Abstract
Background
Robotic surgery has been widely adopted for complex procedures to overcome technical limitations of open or laparoscopic methods. However, evidence of any subsequent benefit is lacking. This study was undertaken to compare open, laparoscopic, and robotic gastrectomy in technically demanding procedure—D2 dissection in obese patients with gastric cancer.
Methods
Data collected between 2010 and 2018 on D2 gastrectomy in obese patients with gastric cancer were used to conduct retrospective analysis, comparing short- and long-term outcomes of open, laparoscopic, and robotic techniques.
Results
In a total of 185 patients, there were 69 open, 62 laparoscopic, and 54 robotic gastrectomy procedures. Median ages for respective surgical groups were 66 (interquartile range [IQR]: 61–64 years), 63 (IQR: 59–63), and 59 years (IQR: 56–60 years) (p = 0.009). Early-stage gastric cancer ranked proportionately higher in the laparoscopic group (p = 0.005), but operative times were similar among groups. Estimated blood loss (p < 0.001) and drainage volumes (p = 0.001) were higher in the open group, relative to others. Although a robotic approach performed best in overall compliance and in mean number of retrieved lymph node, observed rates of early or late complications did not differ by technique. The open group experienced significantly poorer overall (p = 0.039) and relapse-free (p < 0.001) survival compared with the laparoscopic or robotic group. Robotic surgery emerged from multivariable Cox regression as a protective factor for relapse-free survival (HR = 0.314, 95% CI 0.116–0.851).
Conclusions
In obese patients with gastric cancer, robotic gastrectomy with D2 lymphadenectomy proved comparable to open or laparoscopic technique short-term, yielding better long-term outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of death from cancer worldwide.1 In recent years, minimally invasive surgery (MIS) has become a widely accepted form of treatment. Outcomes of several large-scale, randomized, and controlled trials have validated laparoscopic gastrectomy, proving it technically feasible and safe, with better short-term outcomes and similar long-term oncologic results than open distal gastrectomy.2,3,4,5 However, if greater technical demands (as in obese patients) and procedural complexities (i.e., D2 lymphadenectomy) are imposed, it is not broadly accepted. Excess abdominal fat obscures dissection fields, limiting tactical mobility and hampering intricate perivascular nodal dissections.6 When approaching dorsal aspect of pancreas or splenic hilum, the restricted wrist motion of conventional laparoscopic instruments makes adequate D2 lymphadenectomy problematic.7
To overcome the technical constraints of laparoscopic surgery, robotic systems have incorporated strategic remedies, including 3D high-definition vision, wristed instruments, and better ergonomics.8 These advantages have been shown through numbers of studies to provide better operative control during complex procedures (D2 dissection, total or completion total gastrectomy) or when operating on obese patients.6,9,10,11,12,13 Nonetheless, few researchers have conducted simultaneous technical investigations to discern the benefits of robotic surgery compared with open or laparoscopic surgery.14,15
Herein, we examined a known MIS-challenged cohort (i.e., obese patients requiring D2 dissections for advanced gastric cancer) to assess the merits of open, laparoscopic, and robotic gastrectomy techniques, analyzing short- and long-term surgical outcomes.
Materials and Methods
Patient Selection
Using data prospectively collected between March 2009 and June 2018, we retrospectively reviewed patients with gastric cancer. Each underwent radical gastrectomy, performed by a single surgeon. Those qualifying as obese (body mass index [BMI] ≥25) and subjected to D2 lymphadenectomy were eligible for study, unless resection was non-curative or neoadjuvant chemotherapy was given. Of 1,932 patient candidates, 185 undergoing open (69/465, 14.8%), laparoscopic (62/936, 6.6%), or robotic (54/531, 10.16%) gastrectomy were enrolled for study.
Gastric adenocarcinoma was confirmed by upper endoscopic biopsy. In patients with advanced disease, open gastrectomy was advised. Before 2011, MIS was limited to tumors lacking serosal or extra-perigastric lymph node involvement in preoperative evaluations. Such restrictions were later abandoned (after 2016 for robotics; after 2018 for laparoscopy), allowing advanced gastric cancers with serosal positivity or extensive perigastric nodal spread. Patients with MIS-amenable tumors were provided detailed descriptions of the various procedures, including open gastrectomy. All subjects granted written, informed consent for chosen procedures. Patient characteristics, including age, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) score, were retrieved from warehoused data. Prognostic nutritional index (PNI) was calculated as 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3), as previously described.16 This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine (4-2020-1267).
Operative Strategy
Endoscopic injection of indocyanine green (ICG; Dongindang Pharmaceutical, Inchon, South Korea) was performed for fluorescent lymphography.17 One day before surgery, we injected gastric submucosa with ICG solution (0.625 mg/mL) at four points along primary tumor perimeters (total of 1.5 mg). For robotic surgery, the Firefly technology for ICG fluorescence lymphography was used, which can overlay fluorescence images in a synchronous fashion for the integration of visible light and near-infrared image. During surgery, the surgeon frequently switched on near-infrared imaging modes to enable adequate lymphadenectomy at each LN station. Indocyanine green lymphography was used since 2014.
The type of gastrectomy (distal vs. total) performed was dictated by tumor location, adhering to guidelines of the Japanese Gastric Cancer Association (JGCA) and the Korean Gastric Cancer Association for the extent of lymph node dissection.18,19 All reconstruction methods were determined by tumor locations and remnant stomach dimensions. Tumor stage was defined according to the 8th edition of American Joint Committee on Cancer staging system.20
Postoperative Pathologic Examinations
Lymph node stations were dissected immediately upon surgical removal, following JGCA guidelines for dividing and sorting nodes at each station.18 All nodal specimens were then assembled and directly transported to the pathology department. Noncompliance typically is defined as no yield of lymph nodes from indicated stations.21 In this study, we evaluated compliance rates station-by-station, defined as number of successful yields divided by number of attempted dissections.
Short-Term Operative Outcome Measures
Perioperative parameters, including operative time, estimated blood loss, length of hospital stay, and postoperative complications, were extracted from the database. All complications were classified using the Clavien-Dindo system.22 Only grade III or higher early and late complications were formally investigated. Early complications developed during hospitalizations or within 30 days after dates of operation. All adverse events beyond these points were otherwise deemed late complications. Operative mortality was defined as postoperative death within 30 days or during the same hospitalization.
Long-Term Oncologic Outcomes (Recurrence and Survival)
All patients were followed in an outpatient clinic, scheduling visits 2 weeks after discharge, quarterly for the first year after surgery, biannually for the next 2 years, and annually thereafter. Abdominopelvic computed tomography and endoscopy were performed at least once yearly. Adjuvant chemotherapy (5-fluorouracil based) was recommended to eligible patients with advanced (stage II or III) cancers.
Recurrences were categorized as locoregional, peritoneal, hematogenous, lymph node, or mixed pattern.23 In locoregional recurrence, adjacent organs, remnant stomach, anastomotic site, duodenal stump, or regional lymph nodes were involved. Peritoneal seeding and Krukenberg tumors constituted peritoneal recurrence, with spread to liver, lung, bone, or other distant organs reflecting hematogenous recurrence; and lymph node recurrence corresponded with aortocaval, extra-abdominal, or distant nodal metastasis. More than one pattern presenting at time of diagnosis signified mixed recurrence.
Statistical Analysis
Statistical computations were driven by standard software (SPSS Statistics v25.0; IBM Corp, Armonk, NY), setting significance at p < 0.05. To compare categorical variables, chi-square test was applied, using Kruskal–Wallis analysis of variance by ranks for continuous variables. Numeric variables were expressed as median values (IQR, interquartile range) and categorical variables as number (%) designations. Bonferroni correction served for post hoc analysis, and Kaplan-Meier estimates of survival (overall [OS] and relapse-free [RFS]) were generated, assessing survival probabilities by log-rank test. Predictors of OS and RFS were explored via Cox proportional hazard model. The final model included predictors of significance (p < 0.05) after backward selection.
Results
Clinicopathologic Patient Characteristics
A total of 185 patients were selected for study. The median age of the patients was 62 years and 71.4% of patients were male. Median BMI was 26.5 kg/m2. Clinicopathologic characteristics of the patient population are shown by surgical group in Table 1. Age differed significantly among groups (p = 0.009), the highest and lowest proportions of young patients found in robotic and open groups, respectively (p = 0.009, post hoc). PNI values in the open group (vs. others) also differed significantly (p < 0.001). No group differences were evident in terms of sex, BMI, or ASA score. The three groups displayed differing TNM stages and T or N classifications (p = 0.005, p = 0.001, p = 0.012, respectively) stemming from open and laparoscopic data (p = 0.006, p = 0.001, p = 0.048, post hoc, respectively). Open and laparoscopic groups likewise accounted for extremes (peak and nadir, respectively) in proportionate cases of advanced-stage cancer.
Lymph node compliance rates at each station, indicating dissection success, are depicted by surgical group in Fig. 1a. Overall compliance rates at each station tended to be lowest in the laparoscopy group, reaching significance in open vs laparoscopic group at stations 7. One exception of better yield of laparoscopic group than open group was observed at station 5. The robotic group fared significantly better than the open group in compliance at station 5 and achieved a significant lead in retrieved lymph node count (laparoscopic vs. robotic: p < 0.001; open vs. robotic: p = 0.003) (Table 1). Retrieved and metastatic lymph node counts are detailed by station in Fig. 1b. Observed distributions of nodal retrieval, marked by significant differences at stations 6 and 9 (open vs robotic) and at stations 1, 6, and 7 (laparoscopic vs. robotic), underscore the favorability of robotic method. Metastatic nodal yields in the laparoscopic (vs. open) group were significantly poorer at stations 4d and 11p. Retrieved and metastatic nodal counts are shown by stage in the online version (Supplementary Fig. 1). The number of retrieved lymph nodes was visualized in terms of the case load, approaches, and use of NIR imaging (Supplementary Fig. 2). Subgroup analysis of robotic groups of patients who used fluorescence (n = 42) versus those who did not (n = 12) revealed significantly higher number of metastatic (p = 0.007, median 2 vs. 0) and retrieved (p < 0.001, median 61.5 vs. 33) lymph nodes.
Perioperative Parameters
Perioperative parameters are chronicled by surgical group in Table 2. Most laparoscopic procedures (95.2%) were subtotal gastrectomies (p = 0.003). Incidences of combined resection (due to cancer progression) or of concurrent resection (due to associated disease) did not differ among groups, nor did operative times (p = 0.625) or transfusion rates (p = 0.197). Estimated blood loss was the exception (p < 0.001), the open group significantly surpassing both laparoscopic (p < 0.001) and robotic (p < 0.001) groups (which were similar). In the robotic group, near-infrared (NIR) guidance was used for most patients (77.8%).
Short-Term Operative Outcomes
No significant differences in early complications were identified among the three groups (Table 3). In the open group, there was one perioperative death related to remnant stomach infarction, and three radiologic interventions were undertaken for pleural effusion, intra-abdominal fluid collection, and intra-abdominal bleeding. One death also occurred in the laparoscopic group as a consequence of intra-abdominal bleeding. In the robotic group, there was one death due to aspiration pneumonia. The robotic group recorded two grade IV (anastomotic leakage and arrhythmia) complications. Two patients required reoperations as well, due to intestinal strangulation and obstruction, and radiologic intervention was needed in one patient with pleural effusion. Although there were no late complications after robotic procedures, significance was lacking (p = 0.070). The laparoscopic group accrued five reoperations due to adhesions (n = 3) or ventral hernia (n = 2) and one radiologic intervention for abdominal fluid collection. In the open group, four reoperations were associated with adhesions (n = 1) or ventral hernia (n = 3).
Long-Term Oncologic Outcomes (Recurrence and Survival)
The median follow-up period in surviving patients was 56 months (range, 3–120 months). OS curves are plotted by surgical group in Fig. 2a. A significant survival difference was noted in the robotic group, relative to others (p = 0.039). During follow-up, there were 31 deaths (16.8%), distributed by group as follows: open, 17 (24.6%); laparoscopic, 10 (16.1%); and robotic, 4 (7.4%).
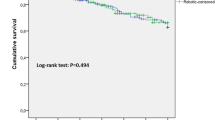
In Fig. 2b, RFS curves are plotted by surgical group and show a significant survival difference in the open group, relative to MIS techniques (p < 0.001). During follow-up, tumor recurred in 42 patents (22.7%), distributed by as follows: open, 26 (37.7%); laparoscopic, 10 (16.1%); and robotic, 6 (11.1%) (p = 0.001). A high recurrence rate in the open group created significant group differences (open vs. laparoscopic: p = 0.018; open vs. robotic: p = 0.003), laparoscopic and robotic groups showing similarity in this regard (p = 0.434). Stratification of patient survival by disease stage is available in the online version (Supplementary Fig. 3).
Recurrence patterns were largely hematogenous or mixed type (10.1%, each) in the open group, with peritoneal seeding predominating in both laparoscopic (4.8%) and robotic (5.6%) groups (Supplementary Table 1).
Uni- and Multivariate Analyses of OS and RFS
Parameters implicated in OS and RFS were identified using a Cox regression model (Table 4). In univariate analysis, robotic technique, total gastrectomy, major complication, tumor size, and stage III disease significantly correlated with OS (p < 0.05). However, only major complication, tumor size, and disease stage emerged from multivariate analysis as independent predictors of OS. PNI, MIS, total gastrectomy, tumor size, and stage III disease were all associated with RFS (p < 0.05) in univariate analysis. Multivariate analysis identified robotic approach and stage III disease as independent risk factors for RFS.
Discussion
Findings of the present study, based on clinical data from radical gastrectomies with D2 dissection (open, laparoscopic, and robotic) in obese patients, have shown the utility implicit in each technique. Given the demands of D2 dissections in this setting, a laparoscopic approach (6.6%) was seldom elected, opting instead for open (14.8%) or robotic (10.16%) methods. Patients in the laparoscopic group also were the least demanding in terms of disease severity (early stage) and technical difficulty (subtotal gastrectomy), yet they displayed surgical outcomes comparable or inferior to those of open or robotic group.
The robotic group, however, claimed robust short-term surgical outcomes, with minimal blood loss and good lymphadenectomy performance, demonstrating significantly better RFS than the open group (at comparable disease levels) and surpassing the laparoscopic group in OS and RFS. Given the less extensive disease consigned to laparoscopy, these robotic successes were notable. Overall, robotic technique conferred the blood-conserving benefit of MIS and rivaled or exceeded open procedures in lymph node dissections, achieving favorable long-term survival rates.
Use of laparoscopic instrumentation for D2 dissections was a serious drawback in our obese subjects, prompting gravitation towards open or robotic gastrectomy for highly advanced gastric cancers (requiring D2 dissection) or those involving upper gastric body (warranting total gastrectomy). The bulk and fragility of abdominal fat often hampers differentiation of pancreatic tissue, fatty deposits, and lymph nodes, impeding nodal dissections.24 Exudation of tissue and blood may obscure vessel and lymph node exposures as well and wrongly divert intraoperative dissection planes. Thus, visceral obesity tends to impair node retrieval and lower counts.25,26 Use of unwristed laparoscopic instruments to handle fragile tissue in a narrow surgical field further exacerbates these vulnerabilities.
An enhanced robotic approach, especially one with wristed instruments, may overcome the technical limitations of laparoscopic gastrectomy.7 Although our open and robotic groups showed comparable disease staging (p = 0.072), observed survival differences (OS: p = 0.039; RFS: p = 0.003) were significant, perhaps boosted by better-suited wristed instruments during lymphadenectomy. Adequate lymphadenectomy is clearly an important issue, particularly in patients at high risk of nodal metastasis from advanced gastric cancer. To assess lymphadenectomy performance, we examined compliance rates and mean retrieved lymph node counts, both commonly used as surrogate markers of oncologic safety.21,27,28 Robotic technique delivered the highest compliance rates at all stations and yielded significantly higher counts of retrieved lymph nodes; therefore, its apparent favorability is not surprising. Previous study reports have corroborated the above, citing excellent lymphadenectomy results for surgery of splenic hilum, suprapancreatic area, and infrapyloric region.12 Furthermore, the benefits of D2 lymphadenectomy during robotic gastrectomy have been documented by others in patients with high BMI values compared with laparoscopic gastrectomy.6
Aside from mechanical features, the superior lymphadenectomy performance of robotics may be credited to the proper lymphatic chain visualization that 3D video systems and ICG fluorescent lymphography afford. Fluorescent lymphography is known to improve regional lymph node detection rates, enabling high compliance rates in the treatment of gastric cancer.17,29 This technology is embedded in robotic systems as Firefly (Intuitive Surgical, Sunnyvale, CA) and was applied exclusively in our robotic gastrectomies. Because fluorescent lymphography is now an added laparoscopic adjunct, improved dissection results may follow.
The present efforts have subsequently distinguished a specific patient subset that stands to benefit from robotic surgery. In our cohort of obese patients, robotic gastrectomy with D2 dissection proved comparable to or better than an open approach in terms of oncologic merit (RFS), empowered by MIS capabilities. The wristed arms that augment tissue handling proficiency and critical heightening of visualization through 3D viewing and fluorescent lymphography may bolster surgical outcomes in such patients.
There were several limitations to this study; the first is its retrospective nature. A degree of bias also was implicit in selection of technique. Laparoscopy was not our surgeon’s preference for highly advanced cancer or total gastrectomy, and patients of younger age or higher socioeconomic status clearly favored robotics. Also, the robot was more frequently applied in the later phase. The “learning effect” through the performance of all gastrectomies by a single surgeon was another factor that perhaps restricted the general applicability our findings. Nonetheless, it did ensure consistency in surgical protocol and quality and in patient management during the period of study, impacting data and outcomes. Finally, this study is seemingly the first comprehensive analysis of its kind, aimed at three surgical approaches of treating gastric cancer.
Conclusions
The data in this study should be interpreted with caution due to the unbalanced patient group, surgeon’s preference, and learning effect. However, this study shows the possibility that a robotic approach aided by fluorescence guided lymphadenectomy may help overcome the mechanical drawbacks of open and laparoscopic surgery during complicated gastric resections. For problematic (i.e., obese) surgical candidates facing D2 gastrectomies, the technologic aspects that facilitate tissue handling and visualization of operative fields and lymphatic chain seem beneficial, encouraging favorable long-term outcomes.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18.
Kim HH, Han SU, Kim MC, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–13.
Hyung WJ, Yang HK, Park YK, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020;38:3304–13.
Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39:2734–41.
Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. 2015;29:3251–60.
Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today. 2016;46:528–34.
Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–92.
Ye SP, Shi J, Liu DN, et al. Robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer based on propensity score matching: short-term outcomes at a high-capacity center. Sci Rep. 2020;10:6502.
Yang C, Shi Y, Xie S, et al. Short-term outcomes of robotic- versus laparoscopic-assisted Total Gastrectomy for advanced gastric Cancer: a propensity score matching study. BMC Cancer. 2020;20:669.
Alhossaini RM, Altamran AA, Cho M, et al. Lower rate of conversion using robotic-assisted surgery compared to laparoscopy in completion total gastrectomy for remnant gastric cancer. Surg Endosc. 2020;34:847–52.
Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–15.
Kim YW, Reim D, Park JY, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc. 2016;30:1547–52.
Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–7.
Yang SY, Roh KH, Kim YN, et al. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24:1770–7.
Guner A, Kim SY, Yu JE, et al. Parameters for predicting surgical outcomes for gastric cancer patients: simple is better than complex. Ann Surg Oncol. 2018;25:3239–47.
Kwon IG, Son T, Kim HI, Hyung WJ. Fluorescent lymphography-guided lymphadenectomy during robotic radical gastrectomy for gastric cancer. JAMA Surg. 2019;154:150–8.
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01042-y
Guideline Committee of the Korean Gastric Cancer Association DWG, Review P. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48
Edge SB, Edge SB. AJCC Cancer Staging Manual. 8th edn. Berlin: Springer; 2017.
de Steur WO, Hartgrink HH, Dikken JL, Putter H, van de Velde CJ. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg. 2015;102:1388–93.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Song J, Lee HJ, Cho GS, et al. Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol. 2010;17:1777–86.
Park JY, Ryu KW, Reim D, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg. 2015;39:1789–97.
Miyaki A, Imamura K, Kobayashi R, Takami M, Matsumoto J. Impact of visceral fat on laparoscopy-assisted distal gastrectomy. Surgeon. 2013;11:76–81.
Ueda J, Ichimiya H, Okido M, Kato M. The impact of visceral fat accumulation on laparoscopy-assisted distal gastrectomy for early gastric cancer. J Laparoendosc Adv Surg Tech A. 2009;19:157–62.
Claassen YHM, de Steur WO, Hartgrink HH, et al. Surgicopathological quality control and protocol adherence to lymphadenectomy in the CRITICS Gastric Cancer Trial. Ann Surg. 2018;268:1008–13.
Lin GT, Chen QY, Zhong Q, et al. Intraoperative surrogate indicators of gastric cancer patients’ long-term prognosis: the number of lymph nodes examined relates to the lymph node noncompliance rate. Ann Surg Oncol. 2020;27:3281–93.
Lin GT, Chen QY, Zheng CH, et al. Lymph node noncompliance affects the long-term prognosis of patients with gastric cancer after laparoscopic total gastrectomy. J Gastrointest Surg. 2020;24:540–50.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1H1A2079953).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Seohee Choi, Jeong Ho Song, Sejin Lee, Minah Cho, Yoo Min Kim, Hyoung-Il Kim have no conflicts of interest or financial ties to disclose. Dr. Woo Jin Hyung received research grants from Medtronic and GC Pharma. These funding sources did not influence our study design and played no part in its execution, analytics, and data interpretation or in our decision to submit results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, S., Song, J.H., Lee, S. et al. Surgical Merits of Open, Laparoscopic, and Robotic Gastrectomy Techniques with D2 Lymphadenectomy in Obese Patients with Gastric Cancer. Ann Surg Oncol 28, 7051–7060 (2021). https://doi.org/10.1245/s10434-021-09952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09952-6