Abstract
Background
Multiple adjuvant therapies for melanoma have been approved since 2015 based on randomized trials demonstrating improvements in recurrence-free survival (RFS) with adjuvant therapy after surgical resection of high-risk disease. Inclusion criteria for these trials required performance of a completion lymph node dissection (CLND) for positive sentinel lymph node (pSLN) disease.
Objective
We aimed to describe current practice for adjuvant therapies in patients with pSLN without CLND (active surveillance [AS]), and to evaluate recurrence in these patients.
Methods
Melanoma patients with pSLN between 2016 and 2019 were identified at two institutions. Demographic information, disease and treatment characteristics, and recurrence details were reviewed retrospectively. Patients were stratified by recurrence and patient-, treatment- and tumor-related characteristics were compared using Fisher’s exact test and t test for categorical and continuous variables, respectively.
Results
Overall, 245 SLN biopsies were performed, of which 36 (14.7%) were pSLN. Of 36 pSLN, 4 underwent CLND and 32 underwent AS, of whom 22 (68.8%) received adjuvant therapy with the anti-programmed death-1 (PD1) inhibitor nivolumab (16/22), anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor ipilimumab (3/22), or BRAF/MEK inhibitors (3/22). At a median follow up of 13.3 months, 7/32 (21.9%) patients on AS recurred, including 4/22 (18.2%) who received adjuvant therapy and 3/10 (30.0%) who did not. Tumor ulceration was significantly associated with recurrence. While not significant, acral lentiginous subtype appeared more common among those with recurrence.
Conclusion
The majority (68.8%) of patients with pSLN managed without CLND were treated with adjuvant therapy. The 1-year RFS for patients managed with adjuvant therapy without CLND was 82%, which is similar to modern adjuvant therapy trials requiring CLND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, the management of melanoma sentinel lymph node (SLN) metastases has changed dramatically. While completion lymph node dissection (CLND) was previously standard management for positive SLN disease, the German Dermatologic Cooperative Oncology Group trial (DeCOG-SLT) and the Multicenter Selective Lymphadenectomy Trial II (MSLT-II), published in 2016 and 2017, respectively, both showed no melanoma-specific survival benefit for patients undergoing CLND for SLN metastases.1,2 As a result, CLND is no longer considered standard for all patients with SLN-positive disease. Concurrently, multiple adjuvant systemic therapies have been approved in patients after surgical resection of sentinel node metastases, and many patients with SLN metastases are now being managed with active nodal surveillance (AS) and are being considered for systemic adjuvant therapy without CLND.
Adjuvant systemic therapies have been approved since 2015 and now include the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor ipilimumab, programmed death-1 (PD1) inhibitors pembrolizumab and nivolumab, and the targeted BRAF/MEK inhibitor combination of dabrafenib and trametinib.3,4,5 These therapies were approved following several randomized clinical trials showing significant improvements in disease-free survival, and, in some cases overall survival, when used in the adjuvant setting for resected high-risk stage III or IV melanoma. Importantly, each of these trials mandated that complete resection of disease, including a CLND, be performed prior to initiation of adjuvant therapy. On the basis of these trials, national guidelines now recommend that patients with resected stage III and IV disease, including SLN metastases with > 1 mm tumor foci, should be considered for systemic adjuvant therapy.
As CLND is no longer standard for patients with SLN metastases, adjuvant therapies are now being used routinely for patients with SLN metastases without CLND; however, these adjuvant therapies have not been rigorously studied in the adjuvant setting for patients with positive SLN who have not undergone CLND. We hypothesized that patients with SLN-positive melanoma managed with active surveillance and adjuvant therapy in the post-MSLT-II era will have similar outcomes as patients managed with CLND and adjuvant therapy in modern trials. In this study, we aimed to describe modern adjuvant therapy use in patients with SLN metastases managed without CLND, and assess recurrence rates for these patients.
Methods
This study was approved by the Duke University Hospital (DUH) and University of Texas Medical Branch (UTMB) Institutional Review Boards. Duke University served as the coordinating center and performed all analyses. Data collection was performed retrospectively and independently at each institution, and was then deidentified and shared with the coordinating institution.
All patients with positive SLN biopsies (SLNB) managed at each participating institution between 2016 and 2019 were included. All patients underwent wide local excision of the primary tumor in addition to SLNB. Data collection included patient demographic information; primary tumor characteristics, including location, histologic subtype, BRAF mutation status, Breslow depth, and presence of ulceration and microsatellites; nodal metastasis characteristics, including nodal basin(s) assessed and involved, number of nodes assessed, number of nodes positive for metastatic disease, and diameter of nodal metastasis; American Joint Committee on Cancer (AJCC) version 8 staging; performance of CLND; systemic adjuvant therapy use; type of adjuvant therapy; duration of adjuvant therapy; recurrence; time to recurrence from SLNB; location of recurrence; additional therapy for recurrence; type of additional therapy for recurrence; and overall survival (OS). Recurrence-free survival (RFS) and OS were computed from the time of SLNB.
Given the small sample size, the analysis was primarily descriptive. Patients were stratified by recurrence of melanoma. Patient-, tumor-, and treatment-related characteristics were compared using Fisher’s exact test and t test for categorical and continuous variables, respectively. Data are presented as count (percentage) or median (interquartile range) unless otherwise noted. A two-sided p value ≤ 0.05 was considered significant. All analyses were performed using R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
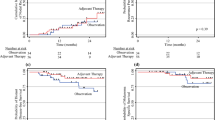
Overall, 245 SLNBs were performed during the study period, of which 36 (14.7%) were positive for metastatic disease (pSLN). Of these 36 patients, 4 underwent CLND and 32 were managed with AS (Fig. 1). Three of four patients who received CLND also received adjuvant therapy (but one patient did not), consisting of anti-PD1 (nivolumab, n = 2) or combination anti-PD1 and anti-CTLA4 inhibitor therapy (nivolumab + ipilimumab, n = 1). At a median follow-up of 20.8 months for the four patients managed with CLND, the RFS was 75%, with one of four patients recurring 4 months after SLNB with in-transit and central nervous system metastases, leading to death due to melanoma.
Flow diagram showing patients managed with SLNB between 2016 and 2019 at Duke University Hospital and University of Texas Medical Branch, stratified by nSLN or pSLN and management with CLND or active surveillance and receipt of adjuvant therapy, including anti-PD1, anti-CTLA4, and targeted therapy with BRAF/MEK inhibitors. SLNB sentinel lymph node biopsy, nSLN negative sentinel lymph node, pSLN positive sentinel lymph node, CLND completion lymph node dissection, PD1 anti-programmed death-1, CTLA4 anti-cytotoxic T-lymphocyte-associated protein 4
After a pSLNB, 32 of 36 patients were managed with AS. The AS methods were not consistent across time or institution; early in the experience, AS at Duke largely consisted of physical examination with computed tomography (CT) and/or positron emission tomography (PET)/CT every 3–6 months following SLNB, and, in more recent patients, physical examination with ultrasounds every 4–6 months, with alternating cross-sectional imaging every 6 months. At UTMB, physical examinations are used, with nodal ultrasound performed for palpable abnormalities, as well as CT or PET/CT every 4 months for the first 12 months and every 6 months after 1 year. Twenty-two of the 32 (68.8%) patients on AS received systemic adjuvant therapy with anti-PD1 therapy (nivolumab, n = 16), anti-CTLA4 therapy (ipilimumab, n = 3), or BRAF/MEK inhibitor therapy (n = 3). The remaining 10 patients on AS did not receive adjuvant therapy (Fig. 1).
At a median follow-up of 10.7 months for the 32 patients managed with AS with or without adjuvant therapy, 7 (21.9%) patients had recurred, resulting in an overall RFS rate of 78.1%. For the 22 patients on AS who received adjuvant therapy, 4 (18.2%) recurred, for an RFS of 81.8%. For the 10 patients on AS who did not receive adjuvant therapy, 3 (30.0%) recurred, for an RFS of 70.0%. Median time to recurrence was 10.7 months from SLNB. Among the seven patients with recurrence, five recurred in the nodal basin alone, one recurred with simultaneous nodal basin recurrence and pulmonary metastatic disease, and one recurred with visceral metastasis alone. Five patients had recurrence detected on imaging (CT and/or PET/CT) and two had recurrence detected on clinical examination. Additional treatment of recurrence included surgical resection (n = 5), systemic therapy (n = 4), and radiation (n = 2). Only one patient in the AS group died during follow-up as a result of non-melanoma-related causes 1.7 years after SLNB.
In Table 1, we examined factors associated with recurrence. Ulceration of the primary tumor was significantly associated with recurrence (Table 1). While not significant, patients with recurrence had higher rates of acral melanoma and a higher range of nodal metastasis diameter.
Discussion
The management of AJCC stage III melanoma has undergone recent dramatic changes. Modern agents, including checkpoint inhibition and targeted therapy, found first to be efficacious in the treatment of stage IV metastatic melanoma, have also now demonstrated efficacy in the adjuvant setting after surgical resection of high-risk melanoma. Several effective adjuvant therapy regimens after complete surgical resection of stage III and IV disease have been approved since 2015 based on results from large multicenter randomized studies showing improvements in disease-free survival with these modern systemic agents.3,5,6 While patient populations varied among trials, patients who are SLNB-positive and have > 1 mm of tumor foci should now be considered for adjuvant therapy, as adjuvant therapy decreases recurrence in these patients by about 50%.3,5,6 Inclusion criteria for these adjuvant trials required CLND prior to initiation of adjuvant therapy. However, two recent randomized trials have now shown no survival benefit for routine CLND after a positive SLNB, and the practice of routine CLND after a positive SLNB has appropriately diminished across many institutions.1, 2 Consequently, patients who are SLNB-positive and have > 1 mm of tumor foci are now often treated with adjuvant therapy without CLND, which was not the practice in the randomized controlled trials of adjuvant therapy. This has raised the question of the applicability of adjuvant therapy trial results in patients with a positive SLNB not receiving CLND. In this study, we found that adjuvant therapy after a positive SLNB without CLND was an effective strategy to decrease rates of recurrent melanoma.
Although CLND may reduce the rate of regional recurrence, ultimately there is no survival advantage, likely because patients with stage III disease are also at elevated risk of distant recurrence. In contrast, effective systemic adjuvant therapy would be expected to improve both regional and distant RFS. Using modern immune checkpoint and targeted therapy agents, adjuvant therapy has now been shown to decrease RFS in stage III melanoma patients after complete surgical resection, and these promising results are being extrapolated, in current clinical practice, to patients who have not undergone CLND. Further evidence is needed to support this practice. However, given gold-standard evidence that adjuvant therapy improves RFS, there would not be equipoise in randomizing patients with a positive SLNB and no completion to a novel therapeutic adjuvant agent compared with placebo in future study. Similarly, given two large randomized trials showing no OS benefit for CLND for positive SLN disease, and a 25% rate of lymphedema in patients who had a CLND, designing a trial where one group received CLND plus adjuvant therapy, while the other group received active surveillance plus adjuvant therapy to test the added value of CLND, also lacks clinical equipoise. The current practice of adjuvant therapy use without CLND can therefore best be explored in a prospective fashion.
Current patient selection for adjuvant therapy is based on data showing a 1–2 mm cut-off of tumor foci diameter in the SLNB correlated with survival; this was also used for inclusion in many of the adjuvant therapy trials.7 For patients with < 1 mm of tumor in the SLNB, the risk of locoregional and distant recurrence remains low, and the risks of toxicity from adjuvant therapy may outweigh the benefits. In our study, patients with a smaller maximal diameter had lower rates of recurrence that were not statistically significant, due in part to the small number of patients in our study. However, the presence of ulceration was significantly associated with recurrence in our study. Clinical features, including the size of nodal metastasis, presence of extranodal extension, primary tumor ulceration, and Breslow thickness, will continue to be important factors in weighing the risks of recurrence and the decision to pursue adjuvant therapy. Adjuvant therapy has significant associated toxicities, making patient selection critical to maximize potential benefits and minimize risks for the individual patient. There is an unmet need to further define the patient population that benefits from adjuvant therapy. The size of the nodal metastasis, along with the discovery of novel biomarkers, may help us refine the appropriate patients for adjuvant therapy.
This study found that adjuvant therapy in patients with a positive SLNB who did not undergo CLND had a similar RFS as patients included in recent adjuvant therapy trials who required CLND. However, our study is limited by the low number of patients, limited follow-up time, and selection bias given its retrospective nature. The follow-up time for patients in our study was also different between patients with no recurrence (8.1 months) to no recurrence (20.5 months). These limitations preclude meaningful comparisons and limit our conclusions. Furthermore, the active surveillance protocols of the institutions in this study differed from those used in MSLT-II in that CT and/or PET/CT were more often used than ultrasound surveillance, although more recent patients in the study were more likely to be assessed with nodal basin ultrasounds. However, we can and should continually evaluate the practice of active surveillance plus adjuvant therapy after a positive SLNB. Additional, larger studies are needed to support this practice.
Conclusion
In this multi-institutional study, we found that active surveillance with adjuvant therapy after a positive SLNB was effective at reducing the risk of recurrence of melanoma compared with active surveillance alone.
References
Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–22.
Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–67.
Weber J, Mandalà M, Del Vecchio M, et al. Adjuvant therapy with nivolumab (NIVO) versus ipilimumab (IPI) after complete resection of stage III/IV melanoma: updated results from a phase III trial (CheckMate 238) [abstract no. 9502]. J Clin Oncol. 2018;36(Suppl):9502.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10.
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–23.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801.
van der Ploeg AP, van Akkooi AC, Schmitz PI, Koljenovic S, Verhoef C, Eggermont AM. EORTC Melanoma Group sentinel node protocol identifies high rate of submicrometastases according to Rotterdam Criteria. Eur J Cancer. 2010;46(13):2414–21.
Acknowledgments
Drs. Farrow and Raman are supported by a National Institutes of Health T-32 Grant (T32-CA093245) for translational research in surgical oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Georgia M. Beasley was a one-time consultant for Regeneron in 2019.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farrow, N.E., Raman, V., Williams, T.P. et al. Adjuvant Therapy is Effective for Melanoma Patients with a Positive Sentinel Lymph Node Biopsy Who Forego Completion Lymphadenectomy. Ann Surg Oncol 27, 5121–5125 (2020). https://doi.org/10.1245/s10434-020-08478-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08478-7