Abstract
Background
Our study investigated the effect of lymph node (LN) noncompliance on the long-term prognosis of patients after laparoscopic total gastrectomy (LTG) and explored the risk factors of LN noncompliance.
Methods
The clinicopathological data of gastric cancer (GC) patients who underwent LTG with D2 lymphadenectomy from June 2007 to December 2013 were prospectively collected and retrospectively analyzed. The effects of LN noncompliance on the long-term prognosis of patients with GC after LTG were explored.
Results
The overall LN noncompliance rate was 51.9%. The survival rate of patients after LTG with LN compliance was significantly superior to that of patients with LN noncompliance (p = 0.013). The stratified analysis of TNM stage indicated that there was no difference between the OS of stage I patients with LN compliance and those with LN noncompliance; OS of stage II/III patients with LN compliance was significantly better than that of those with LN noncompliance. Cox regression analyses showed that LN noncompliance was an independent risk factor for OS. Logistic regression analysis showed that high BMI (≥ 25 kg/m2) was an independent risk factor for preoperative prediction of LN noncompliance in cStage II/III patients. Patients with a high BMI were more likely to have LN noncompliance during surgery, especially during the dissections of #6, #8a, and #12a LN stations.
Conclusions
LN noncompliance was an independent risk factor for poor prognosis in patients with advanced gastric cancer (AGC) after LTG. Patients with high BMI were more likely to have LN noncompliance, especially during the dissections of #6, #8a, and #12a LN stations. LN tracing was recommended for these patients to reduce the rate of LN noncompliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Gastric cancer (GC) ranks second in cancer mortality worldwide.1 Lymph node (LN) metastasis is the main pattern of gastric cancer metastasis.2 Lymphadenectomy is the key to gastric cancer surgery, and adequate lymphadenectomy is helpful for accurate postoperative pathological staging and improving the prognosis of patients with GC.3, 4 Since Japanese scholars proposed standardized guidelines for GC surgery, the D2 lymphadenectomy in patients with advanced gastric cancer (AGC) has become the consensus approach in Asian countries.5 The 15-year follow-up results of Dutch Gastric Cancer Trial (DGCT) based on a Western population also indicated that the D2 lymphadenectomy group had a lower local recurrence rate and tumor-related mortality than the D1 group, which laid a solid foundation for the recommendation of D2 lymphadenectomy as the standard operation for GC in the European guidelines.6, 7 In 1994, Kitano first used laparoscopic surgery for early gastric cancer (EGC).8 With the rapid development of laparoscopic techniques and instruments, the advantages of minimally invasive surgery make this approach increasingly accepted as the standard of care in many hospitals globally.9,10,11 However, D2 complete nodal dissection in laparoscopic surgery, especially in LTG, is known to be a technically challenging surgical procedure, and surgical quality varies among surgeons. Therefore, efforts to standardize the efficient completion of D2 extended lymphadenectomy using laparoscopy to achieve radical tumor removal have received increasing attention in recent years. LN noncompliance has been proposed as a way to assess the quality of D2 lymphadenectomy. In previous studies, LN noncompliance was used as the quality measure for D2 radical resection during open gastrectomy.12,13,14,15,16,17 A phase II multicenter trial named COACT1001 used LN noncompliance to evaluate the feasibility of laparoscopic distal gastrectomy (LDG),18 but there were no reports describing LN noncompliance in LTG. To evaluate the status of LN noncompliance in LTG in a way that enhances patient survival, high-volume, multicenter, prospective, randomized controlled trials are eventually needed. Retrospective research could provide reference values. Therefore, the purpose of this study was to explore the effect of LN noncompliance on the long-term prognosis of patients after LTG and to explore the risk factors of LN noncompliance through a high-volume retrospective study to guide both clinical decision-making and outcome research.
PATIENTS AND METHODS
Patients
This study retrospectively analyzed a prospective database containing 2401 patients with GC who had undergone D2 radical gastrectomy by the same group of surgeons from June 2007 to December 2013 at Fujian Medical University Union Hospital in China. The following inclusion criteria were used: (1) preoperative endoscopic biopsy-proven GC, (2) D2 lymphadenectomy, and (3) no distant metastases or adjacent organ invasion (pancreas, spleen, liver, colon, etc.) prior to surgery. Patients were excluded due to a preoperative diagnosis of T4b or distant metastasis, exploratory or palliative surgery, preoperative chemotherapy, combined organ resection, histological identification of a tumor type other than adenocarcinoma, incomplete histopathological data, or remnant GC. The current study excluded 1058 patients with distal gastrectomy and 395 patients with open total gastrectomy and included 948 patients with laparoscopic total gastrectomy. All patients signed informed consent forms prior to surgery. This retrospective study was approved by the ethics committee of Fujian Medical University Union Hospital.
Preoperative imaging studies were routinely performed following endoscopic and upper gastrointestinal examinations with contrast to confirm the tumor location and included computed tomography (CT) scanning, endoscopic ultrasound (EUS), and positron emission tomography-computed tomography (PET-CT) as needed to evaluate the clinical stage. We used CT scans, EUS, and the 7th edition of the International Union Against Cancer (UICC) classification system to assess the clinical and pathologic stages. Noncompliance was defined as patients with more than one LN station absence as described in the protocol for D2 lymphadenectomy in the Japanese Gastric Cancer Association (JGCA).2, 19 Based on the criteria of obesity released by 2004 World Health Organization (WHO), that is, < 25 kg/m2(normal), 25–29.9 kg/m2 (pre-obesity), 30–34.9 kg/m2 (obesity class I), ≥ 35 kg/m2 (obesity class II), patients were classified into two groups according to their body mass index (BMI). Patients with BMI < 25 kg/m2 were designated as the low-BMI group, while patients with BMI ≥ 25 kg/m2 were designated as the high-BMI group in this study.
Surgical Procedures
The following sequence of lymphadenectomies during LTG was performed: No. 6 → Nos. 7, 9, 11p → Nos. 8a, 12a, 5 → No. 1 → No. 4sb → Nos. 10, 11d → No. 2. For details, please see the indicated references.20,21,22
Postoperative Pathological Examination
Each station of lymph nodes was immediately dissected according to the location of blood vessel clips retained in the specimens in the operation room after the specimens were removed by surgeons. Lymph nodes were divided and sorted into stations according to the protocol for D2 lymphadenectomy in the JGCA. All lymph node specimens were assembled and immediately sent to the department of pathology for examination by at least two experienced pathologists through palpation and microscopy. If more than one station of lymph nodes were not detected, this specimen was considered lymph node noncompliance. All pathological examinations were performed in a standard manner.
Follow-Up
Postoperative follow-up was performed in the outpatient department every 3 months for the first 2 years, every 6 months during years 3 to 5, and once a year after year 5. Most routine patient follow-up appointments included a physical examination, laboratory tests (including assessment of CA19–9, CA72–4, and CEA levels), chest radiography, abdominopelvic US or CT, and an annual endoscopic examination. The OS was calculated from the day of surgery until death or until the final follow-up date, whichever occurred first.
Statistical Analysis
All statistical analyses were performed using SPSS v. 20.0 for Windows (SPSS Inc., Chicago, IL, USA). All continuous variables are presented as their mean ± standard deviation. Chi-square or Fisher’s exact tests were used to analyze categorical variables. Cumulative survival rates were compared using the Kaplan–Meier method and Log-rank test. Regression analysis was performed using the Cox proportional hazards regression model in multivariate analyses. Logistic regression analysis was used to analyze risk factors. Stepwise backward variable removal was applied to the multivariate model to identify the most accurate and parsimonious set of predictors.23 Values of p < 0.05 were considered statistically significant.
RESULTS
Patient Characteristics and LN Noncompliance
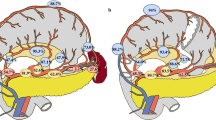
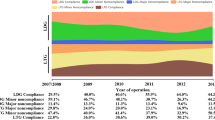
Of the 948 patients with gastric cancer who underwent LTG, 492 (51.9%) had LN noncompliance. LN noncompliance decreased over time, starting at 73.3% in 2007 and reducing to 31.6% in 2013 (Supplementary Fig. 1). Figure 1 shows the status of LN dissection in all stations during the procedure of LTG. Meanwhile, the rate of LN noncompliance in #10, #5, and #12a is high at 68.25%, 50.21%, and 33.44%, respectively, and the rate of LN noncompliance in #3, #4, and #7 is low at 4.22%, 9.39%, and 9.49%. Table 1 shows the clinicopathological data of patients, including 80.3% of patients with cStage II/III and 82.6% of patients with pStage II/III. Age, BMI, ASA score, tumor size, tumor location, macroscopic type, TNM stage, and the total number of retrieved LNs were significantly correlated with LN noncompliance (all p values were < 0.05).
OS Analysis of all Patients
The last follow-up of all patients was in January 2017. The overall follow-up rate was 90.3%, and the 5-year OS of all patients was 49%. The Kaplan–Meier curve of all patients revealed that OS was significantly higher in LN compliant patients than in LN noncompliant patients (P = 0.013), of which 5-year OS was 53 and 45%, respectively (Fig. 2). The Kaplan–Meier survival curve suggested that among the patients with more than 15 LNs retrieved, the prognosis of patients with LN noncompliance were still worse than those with LN compliance and the difference was statistically significant (p = 0.045) (Supplementary Fig. 2).
Stratification analysis of cTNM stage showed that for cStage I patients, the OS of LN compliant patients was similar to that of LN noncompliant patients (p = 0.484). Regarding cStage II patients, the OS of LN compliant patients was slightly, but not significantly, higher than that of LN noncompliant patients (p = 0.138). However, compared with LN noncompliant patients, LN compliant patients showed a significant OS benefit in the cStage III group (p < 0.001). The OS of LN compliant patients with cT3 and cT4 was significantly better than that of LN noncompliant patients (p values were 0.008 and 0.001, respectively). The OS of LN compliant patients with cN+ was superior to that of LN noncompliant patients (p = 0.003), while the OS of LN compliant patients with cT1–2 and cN0 was not significantly different from that of LN noncompliant patients (all p values were > 0.05) (Fig. 3).
According to pTNM stage stratification analysis, the OS of pStage I LN compliant patients was similar to that of LN noncompliant patients (p = 0.890), while the survival of stage II and stage III LN compliant patients was significantly superior to that of LN noncompliant patients (all p values were < 0.05). The OS of pT4a or pN2-3b patients with LN compliance was significantly better than that of patients with the same staging and LN noncompliance (all p values were < 0.01), while the survival of patients with pT1–3 and pN0–1 was not affected by the status of LN dissection (all p values were < 0.05) (Supplementary Fig. 3). The results of the forest plot also indicated that the OS of stage II/III patients with LN compliance was significantly better than that of stage II/III patients with LN noncompliance (Supplementary Fig. 4).
Univariate and Multivariate Cox Regression Analysis
Univariate Cox regression analysis of all patients showed that age, tumor size, tumor location, macroscopic type, histological type, staging (cT, cN, pT, and pN classification), lymphovascular invasion, and LN noncompliance affected OS (all p values were < 0.05). Further, multivariate Cox regression analysis showed that age, macroscopic type, pT classification, pN classification, and LN noncompliance were independent prognostic factors of OS (all p values were < 0.05) (Table 2).
Preoperative Predictors of LN Noncompliance in cStage II/III Patients
A total of 761 patients with cStage II/III accounted for 80.3% of all patients. Regarding cStage II/III patients, univariate logistic regression analysis showed that high BMI and ASA score > 1 were risk factors for LN noncompliance (all p values were < 0.05). Multivariate logistic regression analysis indicated that high BMI was the only significant parameter affecting LN noncompliance (p = 0.01) (Table 3).
Subgroup Analysis of BMI and LN Stations in cStage II/III Patients
BMI subgroup analysis indicated that increasing BMI increased the rate of LN noncompliance. When BMI > 30 kg/m2, the rate of LN noncompliance was as high as 86%. The LN noncompliance rate of various LN stations dissected in the high BMI subgroup was more serious than that in the low-BMI subgroup, especially at #6, #8a, and #12a LN stations. In the high-BMI subgroup, the LN noncompliance rates of #6, #8a, and #12a stations were 83%, 83%, and 70%, respectively. In the low-BMI subgroup, the LN noncompliance rates of these three stations were 74%, 75% and 59%, respectively (Fig. 4).
DISCUSSION
As a quantitative index, the LN noncompliance rate has been gradually recognized and applied by multiple RCTs to evaluate the quality of intraoperative lymphadenectomy for GC.14, 18 The clinical status of the patient, the extent of tumor growth, the operating strategy preferred by the surgeon, and the intensity of the scrutiny by the pathologist in assessing the resection specimens can affect LN noncompliance, which explains why some studies reported that the LN noncompliance rate in radical gastrectomy was different, ranging between 43.2 and 84%.12,13,14, 16, 18 However, there is no study that reports the LN noncompliance rate in LTG. We report, for the first time, the LN noncompliance rate in this complex procedure and elucidate its effect on prognosis. In our study, LN noncompliance in LTG was 51.9% and decreased over time due to the accumulation of surgical experience. In the present study, a group of surgeons conducted LN sorting in the operating room, and two or more experienced, senior pathologists conducted palpation examinations of all LNs from the specimens to avoid having the LN assessment process influence LN noncompliance. Therefore, we believe that the main cause of LN noncompliance in this study is the failed intraoperative dissection of LNs at specific stations. The status of LN noncompliance at each station was further analyzed in this study, and the rate of LN noncompliance at #10, #5, and #12a LN stations was relatively high. Oktar Asoglu reported that the LN noncompliance rate at the #5 LN station during D2 lymphadenectomy was the highest, reaching 53%,24 and the LN noncompliance rate at the #5 LN station was 50.21% in our study. In the LN stations with normal perigastric drainage, there is a considerable difference in the number of LNs per station, and sometimes, small stations (#2, #5, and #10) may not contain any LNs at all, which indicates biological variability.25, 26 Therefore, even if the surgeon dissected all the lymph nodes in all stations according to the standard of D2 radical gastrectomy for gastric cancer, there may be no lymph nodes in the dissected specimens. Taking into account this biological variation, the DGCT study considered that lymph node dissection for gastric cancer allowed one station of lymph nodes to be absent, but dissection was ruled out to be noncompliant if more than one station of lymph nodes were not detected. The definition of lymph node noncompliance in this study conforms to this standard. This biological variation may also be reported as another important cause of LN noncompliance. Although the results of prior studies indicate that inadequate LN dissection may potentially affect the prognosis of GC patients,27, 28 studies on the relationship between LN noncompliance and the prognosis of patients after GC surgery are scarce. The current study hypothesized that LN noncompliance was closely related to the long-term prognosis of GC patients after surgery. Compared with LN compliant patients, LN noncompliant patients exhibited poorer survival after LTG for GC. TNM stage stratification analysis showed that the OS of stage I LN compliant patients was consistent with that of LN noncompliant patients, while the survival of stage II and stage III LN compliant patients was significantly better than that of LN noncompliant patients, which was similar to the forest plot results for OS. In theory, based on a lower risk of LN metastases in EGC, LN without metastasis may be left in the patient, so LN noncompliance does not really affect the survival of patients. However, advanced gastric cancer (AGC) has grown into a systemic disease, and the risk of LN metastasis is greatly increased in AGC. Therefore, LN noncompliance, especially when it occurs in these patient groups with high metastasis risk, can significantly influence the survival of patients. This is consistent with the results of this study.
As the prognosis of patients with AGC with LN noncompliance after LTG is significantly poor, it is of great significance to identify preoperative risk factors for these patients. Obviously, compared with the postoperative pathological stage, the study of risk factors for patients with preoperative cStage II/III has more clinical significance. Logistic regression analysis of cStage II/III patients showed that high BMI was the only independent risk factor for LN noncompliance, which was consistent with prior efforts.24 Previous studies reported that parameters affecting surgical difficulty, such as gender, age, and abdominal surgery history, did not significantly affect LN noncompliance.14, 24 To further explore the effect of BMI on the rate of LN noncompliance at each station, it was found that the LN noncompliance rates of #6, #8a, and #12a LN stations in patients with high BMI were significantly higher than those with low BMI. As we have mentioned earlier in this paper, with patients in the overall group, including patients with gastric cancer in cStages I–III, the noncompliance of LNs in each station was analyzed, and it was found that the noncompliance rates of LNs in stations #10, #5, and #12a were relatively high. However, further analysis of patients with cStages II–III advanced gastric cancer found that the patients with BMI ≥ 25 kg/m2 had the highest noncompliance rate in station #6, #8a, and #12a. Differences in the study population led to differences in lymph node stations with high noncompliance rates. In recent years, a number of studies have reported that high BMI or increased intra-abdominal fat would lead directly to a reduced number of LNs detected.29,31,32,33,33 High-BMI patients often have massive adipose tissue accumulation in the abdomen, and it is often difficult to distinguish the relationship between pancreatic tissue, fat tissue, and LNs during surgery, which makes LN dissection more difficult. Moreover, in the process of dissection, there is more exudation of tissue and blood, which affects the exposure of LNs and the resection plane under laparoscopy to the surgeon and assistant. In particular, high-BMI patients have very deep LNs in specific areas, such as the celiac trunk and around the head of the pancreas, further increasing the difficulty of accurate localization and dissection. Reducing the noncompliance rate of LN dissection in such patients may become the focus of further research. Previous studies have shown that LN tracing techniques, such as Indocyanine Green (ICG) and nanocarbon tracers, can improve the detection rate of regional LNs in gastric cancer, breast cancer, prostate cancer, and other cancers.34,36,37,38,39,39 Therefore, we think that, in these high-risk LN noncompliant patients, using the LN tracer technique intraoperatively may be helpful to dissect LNs and blood vessels and identify LNs within fat tissue, thus finding the right LN dissection plane and improving the rate of LNs retrieved. Surgeons should use a tracer to pay more attention to the #6, #8a, and #12a LN stations to reduce the LN dissection noncompliance rate. However, further exploration in prospective research is still needed.
Similar to other retrospective studies, our study also has several limitations. First, our study was a single-center retrospective study; therefore, it may be somewhat unavoidably biased. Multicenter clinical trials are needed to confirm the results of our study. Second, social and economic factors may have influenced the results. In such a country with vast territory like China, economic level, medical service, and patients’ beliefs and medical consciousness vary sharply from one region to another region. Some of the patients in vulnerable situations, being elderly or underprivileged, tend to refuse to accept postoperative adjuvant chemotherapy, which will affect the implementation of the comprehensive treatment strategies for gastric cancer. These social and economic factors may act as limiting factors and may influence short-term or long-term outcomes. Third, our study lacks historical institutional data from the “open era.” We look forward to a further multicenter prospective control study on the LN noncompliance rate in laparotomy and laparoscopic surgery, which will be further elaborated in the subsequent series of articles on the LN noncompliance rate. However, our research is the first study to confirm that LN noncompliance is an independent risk factor for predicting the long-term prognosis of patients undergoing LTG for GC.
References
Torre L, Bray F, Siegel R, Ferlay J, Lortettieulent J, Jemal A. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Bunt AM, Hermans J, Boon MC, Cj VDV, Sasako M, Fleuren GJ, Bruijn JA. Evaluation of the extent of lymphadenectomy in a randomized trial of Western- versus Japanese-type surgery in gastric cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1994;12:417–422.
Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D 1 and D 2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999;79:1522–1530.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608.
Glynne-Jones R, Northover JM, Cervantes A, Group EGW. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2010;21(Suppl 5(5)):v50.
Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077.
Songun I, Putter H, Kranenbarg EM, Sasako M, Van CDV. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449.
Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148.
Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168.
Han HS, Kim YW, Yi NJ, Fleischer GD. Laparoscopy-assisted D2 subtotal gastrectomy in early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2003;13(6), 361–365.
Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763.
Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, Van EP, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345(8952):745–748.
Bonenkamp JJ, Hermans J, Sasako M, Welvaart K, Songun I, Meyer S, Plukker JTM, Van Elk P, Obertop H, Gouma DJ. Extended Lymph-Node Dissection for Gastric Cancer. N Engl J Med. 1999;340:908–914.
Bonenkamp JJ, Hermans J, Sasako M, van De Velde CJ. Quality control of lymph node dissection in the Dutch randomized trial of D1 and D2 lymph node dissection for gastric cancer. Gastric Cancer. 1998;1:152–159.
de Steur WO, Hartgrink HH, Dikken JL, Putter H, van de Velde CJ. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg. 2015;102:1388–1393.
Claassen YHM, De Steur WO, Hartgrink HH, Dikken JL, Van Sandick JW, Van Grieken NCT, et al. Surgicopathological quality control and protocol adherence to lymphadenectomy in the critics gastric cancer trial. Ann Surg. 2018;268:1008–1013.
Claassen YHM, van Sandick JW, Hartgrink HH, Dikken JL, De Steur WO, van Grieken NCT, Boot H, Cats A, Trip AK, Jansen EPM, Meershoek-Klein Kranenbarg WM, Braak J, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH. Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. Br J Surg. 2018;105:728–735.
Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, Jeong O, Yoon KY, Lee JH, Lee SE, Yu W, Jeong SH, Kim T, Kim S, Nam BH, Group C. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg. 2018;267:638–645.
Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100.
Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Jun L, Chen QY, Lin M, Tu R. Evaluation of laparoscopic total gastrectomy for advanced gastric cancer: results of a comparison with laparoscopic distal gastrectomy. Surg Endosc. 2016;30:1988–1998.
Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:1–8.
Tu RH, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M. Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur J Surg Oncol (EJSO). 2016;43(2);485–492.
Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526.
Asoglu O, Matlim T, Kurt A, Onder SY, Kunduz E, Karanlik H, Sam B, Kapran Y, Bugra D. Guidelines for extended lymphadenectomy in gastric cancer: a prospective comparative study. Ann Surg Oncol. 2013;20:218–225.
Wagner PK, Ramaswamy A, Rüschoff J, Schmitz-Moormann P, Rothmund M. Lymph node counts in the upper abdomen: Anatomical basis for lymphadenectomy in gastric cancer. Br J Surg. 2010;78:825–827.
Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–425.
Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Jpn J Surg. 1981;11:140–145.
Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130–3136.
Kim MG, Kim KC, Kim BS, Kim TH, Kim HS, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index>/=30). World J Surg. 2011;35:1327–1332.
Miyaki A, Imamura K, Kobayashi R, Takami M, Matsumoto J. Impact of visceral fat on laparoscopy-assisted distal gastrectomy. Surgeon. 2013;11:76–81.
Sugimoto M, Kinoshita T, Shibasaki H, Kato Y, Gotohda N, Takahashi S, Konishi M. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc. 2013;27:4291–4296.
Lee HJ, Kim HH, Kim MC, Ryu SY, Kim W, Song KY, Cho GS, Han SU, Hyung WJ, Ryu SW. The impact of a high body mass index on laparoscopy assisted gastrectomy for gastric cancer. Surg Endosc. 2009;23:2473–2479.
Ueda J, Ichimiya H, Okido M, Kato M. The impact of visceral fat accumulation on laparoscopy-assisted distal gastrectomy for early gastric cancer. J Laparoendosc Adv Surg Tech. 2009;19(2), 157–162.
Cabanas RM. An approach for the treatment of the penile carcinoma. Cancer. 1977;39(2):456–466.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. Ann Surg. 188:391–398.
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch Surg. 1992;27:392–399.
Joosten JJA, Strobbe LJA, Wauters CAP, Pruszczynski M, Ruers TJM. Intraoperative lymphatic mapping and the sentinel node concept in colorectal carcinoma. Br J Surg. 1999;86:482–486
Yano K, Mitsumori N, Takahashi N, Kashiwagi H, Yanaga K. The efficiency of micrometastasis by sentinel node navigation surgery using indocyanine green and infrared ray laparoscopy system for gastric cancer. Gastric Cancer. 2012;15:287–291.
Samorani D, Fogacci T, Panzini I, Frisoni G, Accardi FG, Ricci M, Fabbri E, Nicoletti S, Flenghi L, Tamburini E. The use of indocyanine green to detect sentinel nodes in breast cancer: a prospective study. Eur J Surg Oncol. 2015;41:64–70.
Funding
This work was supported by scientific and technological innovation joint capital projects of Fujian Province, China (No.2016Y9031). Minimally invasive medical center of Fujian Province (No. [2017]171). National key clinical specialty discipline construction program of China (No. [2012]649).
Author information
Authors and Affiliations
Contributions
GTL, CMH, and CHZ conceived and designed the study. QYC, PL, JWX, JBW, and JXL performed the study. GTL and QYC analyzed the data. ZNH, LLC, ML, RHT, and JLL contributed the reagents/materials/analysis tools. GTL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lin, GT., Chen, QY., Zheng, CH. et al. Lymph Node Noncompliance Affects the Long-Term Prognosis of Patients with Gastric Cancer after Laparoscopic Total Gastrectomy. J Gastrointest Surg 24, 540–550 (2020). https://doi.org/10.1007/s11605-019-04199-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04199-9