Abstract
Introduction
For patients with stage III melanoma with occult lymph node metastasis, the use of adjuvant therapy is increasing, and completion lymph node dissection (CLND) is decreasing. We sought to evaluate the use of modern adjuvant therapy and outcomes for patients with stage III melanoma who did not undergo CLND.
Methods
Patients with a positive SLNB from 2015 to 2020 who did not undergo CLND were evaluated retrospectively. Nodal recurrence, recurrence-free survival (RFS), distant metastasis-free survival (DMFS), and melanoma-specific survival were evaluated.
Results
Among 90 patients, 56 (62%) received adjuvant therapy and 34 (38%) underwent observation alone. Patients who received adjuvant therapy were younger (mean age: 53 vs. 65, p < 0.001) and had higher overall stage (Stage IIIb/c 75% vs. 54%, p = 0.041). Disease recurred in 12 of 34 patients (35%) in the observation group and 11 of 56 patients (20%) in the adjuvant therapy group. The most common first site of recurrence was distant recurrence alone (5/34 patients) in the observation group and nodal recurrence alone (8/90 patients) in the adjuvant therapy group. Despite more adverse nodal features in the adjuvant therapy group, 24-month nodal recurrence rate and RFS were not significantly different between the adjuvant and observation cohorts (nodal recurrence rate: 26% vs. 20%, p = 0.68; RFS: 75% vs. 61%, p = 0.39). Among patients with stage IIIb/c disease, adjuvant therapy was associated with a significantly improved 24-month DMFS (86% vs. 59%, p = 0.04).
Conclusions
In this early report, modern adjuvant therapy in patients who forego CLND is associated with longer DMFS among patients with stage IIIb/c disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The management of regional nodes in melanoma has changed significantly over the past several decades. The first Multicenter Selective Lymphadenectomy Trial (MSLT-I) demonstrated the importance of sentinel lymph node biopsy (SLNB) in accurately staging the regional nodal basin in patients with intermediate-thickness melanoma.1 Completion lymph node dissection (CLND) among patients with a positive SLNB remained debated until the publication of two large randomized trials comparing CLND versus observation in patients with a positive SLNB (DeCOG-SLT, MSLT-II).2,3 DeCOG-SLT and MSLT-II both demonstrated that CLND does not improve overall survival compared with observation alone in patients with a positive SLNB. These results, and the morbidity associated with CLND, have resulted in a reduction in the use of CLND nationally.4
Coinciding with the de-escalation of regional nodal surgery for stage III melanoma was the approval of effective modern adjuvant therapies beginning with ipilimumab in 2015.5 Among patients with completely resected stage III melanoma, adjuvant molecular targeted therapy with dabrafenib plus trametinib for patients with BRAF V600E/K mutations, or immunotherapy with immune checkpoint blockade significantly improved recurrence free survival rates.5,6,7,8 Notably, these new adjuvant therapies were evaluated in clinical trials that required CLND for enrollment.
The concurrent paradigm changes in de-escalating surgical management of the regional nodal basin, in conjunction with the progress in effective adjuvant therapies, have changed the treatment landscape for stage III melanoma. However, outcomes following adjuvant therapy have not been reported for patients with a positive SLNB who did not undergo CLND. The purpose of this study was to evaluate the use of adjuvant immunotherapy and molecular targeted therapy and outcomes for patients with stage III melanoma who did not undergo CLND.
Methods
This is a retrospective cohort study of a prospectively maintained database of adult patients who underwent wide local excision (WLE) and SLNB for melanoma at a large academic medical center from January 2015 through January 2020. This study was approved by the Colorado Multiple Institutional Review Board. Inclusion criteria were all consecutive cases with clinically negative nodes, no evidence of distant metastasis, and a positive SLNB. Exclusion criteria included undergoing an immediate CLND and follow up less than 5 months.
All patients underwent WLE with appropriate margins based on tumor depth. SLNB was performed with preoperative planar lymphoscintigraphy. For patients with head and neck melanoma, preoperative single-photon emission computed tomography (SPECT-CT) also was performed for the majority of patients as previously described.9 Methylene blue was injected intraoperatively for additional localization. All hot, blue, or pathologic lymph nodes were excised and subject to standard pathologic assessment.
Patient, tumor, and nodal characteristics were compared between patients who received adjuvant therapy versus observation alone. Continuous variables were expressed as means and standard deviations and categorical variables were expressed as absolute numbers and percentages. Differences between groups were compared using chi-square and student’s t-test for categorical and continuous variables, respectively.
All patients underwent routine follow-up with medical oncology which included physical examination, CT, PET/CT, and ultrasound at the discretion of the treating physician. The first site of documented disease recurrence was documented for all patients.
Recurrence outcomes included regional nodal recurrence, recurrence-free survival (RFS), distant metastasis-free survival (DMFS), and melanoma-specific survival (MSS). RFS was defined as the duration from the time of WLE and SLNB until the time of first disease recurrence, including local, in-transit, regional, or distant metastasis. DMFS was defined as the duration from the time of WLE and SLNB to distant metastasis. Melanoma-specific survival (MSS) was defined as duration of time from WLE and SLNB to death due to melanoma recurrence. Disease-free survival functions were calculated by using the Kaplan-Meier method. The log-rank test was used to evaluate differences in recurrence between patients who received adjuvant therapy versus observation alone. Univariable cox-proportional hazard analysis was used to evaluate the association between patient and disease characteristics with melanoma recurrence. The proportional hazards assumption was checked for each variable graphically using log-log plots.
A two-sided significance level of 0.05 was used for all statistical tests. All analyses were performed using STATA version 15.1 (STATA Corp, College Station, TX).
Results
Patient Population
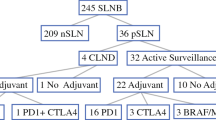
From January 2015 through January 2020, 131 patients with primary cutaneous melanoma underwent WLE with positive SLNB. There were 39 of 131 (30%) patients who underwent immediate CLND and 2 patients with less than 30 days of follow-up who were excluded. The remaining 90 patients who did not undergo CLND were subject to further analysis. This included all 69 patients treated following the publication of MSLT-II in 2017.3
The mean primary tumor depth was 2.05 mm (standard deviation [SD] 1.48); 41 patients (46%) had an ulcerated primary tumor. On SLNB, the median number of sentinel nodes removed was 2 (interquartile range [IQR] 1–3). There were 75 patients (83%) with one positive sentinel node and 15 patients (16.7%) with two positive sentinel nodes. The largest sentinel node tumor deposit was <1 mm in 58 patients (64%).
There were 56 patients (62%) who received adjuvant therapy and 34 patients (38%) who underwent observation alone. Adjuvant therapy consisted of immunotherapy in 53 patients and molecular targeted therapy (BRAF and MEK inhibitors) in 3 patients; 43/53 (81%) patients received nivolumab, 6/53 (11%) patients received pembrolizumab, and 4/53 (7.5%) patients received ipilimumab. Patients who received adjuvant therapy were younger (mean age: 52.9 vs. 64.6 years, p < 0.001), more commonly had a nodular histologic subtype (45% vs. 18%, p = 0.038), had greater sentinel lymph node disease burden (>1 positive SLN: 25% vs. 3%, p = 0.006; proportion of positive nodes ≥2/3rds: 53.6% vs. 29.4%, p = 0.025), higher nodal stage (N2/N3: 33.9% vs. 5.9%, p = 0.009), and higher overall stage (stage IIIb/c 75% vs. 54%, p = 0.041; Table 1).
Patterns of Recurrence and Therapy After Recurrence
The median follow-up was 19 (IQR 11–28) months for the entire cohort. Disease recurrence occurred in 12 of 34 patients (35%) in the observation cohort and 11 of 56 patients (20%) in the adjuvant therapy cohort. The location of first site of disease recurrence and therapy after recurrence is summarized in Table 2. In the observation cohort, distant disease with or without regional lymph node recurrence was the first site of recurrence in 7 of 34 patients (21%) compared with 3 of 56 patients (5%) in the adjuvant therapy cohort. In the observation cohort, regional lymph node recurrence without distant recurrence was the first site of recurrence in 4 of 34 patients (12%) compared with 8 of 56 patients (14%) in the adjuvant therapy cohort (Table 2).
Therapy after first recurrence consisted of CLND, molecular targeted therapy, and/or immunotherapy. In the observation cohort, 9 of 12 (75%) patients who developed recurrence received therapy after the recurrence, consisting of CLND (n = 3), molecular targeted therapy (n = 2), and immunotherapy (n = 8). In the adjuvant therapy cohort, 9 of 11 (82%) patients who developed recurrence received additional therapy after the recurrence, including CLND (n = 4), molecular targeted therapy (n = 2), and immunotherapy (n = 8).
Incidence of Nodal Recurrence
The estimated rate of regional nodal recurrence in the entire cohort was 8% (95% CI 3–16%) at 12 months and 22% (95% CI 13–36%) at 24 months. There was no difference in the rates of regional nodal recurrence between the observation and adjuvant therapy groups on univariable analysis (p = 0.68; Fig. 1a). In the observation group, the estimated rate of regional nodal recurrence at 24 months was 20% (95% CI 9–43%) compared with the adjuvant therapy group at 25% (95% CI 12–49%).
Survival
At 24 months follow-up, there was no difference in the rates of RFS between the observation group (80%, 95% CI 57–91%) and the adjuvant therapy group (75%, 95% CI 51–88%, p = 0.39). Additionally, there was no difference in the MSS (observation: 82%, 95% CI 59–93%; adjuvant therapy: 90%, 95% CI 71–97%, p = 0.61). There was, however, a nonsignificant trend toward improved DMFS at 12 and 24 months follow-up in the adjuvant therapy group (96%, 95% CI 85–99% and 90%, 95% CI 75–96%, respectively) versus the observation group (87%, 95% CI 69–95% and 74%, 95% CI 52–87%, respectively) (p = 0.07).
In the entire study population, the RFS rate for stage IIIa patients at 24 months was 97% (95% CI 80–99%). Given the low rate of recurrence in stage IIIa patients, we performed a stratified analysis comparing RFS and DMFS between the observation and adjuvant therapy cohorts in patients with stage IIIa and stage IIIb/c disease (Fig. 2). There was no difference in the probability of RFS between the observation and adjuvant therapy groups in patients with stage IIIa or stage IIIb/c disease (p = 0.79 and p = 0.11; Fig. 2a, b). There also was no difference in the probability of DMFS between the observation and adjuvant therapy groups in patients with stage IIIa disease (p = 0.33; Fig. 2c). There was, however, a difference in the DMFS at 24 months among patients with stage IIIb/c disease favoring the adjuvant therapy group (86%, 95% CI 95–95% vs. 59%, 95% CI 30–79%, p = 0.04; Fig. 2d).
Patient, tumor, and nodal characteristics were evaluated to determine factors associated with RFS among patients who underwent observation and those who received adjuvant therapy (Table 3). In both groups, increased primary tumor depth was associated with worse RFS (observation: hazard ratio [HR] 1.17, 95% CI 1.05–1.29; adjuvant therapy: HR 1.52, 95% CI 1.00–2.30). In the adjuvant therapy group, primary tumor ulceration was also associated with worse RFS (HR 7.45, 95% CI 1.56–35.9). We observed no association between nodal characteristics, including number of sentinel lymph nodes removed, number of positive nodes removed, proportion of positive nodes, N stage, or size of largest metastatic deposit and RFS in either group.
Immune-Related Adverse Events
Immune-related adverse events that required discontinuation of immunotherapy occurred in 12 of 53 patients (23%). Immune-related adverse events occurred with all immunotherapy regimens used. Nine of 43 patients (21%) who received nivolumab, 1 of 6 patients (16.7%) who received pembrolizumab, and 2 of 4 patients (50%) who received ipilimumab experienced immune-related adverse events that required discontinuation of therapy.
Discussion
We report our institutional experience among patients with a positive SLNB who did not undergo CLND and compare outcomes for observation versus adjuvant therapy. Patients who received adjuvant therapy were younger and had greater sentinel lymph node disease burden with higher nodal stage, and more frequently had stage IIIb or IIIc disease. However, despite more advanced nodal disease burden, the incidence of nodal recurrence, RFS, DMFS, and MSS were not significantly different between patients who received adjuvant therapy and those who underwent observation alone. Because adjuvant therapy was used more often in higher stage disease patients, we considered that our largely equivalent findings may in fact represent efficacy of adjuvant therapy. For this reason, we performed an additional analysis after stratifying for disease stage and found there was a significantly better 24-month DMFS for patients with IIIb/c disease who were treated with adjuvant therapy (86% vs. 59%). Overall, these early results suggest that adjuvant therapy in patients with a positive SLNB who forego CLND may reduce the risk of distant recurrence in patients with stage IIIb and stage IIIc disease. Additional investigation into the utility of adjuvant therapy in patients with stage IIIa disease who forego CLND is needed due to their overall low risk of recurrence and adverse side effects associated with adjuvant therapies.
The utility of adjuvant therapy in patients who forego CLND is of substantial clinical importance. Concurrent with the de-implementation of immediate CLND following DeCOG-SLT and MSLT-II was the approval of more effective adjuvant therapies for patients with stage III melanoma that were demonstrated to improve RFS, including immune checkpoint blockade therapy and molecular targeted therapy for patients with BRAF V600E/K mutations.6,7,8 Due to accrual periods of DeCOG-SLT and MSLT-II occurring before the approval of modern adjuvant therapies (2006–2014 for DeCOG-SLT and 2004-2014 for MSLT-II), the results from these trials are not entirely representative of modern treatment strategies. Sixty-one percent of patients in DeCOG-SLT received interferon-based adjuvant therapy, whereas only 7% of patients in MSLT-II received any adjuvant therapy.2,3 The National Comprehensive Cancer Network clinical practice guidelines now recommends systemic adjuvant therapy with nivolumab, pembrolizumab, or dabrafenib/trametinib or continued observation in patients with stage III disease following WLE and SLNB with or without CLND.10 For this reason, more patients with a positive SLNB are foregoing CLND and receiving systemic adjuvant therapy despite a lack of formal prospective clinical trials evaluating this treatment paradigm, as all randomized trials evaluating the efficacy of adjuvant therapy in stage III melanoma required CLND for enrollment.5,6,7,8,11 Two recent retrospective studies have evaluated the role of surveillance versus CLND in the post MSLT-II era.12,13 These two separate reports analyzed a modern cohort or patients in which 38% of patients received adjuvant therapy. First, CLND was not associated with improved RFS compared with active surveillance alone.12 Second, in patients with high-risk features, such as microsatellites, extranodal extension, or >3 positive SLNs that would have excluded them from DeCOG-SLT and MSLT-II, there was no difference in RFS or MSS among patients treated with CLND versus observation.13 These studies, using real-world outcomes, are in alignment with prior randomized studies in that CLND is not associated with improvement in RFS, even in high-risk patients.
There is little data comparing adjuvant therapy to observation alone in patients who forego CLND following a positive SLNB. Farrow et al. reported a descriptive analysis of 32 patients with a positive SLNB who were managed without CLND, including 22 patients who received adjuvant therapy and 10 patients who underwent observation alone.14 Overall, their study population consisted of 34% of patients with stage IIIa disease, 12.5% with stage IIIb disease, and 47% with stage IIIc disease. At a median follow-up of 10.7 months, RFS for patients who received adjuvant therapy was 82% versus 70% for those who underwent observation alone, but conclusions and statistical comparison were limited due to the small sample size.14 Mitra et al. reported their single-center experience in the modern adjuvant therapy era of 215 patients with a positive SLNB who did not undergo CLND.15 In their cohort, 47% of patients received modern adjuvant therapy, primarily consisting of immunotherapy. Younger age, tumor lymphovascular invasion, and BRAF mutations were associated with the use of adjuvant therapy. Among their entire cohort, isolated nodal recurrence was most commonly the first site of disease recurrence. Lymphovascular invasion (LVI), increased number of positive sentinel lymph nodes, and tumor deposits >1 mm were all independently associated with increased nodal recurrence rates. On univariate analysis, they did not find an association between adjuvant therapy and nodal control, RFS, or DMFS.15 Similarly, among our entire cohort, we did not demonstrate a significant association between adjuvant therapy and nodal recurrence, RFS, DMFS, or MSS. However, after stratifying by overall stage, we did demonstrate a significant association between adjuvant therapy and improved DMFS. A possible explanation for the lack of an association in outcomes with the use of adjuvant therapy in both studies could be the inclusion of stage IIIa patients with overall low recurrence rates.
Because literature to date is limited that reports the clinical and pathologic factors associated with RFS among patients with stage III melanoma who did not undergo immediate CLND and who received modern adjuvant therapy, we also performed a univariate analysis to identify factors associated with RFS. In the adjuvant therapy group, primary tumor depth and ulceration were significantly associated with worse RFS. Sentinel lymph node tumor burden according to the Rotterdam criteria (< 0. mm, 0.1–1.0 mm, and >1.0 mm tumor burden) has been reported to be a strong prognostic factor for survival in melanoma.16 Our data do not demonstrate an association between nodal characteristics, including number of sentinel lymph nodes removed, number of positive nodes removed, proportion of positive nodes, N stage, or size of largest metastatic deposit and RFS. With a larger sample size, Mitra et al. reported primary tumor depth, microsatellitosis, extracapsular invasion, LVI and greater nodal disease burden are associated with RFS in patients who receive adjuvant systemic therapy without CLND.15
In our study, the most common first site of disease recurrence differed between patients who received adjuvant therapy and those who underwent observation alone. In the observation cohort, the most common first site of recurrence was distant recurrence alone (5/34 patients). In contrast, regional nodal recurrence alone (8/90 patients) was the most common first site of recurrence in the adjuvant therapy group. In the MSLT-II trial, in the observation cohort who did not undergo CLND, initial site of recurrence was most commonly distant recurrence alone which is consistent with our cohort of patients who did not receive adjuvant therapy.3 Distant recurrence without regional recurrence was also the most common type of recurrence reported in DeCOG-SLT.2 In contrast to these prior reports among patients who largely did not receive adjuvant therapy, our findings of regional nodes being the most common site of first recurrence are in agreement with recent reports of recurrence patterns among patients who did not receive CLND in the era of modern adjuvant therapy.15 These data suggest that the increased use of adjuvant immunotherapy may impact recurrence patterns and recurrence in regional draining lymph nodes may be the most common site of recurrence in those receiving adjuvant therapy.
There are several limitations to this study inherent to its retrospective design and small sample size. Due to provider and patient selection, there were significant differences in the patient, tumor, and nodal characteristics between patients who received adjuvant therapy versus observation alone. We were unable to perform a propensity weighted or matched comparison due to insufficient common support between groups. Additionally, multivariable analyses were not performed due to an insufficient sample size and number of events.
Several key questions still remain regarding the use of adjuvant therapy in patients with stage III melanoma who do not undergo CLND. First, prognosis varies greatly for patients with stage IIIa disease versus stage IIIb or IIIc disease. Five-and 10-year MSS for stage IIIa disease is approximately 93% and 88%, respectively, compared with 83% and 77% for stage IIIb disease and 69% and 60% for stage IIIc disease.17 Our results demonstrate a low rate of recurrence in patients with stage IIIa disease at a median follow-up 19 months with no difference in recurrence between observation and adjuvant therapy groups. Given the nonnegligible rate of immune-related adverse events with immunotherapy, further work is needed to determine the efficacy of upfront adjuvant therapy in patients with stage IIIa disease. Additionally, we identified primary tumor depth and tumor ulceration as significant risk factors for disease recurrence, while no nodal characteristics were significantly associated with RFS. Future work should attempt to identify additional prognostic and predictive variables to identify patients who will most likely attain the greatest benefit from adjuvant therapy.
Conclusions
Modern adjuvant therapy may mitigate the risk of nodal recurrence in patients who forego CLND, and the use of adjuvant therapy is associated with increased DMFS in patients with stage IIIb/c disease. The use of adjuvant therapy for stage IIIa patients may be overtreatment given their overall lower risk of recurrence and the risk of adverse effects with additional therapy. In the post-MSLT-II era, immediate CLND should not be considered a prerequisite for adjuvant therapy, but these findings strongly support that additional investigation is needed evaluating the utility of adjuvant therapy in patients with stage III disease who forego CLND.
References
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609.
Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–67.
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–22.
Herb JN, Dunham LN, Ollila DW, Stitzenberg KB, Meyers MO. Use of completion lymph node dissection for sentinel lymph node-positive melanoma. J Am Coll Surg. 2020;230(4):515–24.
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30.
Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–23.
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801.
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35.
Chapman BC, Gleisner A, Kwak JJ, Hosokawa P, Paniccia A, Merkow JS, et al. SPECT/CT improves detection of metastatic sentinel lymph nodes in Patients with head and neck melanoma. Ann Surg Oncol. 2016;23(8):2652–7.
Coit DG, Thompson JA, Albertini MR, Barker C, Carson WE, Contreras C, et al. Cutaneous melanoma, version 2.2019. NCCN Clin Pract Guidel Oncol. 2019;17(4):367.
Nijhuis AAG, Spillane AJ, Stretch JR, Saw RPM, Menzies AM, Uren RF, et al. Current management of patients with melanoma who are found to be sentinel node-positive. ANZ J Surg. 2020;90(4):491–6.
Broman KK, Hughes T, Dossett L, Sun J, Kirichenko D, Carr MJ, et al. Active surveillance of patients who have sentinel node positive melanoma: an international, multi-institution evaluation of adoption and early outcomes after the multicenter selective lymphadenectomy Trial II (MSLT-2). Cancer. 2021;127(13):2251–61.
Broman KK, Hughes TM, Dossett LA, Sun J, Carr MJ, Kirichenko DA, et al. Surveillance of sentinel node-positive melanoma patients with reasons for exclusion from MSLT-II: multi-institutional propensity score matched analysis. J Am Coll Surg. 2021;232(4):424–31.
Farrow NE, Raman V, Williams TP, Nguyen KY, Tyler DS, Beasley GM. Adjuvant Therapy is effective for melanoma patients with a positive sentinel lymph node biopsy who forego completion lymphadenectomy. Ann Surg Oncol. 2020;27(13):5121–5.
Mitra D, Ologun G, Keung EZ, Goepfert RP, Amaria RN, Ross MI, et al. Nodal recurrence is a primary driver of early relapse for patients with sentinel lymph node-positive melanoma in the modern therapeutic Era. Ann Surg Oncol. 2021;28:3480–9.
van Akkooi ACJ, Nowecki ZI, Voit C, Schäfer-Hesterberg G, Michej W, de Wilt JHW, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248(6):949–55.
Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torphy, R.J., Friedman, C., Ho, F. et al. Adjuvant Therapy for Stage III Melanoma Without Immediate Completion Lymph Node Dissection. Ann Surg Oncol 29, 806–815 (2022). https://doi.org/10.1245/s10434-021-10775-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10775-8