8:281–288. https://doi.org/10.1111/ases.12177
Ida S, Hiki N, Ishizawa T, Kuriki Y, Kamiya M, Urano Y, Nakamura T, Tsuda Y, Kano Y, Kumagai K, Nunobe S, Ohashi M, Sano T (2018) Pancreatic compression during lymph node dissection in laparoscopic gastrectomy: possible cause of pancreatic leakage. J Gastric Cancer 18:134–141. https://doi.org/10.5230/jgc.2018.18.e15
Washio M, Yamashita K, Niihara M, Hosoda K, Hiki N (2020) Postoperative pancreatic fistula after gastrectomy for gastric cancer. Ann Gastroenterol Surg E-pub. https://doi.org/10.1002/ags3.12398
Engström K, Engström KG (2010) Hazards with electrocautery-induced decomposition of fatty acids–in view of lipid embolization. Scand Cardiovasc J 44:307–312. https://doi.org/10.3109/14017431.2010.491553
Okura Y, Shinohara H, Shindoh J, Haruta S, Ueno M, Sakai Y, Udagawa H (2016) A new scoring system using preoperative factors and contour mapping for predicting postoperative complications of laparoscopic gastrectomy. Dig Surg 33:74–81. https://doi.org/10.1159/000442028
Tanaka K, Miyashiro I, Yano M, Kishi K, Motoori M, Seki Y, Noura S, Ohue M, Yamada T, Ohigashi H, Ishikawa O (2009) Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol 16:1520–1525. https://doi.org/10.1245/s10434-009-0391-y
Van Herpen NA, Schrauwen-Hinderling VB (2008) Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav 94:231–241. https://doi.org/10.1016/j.physbeh.2007.11.049
van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ (2010) Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 39:1185–1190. https://doi.org/10.1097/MPA.0b013e3181f6fce2
Smits MM, van Geenen EJ (2011) The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol 8:169–177. https://doi.org/10.1038/nrgastro.2011.4
Paul J, Shihaz AVH (2020) Pancreatic steatosis: a new diagnosis and therapeutic challenging in gastroenterology. Arq Gastroenterol 57:216–220. https://doi.org/10.1590/s0004-2803.202000000-27
Pezzilli R, Calculli L (2014) Pancreatic steatosis: is it related to either obesity or diabetes mellitus? World J Diabetes 5:415–419. https://doi.org/10.4239/wjd.v5.i4.415
Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW (2010) Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg 251:932–936. https://doi.org/10.1097/SLA.0b013e3181d65483
Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD (2007) Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg 246:1058–1064. https://doi.org/10.1097/SLA.0b013e31814a6906
Shinohara H, Kurahashi Y, Ishida Y (2021) Gastric equivalent of the ‘Holy Plane’ to standardize the surgical concept of stomach cancer to mesogastric excision: updating Jamieson and Dobson’s historic schema. Gastric Cancer 24:273–282. https://doi.org/10.1007/s10120-020-01142-9
Shinohara H, Kurahashi Y, Haruta S, Ishida Y, Sasako M (2018) Universalization of the operative strategy by systematic mesogastric excision for stomach cancer with that for total mesorectal excision and complete mesocolic excision colorectal counterparts. Ann Gastroenterol Surg 2:28–36. https://doi.org/10.1002/ags3.12048
Stecco C, Sfriso MM, Porzionato A, Rambaldo A, Albertin G, Macchi V, De Caro R (2017) Microscopic anatomy of the visceral fasciae. J Anat 231:121–128. https://doi.org/10.1111/joa.12617
Shinohara H, Haruta S, Ohkura Y, Udagawa H, Sakai Y (2015) Tracing dissectable layers of mesenteries overcomes embryologic restrictions when performing infrapyloric lymphadenectomy in laparoscopic gastric cancer surgery. J Am Coll Surg 220:e81–e87. https://doi.org/10.1016/j.jamcollsurg.2015.02.037
Shibazaki S, Suda K, Nakauchi M, Nakamura T, Kadoya S, Kikuchi K, Inaba K, Uyama I (2018) Outermost layer-oriented medial approach for infrapyloric nodal dissection in laparoscopic distal gastrectomy. Surg Endosc 32:2137–2148. https://doi.org/10.1007/s00464-018-6111-6
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Kumamoto T, Kurahashi Y, Haruta S, Niwa H, Nakanishi Y, Ozawa R, Okumura K, Ishida Y, Shinohara H (2019) Laparoscopic modified lymphadenectomy in gastric cancer surgery using systematic mesogastric excision: a novel technique based on a concept. Langenbecks Arch Surg 404:369–374. https://doi.org/10.1007/s00423-019-01770-5
Kumamoto T, Kurahashi Y, Niwa H, Nakanishi Y, Ozawa R, Okumura K, Ishida Y, Shinohara H (2020) Laparoscopic suprapancreatic lymph node dissection using a systematic mesogastric excision concept for gastric cancer. Ann Surg Oncol 27:529–531. https://doi.org/10.1245/s10434-019-07700-5
Shinohara H, Kurahashi Y, Kanaya S, Haruta S, Ueno M, Udagawa H, Sakai Y (2013) Topographic anatomy and laparoscopic technique for dissection of no. 6 infrapyloric lymph nodes in gastric cancer surgery. Gastric Cancer 16:615–620. https://doi.org/10.1007/s10120-012-0229-3
Katz DS, Hines J, Math KR, Nardi PM, Mindelzun RE, Lane MJ (1999) Using CT to reveal fat-containing abnormalities of the pancreas. Am J Roentgenol 172:393–396. https://doi.org/10.2214/ajr.172.2.9930790
Soyer P, Spelle L, Pelage JP, Dufresne AC, Rondeau Y, Gouhiri M, Scherrer A, Rymer R (1999) Cystic fibrosis in adolescents and adults: fatty replacement of the pancreas—CT evaluation and functional correlation. Radiology 210:611–615. https://doi.org/10.1148/radiology.210.3.r99mr08611
Matsumoto S, Mori H, Kiyake H, Takaki H, Maeda T, Yamada Y, Oga M (1995) Uneven fatty replacement of the pancreas: evaluation with CT. Radiology 194:453–458. https://doi.org/10.1148/radiology.194.2.7824726
Kawamoto S, Siegelman SS, Bluemke DA, Hruban RH, Fishman EK (2009) Focal fatty infiltration in the head of the pancreas: evaluation with multidetector computed tomography with multiplanar reformation imaging. J Comput Assist Tomogr 33:90–95. https://doi.org/10.1097/RCT.0b013e31815cff0d
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic Surgery (ISGPS) (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 161:584–591. https://doi.org/10.1016/j.surg.2016.11.014
Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA (2008) Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity 16:522–530. https://doi.org/10.1038/oby.2007.110
Ozbulbul NI, Yurdakul M, Tola M (2010) Does the visceral fat tissue show better correlation with the fatty replacement of the pancreas than with BMI? Eur J Med 42:24–27. https://doi.org/10.5152/eajm.2010.08
Bi Y, Wang JL, Li ML, Zhou J, Sun XL (2019) The association between pancreas steatosis and metabolic sysndrome: a systematic review and meta-analysis. Diabetes Metab Res Rev 35:e3142–e3153. https://doi.org/10.1002/dmrr.3142
Unger RH, Orci L (2002) Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 1585:202–212. https://doi.org/10.1016/s1388-1981(02)00342-6
Marks WM, Filly RA, Callen PW (1980) Ultrasonic evaluation of normal pancreatic echogenicity and its relationship to fat deposition. Radiology 137:475–479. https://doi.org/10.1148/radiology.137.2.7433680
Patel K, Trivedi RN, Durgampudi C, Noel P, Cline RA, Delany JP, Navina S, Singh VP (2015) Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am J Pathol 185:808–819. https://doi.org/10.1016/j.ajpath.2014.11.019
Noel P, Patel K, Durgampudi C et al (2016) Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut 65:100–111. https://doi.org/10.1136/gutjnl-2014-308043
Hiki N, Honda M, Etoh T, Yoshida K, Kodera Y, Kakeji Y, Kumamaru H, Miyata H, Yamashita Y, Inomata M, Konno H, Seto Y, Kitano S (2018) Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer 21:162–170. https://doi.org/10.1007/s10120-017-0764-z
Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32:128–134. https://doi.org/10.1038/ng959
Sadler TW, Langman J (2019) Langman’s medical embryology, 14th edn. Lippincott Williams & Wilkins, Philadelphia
Maruyama K, Okabayashi K, Kinoshita T (1987) Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 11:418–425. https://doi.org/10.1007/BF01655804
Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M (2007) Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 30:2916–2921. https://doi.org/10.2337/dc07-0326
van Raalte DH, van der Zijl NJ, Diamant M (2010) Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care 13:478–485. https://doi.org/10.1097/MCO.0b013e32833aa1ef
Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH (2010) Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond) 34:396–400. https://doi.org/10.1038/ijo.2009.245
Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, Zhang S, Unger RH, Szczepaniak LS (2009) Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab 94:4070–4076. https://doi.org/10.1210/jc.2009-0584
Tahtacı M, Algın O, Karakan T, Yürekli ÖT, Alışık M, Köseoğlu H, Metin MR, Bolat AD, Erel O, Ersoy O (2018) Can pancreatic steatosis affect exocrine functions of pancreas? Turk J Gastroenterol 29:588–594. https://doi.org/10.5152/tjg.2018.17696
Wu WC, Wang CY (2013) Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol 12:77–81. https://doi.org/10.1186/1475-2840-12-77
Suliburk JW, Buck QM, Pirko CJ, Massarweh NN, Barshes NR, Singh H, Rosengart TK (2019) Analysis of human performance deficiencies associated with surgical adverse events. JAMA Netw Open 2:e198067. https://doi.org/10.1001/jamanetworkopen.2019.806
Acknowledgements
The authors thank Dr. Ken Motoori at the Department of Radiology, Tsudanuma General Hospital, for specialist evaluation of the CT classification used in this study; Caryn Jones at ThinkSCIENCE for professional editing and language revision; and all the young surgeons at Toranomon Hospital for their assistance in surgical specimen handling.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (KAKENHI Grant Number 19H03735). The sponsor had no role in study design, data collection, data analysis, manuscript preparation, or publication decisions.
Author information
Authors and Affiliations
Contributions
Study conception and design: Kobayashi N and Shinohara H; acquisition of data: Kobayashi N and Haruta S; analysis and interpretation of data: Kobayashi N and Shinohara H; drafting of manuscript: Shinohara H; critical revision of manuscript: Udagawa H and Ueno M.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all subjects for participation in and publication of this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, N., Shinohara, H., Haruta, S. et al. Reducing the risk of postoperative pancreatic fistula in radical gastrectomy: pre-assessment with computed tomography for the diagnosis of pancreatic steatosis. Langenbecks Arch Surg 407, 587–596 (2022). https://doi.org/10.1007/s00423-021-02337-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02337-z







