Abstract
Background
Hepatic resection combined with perioperative chemotherapy is the standard of care for patients with multiple colorectal liver metastases (CLMs). However, the optimal surgical strategy for treating advanced CLMs remains unclear. The role of the two-stage hepatectomy (TSH) strategy in the management of multiple CLMs remains challenging. This study aimed to compare the outcomes of one-step hepatectomy (OSH)-treated and TSH-treated patients with multiple CLMs.
Methods
This single-institution study included 742 consecutive patients who underwent initial liver resection for histologically confirmed CLMs. The study enrolled patients with 10 or more tumors (n = 106). Clinicopathologic characteristics and long-term outcomes were compared between patients who underwent OSH and those who underwent TSH for 10 or more CLMs.
Results
The study planned OSH for 67 patients (63%) and TSH for 39 patients (37%). One of the OSH-planned patients and two of the TSH-planned patients underwent a trial laparotomy because of non-curative factors. Five patients (13%) did not progress to the second stage of TSH. In the entire cohort, the cumulative 3-year overall survival rate was 58.4% for the patients who had 10 or more CLMs treated with OSH compared with 61.1% for the patients treated with TSH (P = 0.746). In the curative resection cohort, the cumulative 1-year recurrence-free survival rate was 18.2% for the patients treated with OSH and 17.9% for the patients treated with TSH (P = 0.640).
Conclusions
Hepatectomy with perioperative chemotherapy for advanced CLMs with 10 or more tumors is feasible and effective. To prolong survival, TSH is a promising option when curative resection with OSH is impossible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Currently, the presence of multiple colorectal liver metastases (CLMs) is no longer a contraindication to surgery1 because of advancements in patient selection, systemic therapy, and liver resection techniques.2 Furthermore, hepatic resection combined with perioperative chemotherapy has become the standard of care for patients with multiple CLMs.3
Resection of bilobar multiple CLMs remains challenging because it can be difficult to achieve a margin-negative resection while preserving sufficient functional liver parenchyma to avoid postoperative hepatic insufficiency (PHI).4 Preoperative portal vein embolization (PVE) is associated with a decreased incidence of PHI after a major hepatectomy.5 Patients with bilobar CLMs, including those for whom extended hemihepatectomy is required, often are treated with one-step hepatectomy (OSH), in which all metastases are resected in a single surgical procedure. For patients with bilobar CLMs, which cannot be resected using OSH, the use of preoperative PVE and two-stage hepatectomy (TSH) is the next option that helps to balance liver functional reserve and curability.6,7
In the current era of multimodality treatment, with the development of advanced surgical techniques and strategies (e.g., PVE, TSH, and/or liver-first sequencing), an increasing number of patients with bilobar CLMs have undergone curative surgical resection.4,8,9,10 Furthermore, tumor distribution (bilobar or unilobar) would not affect the curability of surgery for multiple CLMs based on the results of propensity score-matched analysis.11
The TSH procedure is safe but carries a substantial risk of dropout during the interval between stages 1 and 2 due to tumor progression or insufficient hypertrophy of the future liver remnant (FLR).12
The completion rates for planned first and second liver resections range from 63 to 87 %.13,14 The postoperative morbidity rates of TSH range from 20 to 59%.15,16 The mortality rate was 0–15 %, and the 3-year overall survival (OS) rates in the reported series ranged from 30 to 80%.7,10,13,14,15,16,17,18,19,20,21 Although the number of CLMs is reported to be a strong prognostic factor,22 complete resection offers the chance for cures even for patients with numerous CLMs.23 Furthermore, the role of the TSH strategy in the management of multiple CLMs remains challenging.
In summary, the optimal surgical strategy for advanced CLMs remains unclear. This study aimed to compare the outcomes of OSH- and TSH-treated patients with multiple CLMs (≥ 10 nodules).
Materials and Methods
Study Design and Population
The Institutional Review Board of the Cancer Institute Hospital approved this retrospective study (approval no. 2019–1084). From a prospectively maintained database, we identified 742 consecutive patients who underwent initial liver resection for histologically confirmed CLMs who had 10 or more CLMs between August 2013 and March 2019. Among these patients, 37 underwent TSH, all of whom had more than 10 tumors. Patients with fewer than 10 tumors (n = 636) were excluded, resulting in a final cohort of 106 patients with 10 or more tumors (Fig. 1).
The number of tumors in this study was defined based on the number of intraoperative findings with intraoperative contrast-enhanced ultrasonography using Sonazoid™ (Diaichi-Sankyo, Tokyo, Japan).24 All the patients received preoperative oxaliplatin-based chemotherapy because perioperative chemotherapy was routinely performed for patients with four or more CLMs or CLMs larger than 5 cm in imaging studies after 2010.25
Definition of Resectability
All the patients were evaluated preoperatively with a baseline history and physical examination. Decisions regarding treatment were made collectively at a multidisciplinary liver tumor conference. Perioperative chemotherapy was routinely performed for all patients judged to be either “borderline resectable (BR)” (with ≥4 CLMs, CLMs >5 cm, or resectable extrahepatic metastasis) or “initially unresectable (IR)” (with unresectable extrahepatic metastases or <30 % FLR volume).25
For the patients with IR-CLM, conversion hepatectomy was planned when a documented response to chemotherapy was observed. After preoperative chemotherapy, the patients who underwent re-staging due to tumors that had shrunken and become resectable underwent surgical resection, defined as resectable with negative margin while preserving sufficient FLR volume with or without TSH.
TSH Strategy
For multiple CLMs, OSH including multiple limited hepatectomy or major hepatectomy after percutaneous PVE was considered as the first-line treatment. Otherwise, TSH was suggested to patients with advanced bilateral CLMs that could not be resected in an OSH (± PVE) due to insufficient FLR volume mainly because of multiple tumors in the FLR.
The TSH group in this study comprised all the patients with planned TSH, including PVL, during the first-stage hepatectomy (first Hx) or sequential percutaneous PVE after the first Hx. Regarding the PVL procedure during the first Hx, partial hilar lymphadenectomy was performed to expose the right portal vein, which then was divided between clamps and injected with 20 mL of pure ethanol into the peripheral portal lumen. Sequential percutaneous PVE was indicated for patients whose first Hx was performed laparoscopically or considered too invasive for performance of a simultaneous PVL. These steps were performed using interventional radiology during postoperative hospitalization after the patient’s recovery of general status and liver function.
Volumetric Analysis
All the patients in this study underwent contrast-enhanced computed tomography (CT) images before OSH or first Hx of TSH and 2 weeks after the first Hx of TSH. Total liver volume (TLV) was defined as the normal parenchymal volume minus the tumor volume. Volume calculation was performed using a three-dimensional liver analysis software (Synapse Vincent; FujiFilm, Tokyo, Japan) based on thin-slice CT images.26 Increased rate of the FLR was defined as a percentage, calculated as follows: ([FLR volume post-procedure]−[FLR volume pre-procedure]) × 100 ÷ (FLR volume post-procedure).
Indications for Second-Stage Hepatectomy
At our institution, second-stage hepatectomy (second Hx) was performed while a sufficient FLR (at least 30% of nontumoral remnant liver without potentially ischemic or congested areas) was maintained in the era of upfront surgery.23 However, with the multidisciplinary strategy, we must consider the problem of chemotherapy-induced liver injury.27 Thus, the second Hx should be performed with less than 40% of the FLR volume preserved in the normal liver. If the initial function is injured based on the indocyanine green clearance test or 99mTc-Galactosyl sialyl albumin scintigram, the decision for TSH should be made when less than 50% of the FLR volume is secured. During the second Hx, new lesions found in the remnant liver were additionally resected when we judged that the remnant liver volume was sufficient if these lesions were resected.
The following data were obtained from electronic medical records: sex, age, diagnosis, preoperative chemotherapy cycles and regimens, perioperative outcomes (estimated blood loss, blood transfusion, operative time, and surgical procedure), tumor characteristics (number of CLMs and size of largest metastasis), and rat sarcoma viral oncogene homolog (RAS) mutation status. All the patients in this study were Japanese. The study defined R0 resection as no exposure of tumor cells to the cut surface of the liver. Postoperative complications were reviewed and classified according to the Clavien-Dindo classification. Major complications were those classified as class 3a or higher.28 Postoperative hepatic insufficiency was defined according to the criteria of the International Study Group of Liver Surgery (ISGLS).29
Statistical Analyses
Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test. For intention-to-treat-based analysis of the entire cohort, OS was measured from the date of definitive OSH resection or first Hx to the date of death or last follow-up visit. For analysis within the resection cohort, recurrence-free survival (RFS) was measured from the date of hepatic resection to the date of radiographic detection of recurrence or last follow-up visit. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test.
All tests were two-sided, and statistical significance was set at P value lower than 0.05. Statistical analyses were performed using JMP software (version 12.1.0; SAS Institute Inc., Cary, NC, USA).
Results
Treatment Flow
Of the 106 patients who met the inclusion criteria, OSH was planned for 67 patients (63%) and TSH for 39 patients (37%). One of the OSH-planned patients had surgery with only a trial laparotomy because of peritoneal dissemination. Two TSH-planned patients underwent a trial laparotomy because of peritoneal dissemination or perihepatic lymph node metastases. Five patients (13%) could not proceed to the second Hx of TSH because of unresectable tumor appearance in the FLR of two patients, tumor progression in the lung of two patients, and PHI after the first Hx of one patient (Fig. 1). The 32 patients who had completed the first Hx underwent the second Hx after a median interval of 35 days (range, 16–388 days).
Baseline Characteristics of OSH and TSH
The baseline characteristics and perioperative data are summarized in Table 1 based on the planned strategy. In the OSH group, 60 patients (90%) had bilobar metastases, 65 patients (97%) received preoperative chemotherapy, 4 patients (6%) underwent PVE before hepatectomy, and 24 patients (36%) underwent major hepatectomy.
All the patients in the TSH group had synchronous and bilobar CLMs and received preoperative chemotherapy. The median number of preoperative chemotherapy cycles before first-stage liver resection was six (range, 4–24 cycles). Most of the patients received an oxaliplatin-containing regimen (38 of 39 patients, 97%).
The two groups did not differ significantly in terms of primary tumor location, primary lymph node metastasis, RAS status, or major comorbidity rates. The median numbers of prechemotherapy and preoperative tumors were higher in the patients treated with TSH than in those treated with OSH (pre-chemotherapy median: 18 vs 15 lesions [P = 0.025]; preoperative median: 16 vs 12 lesions [P = 0.021]), and the preoperative maximum tumor size in the TSH group was larger than in the OSH group (2.6 vs 2.2 cm; P = 0.012). The patients treated with TSH had a significantly higher incidence of advanced liver metastasis, judged to be initially unresectable (81% vs 38%; P < 0.0001).
In the OSH group, 12 patients had extrahepatic metastases (6 in the lung, 4 in hilar lymph nodes, and 1 in a paraaortic lymph node), and in the TSH group, two patients had extrahepatic metastases (1 in the lung and 1 in the adrenal gland) (P = 0.077). Furthermore, the OSH group had a longer surgical time than the TSH group had with the second Hx (median, 458 vs 358 min; P = 0.0006), but the two groups did not differ significantly in terms of blood loss or major morbidity.
Feasibility of TSH Strategy
Among the 37 cases in which TSH was attempted, detailed profiles of first Hx of TSH are summarized in Table 2. For 17 patients (46%), resection of the primary tumor was combined with the first stage of TSH. Major complications after the first Hx of TSH occurred for three patients (8%). One patient experienced postoperative liver insufficiency, which hampered proceeding to a second Hx, and two patients underwent reoperation because of wound dehiscence. These patients received more than six courses of preoperative chemotherapy.
The intra- and postoperative outcomes of the 32 patients who completed TSH are summarized in Table 3. Of these 32 patients, 7 (22%) underwent sequential percutaneous PVE. The median rate of increase in future liver remnants was 29.2%. The median number of days from the first Hx to the second Hx was 35 (range, 16–388 days). Major complications (C-D ≥3a) after the second Hx of TSH occurred for seven patients (22%). At the second Hx, 11 patients (34%) underwent additional partial resection of the remnant liver for new lesions. Five patients had bile leak; one patient had ascites; and one patient had postoperative bleeding. Interventions included percutaneous or transfistula drainage for ascites or bile leaks and transcatheter arterial embolization for bleeding. No 90-day mortality occurred after TSH administration.
Survival Compared OSH With TSH
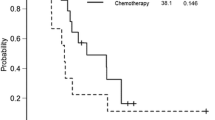
The median observation period was 29.0 months in the OSH group and 16.8 months in the TSH group. Based on intention-to-treat analysis, the cumulative 3-year OS rate was 58.4%, and the median survival time (MST) was 39.2 months for the patients treated with OSH, compared with 61.1% and 40.9 months, respectively, for the patients treated with TSH (hazard ratio [HR], 1.12; P = 0.746; Fig. 2a). Among 66 patients in the OSH group and 32 patients in the TSH group who underwent curative resection, the cumulative 1-year RFS rate and MST were 18.2 % and 7.2 months, respectively, for the patients who had 10 or more CLMs treated with one-step hepatectomy compared with 17.9% and 6.4 months for the patients treated with TSH (HR, 1.12; P = 0.640) (Fig. 2b). The locations of recurrence and the treatment at the time of recurrence are described in Table 3. The rate of repeat hepatectomy tended to be lower in the TSH group.
a Cumulative overall survival (OS) of patients who had multiple (≥10) colorectal liver metastases (CLMs) treated with one-step hepatectomy and of patients who had multiple CLMs treated with two-stage hepatectomy. b Cumulative recurrence-free survival (RFS) of patients who had multiple (≥10) CLMs treated with one-step hepatectomy and of patients who had multiple CLMs treated with two-stage hepatectomy.
Survival Compared With and Without New Lesions in the TSH Group
In the TSH group, the cumulative 3-year OS rate was 70.5% for the patients without additional resection for new lesions, compared with 53.3% for the patients who underwent additional resection (HR, 2.93; P = 0.104; Fig. 3a). The cumulative 1-year RFS rate was 30.3 % for the patients treated without additional resection, compared with 0% for the patients who underwent additional resection (HR, 1.38; P = 0.447; Fig. 3b).
a Cumulative overall survival (OS) of patients who had multiple colorectal liver metastases (CLMs) treated with extra partial resection of the remnant liver at the second stage and of patients who had multiple CLMs treated without extra partial resection of the remnant liver at the second stage. b Cumulative recurrence-free survival (RFS) of patients who had multiple CLMs treated with extra partial resection of the remnant liver at the second stage and of patients who had multiple CLMs treated without extra partial resection of the remnant liver at the second stage.
Discussion
This study aimed to investigate the resection outcomes of multiple CLMs in this era of multidisciplinary treatment, focusing on the differences in surgical strategy. As indicated by the OS rate based on intention-to-treat analysis for the entire cohort and the RFS rate for the curative resection cohort, our tailored approach to bilateral CLMs uses OSH as the first treatment and TSH as the second treatment to achieve R0 resection. Hepatic resection with perioperative chemotherapy for advanced CLMs with severe tumor conditions was proven to be acceptable regardless of the surgical strategy, whether OSH or TSH, from the viewpoints of short- and long-term outcomes.
Patients with advanced bilobar CLMs, even those for whom extended hemihepatectomy is required, often are treated with OSH, in which all metastases are resected in a single surgical procedure.30 Ablation also may be used at the time of OSH to treat lesions that are unresectable because of deep tumor location and/or the small FLR that would result from lesion resection.31
Recently, TSH was reported to be associated with lower rates of major morbidity and postoperative hepatic insufficiency as well as improved OS versus OSH with contralateral resection or ablation.32 If eventual liver volume is sufficient, margin-free resection is the best choice for survival. Therefore, it has been our policy to avoid ablation,33 which never has proved to be efficient for adenocarcinoma, even in cases with deeply located tumor or insufficient FLR.
In the current series, margin-free resection using TSH for severe CLMs provided outcomes similar to those for OSH with less severe conditions of 10 or more CLMs. Furthermore, even for the patients who underwent extra resection at the second Hx, TSH had outcomes comparable with those of patients without extra resection. The results of this study showed that our aggressive surgical strategy for patients with advanced CLMs was valid, and that TSH was a good option for severe tumor conditions in which major hepatectomy with additional contralateral resection or ablation was otherwise required.
For patients with bilobar CLMs that cannot be resected in OSH, TSH offers the best chance for prolonged survival.10 However, TSH is a complicated treatment sequence that consists of preoperative chemotherapy, clearance of one hemi-liver with PVL or postoperative PVE, and eventual hemihepatectomy. Furthermore, patients who undergo an incomplete TSH have worse survival than those who can successfully complete TSH.10 A systematic review showed that the median failure rate of TSH was 23%.34 Therefore, selection criteria for TSH are important. Long courses of preoperative chemotherapy, more than five CLMs, and major complications after the first Hx of TSH are known to be associated with failure of TSH.7,12
In this study, three patients who could not proceed to the second Hx received more than six courses of preoperative chemotherapy, and one patient experienced severe postoperative liver insufficiency. This is consistent with the findings of previous studies that have demonstrated factors associated with the failure of TSH.7,12 In contrast, the failure rate for planned TSH in the current study was 14%.
The short- and long-term outcomes of previous TSH reports, including the current series, are shown in Table 4.7,10,13,14,15,16,17,18,19,20,21 Table 4 indicates that TSH is used at our institution to treat more severe cases than those managed by resection with TSH at other institutions. Our results were comparable even when extra resection of the remnant liver at the second Hx was performed. Our favorable TSH results could be attributed to rigorous selection criteria and advanced surgical navigation and techniques, which have made TSH more certain and effective.24,35,36,37,38 These results suggest that completion of TSH for cases in which radical resection cannot be performed with OSH is an available option for prolonged survival.
Recurrence after hepatectomy for CLMs is frequent, and aggressive treatment such as repeat resection combined with perioperative chemotherapy has been adopted worldwide.39 Table 3 shows no significant difference in the location of recurrence, but a strong tendency for a lower incidence of repeat hepatectomy existed in the TSH group compared with the OSH group. These results suggest that some patients with TSH might have difficulty tolerating an additional hepatectomy or overall disease severity. Nevertheless, recurrence in patients who have previously undergone hepatectomy for multiple CLMs should be managed according to the characteristics of recurrence and tolerance for each treatment.
Recently, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was reported as a novel variant of TSH.40 Baumgart et al.41 reported that a tailored approach to bilateral CLMs uses TSH/PVL/PVE as the first and ALPPS as the second rescue treatment to achieve resectability for patients with extensive tumor burden not amenable to OSH. The meta-analysis showed that overall perioperative safety after TSH was better than that with ALPPS because it resulted in lower overall morbidity, serious morbidity, and perioperative mortality in the TSH group.42
Furthermore, Adam et al.43 reported that OS was significantly worse after ALPPS, although the major complication and 90-day mortality rates of ALPPS were similar to those of TSH. Meanwhile, Hasselgren et al.44 reported that ALPPS seemed to improve survival for patients with CLMs and FLR lower than 30 % compared with TSH. However, only 27 patients (54 %) completed TSH (not including rescue ALPPS).
In our cases, even those with severe tumor conditions compared with those in previous reports (Table 3), only one case required ALLPS. Furthermore, 32 patients (86 %) underwent a second Hx. Based on our results, we believe that ALPPS is unnecessary, and that TSH with rigorous patient evaluation and advanced surgical navigation and techniques are sufficient to treat advanced CLMs where OSH cannot be applied.
The main limitations of this study were its single-institution and retrospective design. As such, it had an inherent risk of selection bias based on our institutional referral pattern, patient population, and tumor board recommendations. In addition, this study included only patients who had planned surgery after successful chemotherapy. Further analyses of larger cohorts or prospective comparisons between surgical and medical cohorts are needed.
Finally, because the minimum number of tumors in the patients who underwent TSH at our institution was 10, patients with more than 10 tumors were included in the analysis to reduce the heterogeneity between the two groups. However, no clear evidence shows that the presence of 10 or more tumors is suitable for the definition of a large number of tumors.
In conclusion, hepatectomy with perioperative chemotherapy for advanced CLMs with severe tumor conditions is acceptable. The completion of TSH for severe tumor conditions in cases for which radical resection is not possible with OSH is an available option for prolonged survival.
References
Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–39.
Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83.
Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729–41.
Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–504 (discussion 1504–1495).
Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7.
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–85.
Passot G, Chun YS, Kopetz SE, et al. Predictors of safety and efficacy of 2-stage hepatectomy for bilateral colorectal liver metastases. J Am Coll Surg. 2016;223:99–108.
Shindoh J, Tzeng CW, Aloia TA, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg. 2013;100:1777–83.
Shindoh J, Tzeng CW, Aloia TA, et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg. 2014;18:45–51.
Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–90.
Omichi K, Shindoh J, Cloyd JM, et al. Liver resection is justified for patients with bilateral multiple colorectal liver metastases: a propensity score-matched analysis. Eur J Surg Oncol. 2018;44:122–9.
Imai K, Benitez CC, Allard MA, et al. Failure to achieve a 2-stage hepatectomy for colorectal liver metastases: how to prevent it? Ann Surg. 2015;262:772–8 (discussion 778–9).
Homayounfar K, Liersch T, Schuetze G, et al. Two-stage hepatectomy (R0) with portal vein ligation: towards curing patients with extended bilobular colorectal liver metastases. Int J Colorect Dis. 2009;24:409–18.
Tsim N, Healey AJ, Frampton AE, et al. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol. 2011;18:1939–46.
Turrini O, Ewald J, Viret F, Sarran A, Goncalves A, Delpero JR. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol. 2012;38:266–73.
Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005.
Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–61 (discussion 1061–1054).
Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–49 (discussion 1049–51).
Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB. 2010;12:262–9.
Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–75.
Muratore A, Zimmitti G, Ribero D, Mellano A, Vigano L, Capussotti L. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol. 2012;19:1310–5.
Beppu T, Sakamoto Y, Hasegawa K, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobil Pancreatic Sci. 2012;19:72–84.
Saiura A, Yamamoto J, Hasegawa K, et al. Liver resection for multiple colorectal liver metastases with surgery up-front approach: bi-institutional analysis of 736 consecutive cases. World J Surg. 2012;36:2171–8.
Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Saiura A. Usefulness of contrast-enhanced intraoperative ultrasound in identifying disappearing liver metastases from colorectal carcinoma after chemotherapy. Ann Surg Oncol. 2014;21(Suppl 3):S390–7.
Ichida H, Mise Y, Ito H, et al. Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. World J Surg Oncol. 2019;17:100–100.
Mise Y, Hasegawa K, Satou S, et al. How has virtual hepatectomy changed the practice of liver surgery? Experience of 1194 virtual hepatectomy before liver resection and living donor liver transplantation. Ann Surg. 2018;268:127–33.
Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–99.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Torzilli G, Procopio F, Botea F, et al. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery. 2009;146:60–71.
Elias D, Baton O, Sideris L, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42.
Mizuno T, Cloyd JM, Omichi K, et al. Two-stage hepatectomy vs one-stage major hepatectomy with contralateral resection or ablation for advanced bilobar colorectal liver metastases. J Am Coll Surg. 2018;226:825–34.
Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23:2619–26.
Chua TC, Liauw W, Chu F, Morris DL. Summary outcomes of two-stage resection for advanced colorectal liver metastases. J Surg Oncol. 2013;107:211–6.
Saiura A, Yamamoto J, Sakamoto Y, Koga R, Seki M, Kishi Y. Safety and efficacy of hepatic vein reconstruction for colorectal liver metastases. Am J Surg. 2011;202:449–54.
Inoue Y, Arita J, Sakamoto T, et al. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg. 2015;262:105–11.
Sato T, Arita J, Inoue Y, Koga R, Takahashi Y, Saiura A. Index of convexity: a novel method for assessing liver functional reserve using technetium-99m-galactosyl human serum albumin liver scintigraphy. Biosci Trends. 2017;11:333–9.
Terasawa M, Ishizawa T, Mise Y, et al. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31:5111–8.
Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100:808–18.
Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14.
Baumgart J, Jungmann F, Bartsch F, et al. Two-stage hepatectomy and ALPPS for advanced bilateral liver metastases: a tailored approach balancing risk and outcome. J Gastrointest Surg. 2019;23:2391–400.
Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg. 2018;42:806–15.
Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1521–9.
Hasselgren K, Røsok BI, Larsen PN, et al. ALPPS improves survival compared with TSH in patients affected of CRLM: survival analysis from the randomized controlled trial LIGRO. Ann Surg. 2021;273:442–8.
Acknowledgments
We thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There are no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omichi, K., Inoue, Y., Mise, Y. et al. Hepatectomy with Perioperative Chemotherapy for Multiple Colorectal Liver Metastases is the Available Option for Prolonged Survival. Ann Surg Oncol 29, 3567–3576 (2022). https://doi.org/10.1245/s10434-022-11345-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11345-2