Abstract
Background
Total neoadjuvant therapy in rectal cancer refers to the administration of chemoradiotherapy plus chemotherapy before surgery. Recent studies have shown improved pathological complete response and disease-free survival with this approach. However, survival benefits remain unproven. Our objective is to present a metaanalysis of oncological outcomes of total neoadjuvant therapy in locally advanced rectal cancer.
Patients and Methods
A comprehensive search was performed on PubMed, Medline, and Google Scholars. Studies comparing total neoadjuvant therapy with standard neoadjuvant chemoradiotherapy were included. Data extracted from the individual studies were pooled and a metaanalysis performed. The outcomes of interest are the rate of complete pathological response, nodal response, resection margin, anal preservation, anastomotic leak, local recurrence, distant recurrence, disease-free survival, and overall survival.
Results
There were 15 comparative studies with 2437 patients in the neoadjuvant chemoradiotherapy group and 2284 in the total neoadjuvant therapy group. The pooled complete pathological response was 22.3% in the total neoadjuvant therapy group, compared with 14.2% in the standard neoadjuvant chemoradiotherapy group (p < 0.001). Even though there was no difference in local recurrence rate, there was a significantly lower rate of distant recurrence (OR 0.81, p = 0.02), and better 3-year disease-free survival (70.6% vs. 65.3%, respectively, p < 0.001) and overall survival (84.9% vs. 82.3%, respectively, p = 0.006), favoring the total neoadjuvant therapy group. Due to significant heterogeneity in the study protocols, there remains uncertainty on the ideal chemotherapy/radiotherapy sequence.
Conclusions
This study provides supporting evidence on the favorable immediate and intermediate oncological outcomes with the use of total neoadjuvant therapy for locally advanced rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Local recurrence in rectal cancer has significantly improved following the inception and standardization of total mesorectal excision (TME) surgery,1 as well as increased uptake of either preoperative long-course chemoradiotherapy (LCCRT) or short-course radiotherapy (SRT).2,3 Despite this multidisciplinary approach to care including the use of adjuvant chemotherapy, the risk of distant relapse remains relatively static and is the leading cause of mortality in this patient population.4 Furthermore, there have been several randomized control trials (RCTs) and one metaanalysis showing a lack of benefit for advocating adjuvant chemotherapy in patients who had received neoadjuvant chemoradiotherapy (nCRT) for rectal cancer.5,6,7,–8 Nevertheless, most national guidelines include this as a standard of care,9,10 perhaps an extrapolation from trial of adjuvant chemotherapy in colon cancer.
Studies aimed at optimizing this paradigm have demonstrated a potential advantage in delaying surgery to maximize the impact of neoadjuvant therapies. A 10- to 12-week wait from the completion of neoadjuvant radiotherapy to surgery, for example, has been shown to decrease local recurrence but with heterogenous effects on postoperative complications and TME quality.11,12,13,–14 This increased time period has provided an opportunity to trial additional preoperative chemotherapy. Delivering systemic chemotherapy in the neoadjuvant setting, either before or after radiotherapy for rectal cancer has been termed total neoadjuvant therapy (TNT). The value of this approach has been studied, and early results have demonstrated high rates of completion and tolerability, complete pathological response (pCR) between 20 and 30%, sphincter sparing surgery, and R0 resection.15,16,–17 This approach attempts to overcome the decreased compliance of adjuvant chemotherapy for rectal cancer compared with colon cancer, which is hypothesized to be one of the reasons why a survival benefit has never been demonstrated.
Other possible benefits of delivering systemic chemotherapy early in the neoadjuvant setting in patients with locally advanced rectal cancer include the early prevention or eradication of micrometastases, minimizing the length of time patients need an ileostomy18 and facilitating the surgical resection by decreasing the bulk of the tumor and nodes. In high-risk patients, intensified neoadjuvant combination chemotherapy has the added benefit of assessing the tumor biology and selecting the best approach for a given patient. In case of marked progression on therapy, a resection might be futile, while a complete clinical response might allow an organ preservation approach in selected patients.
A previous metaanalysis by Petrelli et al. on the subject of TNT is limited by mainly nonrandomized studies.19 Since this last metaanalysis, there have been five additional reported studies, of which three were RCTs.20,21,22,23,–24 With results now available from these additional RCTs, we seek to reassess the potential benefits of the TNT approach in locally advanced rectal cancer. The objective of our present metaanalysis is to evaluate the oncological outcomes of patients with locally advanced rectal cancer, comparing TNT versus standard nCRT or SRT before surgery, specifically the differences in tumor downstaging, resection margins, local recurrence, distant recurrence, and postoperative complications.
Patients and Methods
A systematic search was performed according to the Preferred Reporting Items for Systematic Reviews and Metaanalysis (PRISMA) guidelines. The last date of the search was 7 December 2020 from PubMed, Ovid Medline, and Google Scholars. The following Medical Subject Headings (MeSH) terms were used; “neoadjuvant therapy” OR “total neoadjuvant treatment” OR “neoadjuvant” OR “chemotherapy” OR “chemoradiotherapy” OR “radiotherapy” AND “rectal cancer” OR “rectal adenocarcinoma.” A further search was performed in clinicaltrials.gov using the search terms “neoadjuvant therapy” and “rectal cancer.” A bibliographic search was also performed to identify additional studies from the included studies. Initially, all titles were screened, and abstracts reviewed if there were any uncertainties of study inclusion. Finally, all potential studies for inclusion were retrieved, and full text reviewed. From the clinicaltrials.gov, conference abstracts were retrieved if results had not been published in a peer-reviewed journal. To maintain a high standard in this metaanalysis, conference abstracts were not included in the final analysis.
Therefore, the inclusion criteria were as follow: (a) adult participants ≥ 18 years, (b) published studies comparing standard neoadjuvant therapy (short-course radiotherapy or long-course chemoradiotherapy) versus total neoadjuvant therapy, (c) locally advanced rectal cancer (cT3–4 and/or N+), and (d) rectal cancers measured ≤ 12 cm. The following studies were excluded: (a) if there was an addition of a biologic agent such as anti-epidermal growth factor or anti-vascular endothelial growth factor, (b) studies investigating nonoperative rectal preservation or “watch and wait” strategy, and (c) letters to editor, systematic reviews, and conference abstracts.
Two reviewers (J.C.K. and M.S.) collected the data independently, with any discrepancy (less than 5%) resolved by consensus with S.K.W. There were nine outcomes of interest: the rate of complete pathological response (pCR, defined as T0N0), pN0, R0, anal preservation, anastomotic leak, local recurrence, distant recurrence, disease-free survival (DFS), and overall survival (OS). Data collection includes author, year of publication, study design, neoadjuvant short-course or long-course regimen, total neoadjuvant therapy regimen, patient selection, interval time to surgery, follow-up, and outcome measures.
Interval time to surgery and follow-up timelines were collected as median and categorical data reported as percentage converted to absolute numbers, including OS and DFS. The interval time to surgery was defined as time from last treatment delivered (either chemoradiation or consolidation chemotherapy) to surgery. Induction total neoadjuvant therapy was defined as chemotherapy regimen delivered before radiotherapy, and consolidation total neoadjuvant therapy was defined as chemotherapy regimen delivered after radiotherapy. For studies that did not report the percentage for DFS and OS, an estimated percentage was derived from their respective Kaplan–Meier curves. In data that reported zero events, this was replaced as 0.5 to allow for the computation of statistical calculation. Outcome measures with categorical data such as pCR, pN0, R0, anal preservation, anastomotic leak, local and distance recurrence, for each study their odds ratio (OR) and associated 95% confidence interval (CI) were calculated. As for DFS and OS, the absolute numbers were converted to relative risk and associated 95% CI. Due to potential study heterogeneity, pooled relative risk (RR) was performed using the random-effects (RE) model, whereas pooled OR was performed using the Cochran–Mantel–Haenszel test.
I2 statistics were used to assess for interstudy heterogeneity and can be interpreted as 0–30% for minimal, 30–60% moderate, 60–90% substantial, and 90–100% considerable heterogeneity. Egger’s test was performed to assess for publication bias. The Newcastle–Ottawa scale was used to assess each nonrandomized study’s quality, and a score of ≥ 6 is of good quality.23 The Jadad scale was used to assess the quality of randomized controlled trials. Trials with a Jadad score of > 3 were included. A p value of < 0.05 was considered significant. All data analysis was performed in R Studio Team (2015). RStudio: Integrated Development for R Studio, Inc., Boston, MA, and using the metaphor package for metaanalysis.25
Results
Literature Search Results
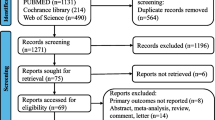
After eliminating duplicates and screening abstracts, 48 articles were assessed for eligibility. After exclusions, 15 comparative studies were included in the final analysis. The full detail of the inclusions process and PRISMA flow chart can be found as eFig. 1 in the online supplement. We identified five retrospective observational studies,18,22,23,26,27 three prospective nonrandomized studies,28,29,–30 and seven randomized controlled trials.16,21,31,32,33,34,35,–36 Table 1 summarizes the characteristics of the included studies. In total, there were 2437 patients treated by standard NCRT, and 2284 patients received TNT. Nine studies used induction TNT, whereas six studies were consolidation TNT. A total of 12 studies used long-course chemoradiotherapy (approximately 50 Gy) in both nCRT and TNT groups. One study used short-course radiotherapy (SRT; 25 Gy in five fractions) in the control and experimental groups.29 Two studies, including the recently published RAPIDO trial, used SRT in combination with FOLFOX or CAPOX in their TNT group, while the patients in the control group received 50.4 Gy LCCRT.31,35 The mean Jadad score was 3.375 for randomized studies, and the mean Newcastle–Ottawa scale score for nonrandomized studies was 6.875. Overall, 11 studies reported their median follow-up, which ranged from 26 to 72.6 months. The interval time to surgery was quite variable in different studies, ranging from 4 to 25 weeks, depending on the number of cycles of chemotherapy in the TNT group and the waiting time after radiation was completed.
Metaanalyses
Complete Pathological Response and Nodal Downstaging
A total of 14 studies reported on pCR, favoring the use of TNT, which showed an increase in odds of pCR by 51% (pooled OR 1.51, 95% CI 1.29–1.78, p < 0.001, I2 = 40.1%; Cochran–Mantel–Haenszel test; Fig. 1). The pooled rate of pCR in the TNT group was 20.5% (15.5–27.5%) versus 14% (12.2–22.2%) in the nCRT group. Ten studies had data on nodal downstaging. Patients who received TNT were less likely to have residual nodal disease on final pathology (pooled OR 0.87, 95% CI 0.73–1.03, p = 0.122, I2 = 67.7%; eFig. 2), although not statistically significant.
Resection Margins
Resection margin status was available in 12 studies for analysis. The patients who received TNT were 34% less likely to have positive surgical margins (0.66, 95% CI 0.51, 0.87, p = 0.003, I2 = 46.1%; eFig. 3)
Anastomotic Leak
Anastomotic leak was the only postoperative morbidity consistently reported through studies suitable for metaanalysis. Six studies reported on the rate of anastomotic leak for comparison. No difference was found in the two groups (pooled OR 0.91, 95% CI 0.53–1.55, I2 = 0%; eFig. 4). The pooled anastomotic leak rate was 8.4% and 8.7% in the TNT and nCRT groups.
Anal Preservation
Data from seven comparative studies were available for analysis on the ability to perform an anal preserving resection after neoadjuvant treatment. Patients in the TNT group were significantly less likely to need an abdominoperineal resection than the nCRT group (OR 0.77, 95% CI 0.62–0.97, p = 0.031, I2 = 58.6%; eFig. 5)
Local and Distant Recurrence
No differences were found in local recurrences after pooling data from seven studies (OR 1.29, 95% CI 0.95–1.74, I2 = 42.1%, eFig. 6). Data on distant recurrences were available in six studies. Albeit moderate to substantial heterogeneity, patients who received TNT were 22% less likely to develop distant recurrences (OR 0.78, 95% CI 0.63–0.96, p = 0.003, I2 = 72.1%; Fig. 2)
Survival Outcomes
Three-year DFS and OS data were available in eight studies for quantitative analysis. The TNT group patients were significantly less likely than the nCRT group to have disease recurrence at three years, 31.9% versus 26.8%, respectively (RR 0.86, 95% CI 0.77–0.96, p < 0.007, I2 = 0.96%; random-effect model; Fig. 3). This DFS advantage also translated in a significant advantage in 3-year OS in favor of TNT (RR 0.83, 95% CI 0.71–0.97, p = 0.019, I2 = 55.3%; Fig. 4). The pooled 3-year survival was 85.5% (76.3–90.2%) and 83.3% (72.5–89.1%) in the TNT and nCRT groups, respectively.
Sub-Metaanalyses
A summary of the outcomes from sub-metaanalyses comparing induction versus consolidation-type TNT can be found in Table 2.
Induction Chemotherapy-Type TNT
All studies in this subgroup analysis used long-course CRT (LCCRT) either alone or after induction chemotherapy. pCR, nodal downstaging, resection margin status, and recurrence analyses were repeated while including only the studies which used induction chemotherapy as part of their TNT protocol. After removing patients who received consolidation chemotherapy, patients in the TNT group were 28% more likely to have complete pathological response (OR 1.28, 95% CI 1.04–1.58, p = 0.022, I2 = 44.5%; eFig. 7), 44% less likely to have residual nodal disease at surgery (OR 0.56, 95% CI 0.41–0.77, p < 0.001, I2 = 33.5%; eFig. 8) and 57% less likely to have positive margins (OR 0.43, 95% CI 0.27–0.66, p = 0.001, I2 = 43.1%; eFig. 9). There were no differences in the rate of local recurrences and distant recurrences, as seen in eFigs. 10 and 11, respectively.
Consolidation Chemotherapy-Type TNT
pCR, nodal downstaging, resection margins status, and recurrence analyses were repeated after eliminating studies that used induction chemotherapy in their TNT protocol. Of note, three of the six studies in this subgroup analysis also used SRT instead of LCCRT. Similar to induction-type TNT, patients who received consolidation-type TNT were 90% more likely to have complete pathological response (OR 1.90, 95% CI 1.48–2.45, p < 0.001, I2 = 0.00%; eFig. 12). Contrary to the results of the induction sequence of TNT, consolidation-type TNT failed to provide better nodal downstaging (OR 1.05, 95% CI 0.85–1.30, p = 0.717, I2 = 66.5%; eFig. 13) and had similar positive margins rates to standard LCCRT (OR 0.87, 95% CI 0.62–1.22, p = 0.472, I2 = 3.82%; eFig. 14). Interestingly, the only studies that had extractable recurrence data in the consolidation TNT subgroup were the three studies that used SRT instead of LCCRT. The pooled data of these three studies, including the recently published RAPIDO trial, demonstrated a significant 27% reduction in the odds of distant recurrence (OR 0.73, 95% CI 0.58–0.92, p = 0.09, I2 = 84.3%; eFig. 15), but at the cost of an 86% higher likelihood of local recurrence (OR 1.86, 95% CI 1.23–2.80, p = 0.04, I2 = 0.00%; Fig. 5). The pooled recurrence rate was 9.3% (6.8–13.4%) versus 5.3% (4.3–7.1%) in the consolidation TNT and non-TNT groups, respectively. Furthermore, this finding of an increased risk of local recurrence had almost no heterogeneity across all studies that use SRT for locally advanced rectal cancer.
Publication Bias
An Egger’s test for publication bias was performed for pCR, given the significant findings with the highest number of publications included in the pooled analysis. It did not show a significant publication bias with p = 0.689, as shown in eFig. 16.
Discussion
The current metanalysis of 15 studies, including 7 randomized control trials, showed favorable outcomes with TNT over standard treatment strategies. An improved nodal response and complete pathological response was observed; a more favorable surgical margin status (R0 resection) was also seen, with no additional morbidity observed. While no difference in local recurrence was seen with TNT, an improved distant recurrence, 3-year DFS, and OS favoring total neoadjuvant therapy are compelling.
The rationale for a TNT strategy is to reduce a patient’s risk of distant recurrence, which was demonstrated in all the pooled studies. The role of adjuvant chemotherapy in the setting of locally advanced rectal cancer treated with preoperative chemoradiation has come into question, with multiple RCTs showing no long-term survival benefits.5,7 The lack of treatment effect and part of the challenge with adjuvant chemotherapy is poor patient tolerance following LCCRT and TME surgery. This was evident in the EORTC 22921 trial, which showed only 43% of patients received the planned dose within the scheduled time interval.5
To overcome this issue, the proponents of TNT seek to improve chemotherapy compliance by delivering all treatment preoperatively rather than postoperatively when patients are disadvantaged from the morbidity and lower performance status associated with major surgery. In this study, compliance was not measured as it was only reported in three studies.18,21,37 From these, both Cercek et al.18 and Fernandez-Martos et al.37 showed a superior compliance rate in the TNT group, achieving a rate of 96% and 94%, respectively, compared with 88% and 57% in the nCRT group. Similarly, the unpublished PRODIGE 23 phase III RCT by Conroy et al. showed a compliance rate for TNT of 91.6% versus 75.3% for adjuvant chemotherapy following nCRT.21 This study also demonstrated a significant improvement in 3-year DSF in favor of the TNT approach (75.7% vs. 68.5%). It should be noted that this advantage could also come from the fact that patients in the TNT group received FOLFIRINOX instead of FOLFOX and received overall, more cycle of chemotherapy than the patients in the CRT group. It is often said that TNT has the advantage of targeting micrometastases early in the disease process. Our analysis confirms this with a lower incidence of distant recurrences which is the main driver of the overall survival advantage observed in our pooled analysis.
The chemotherapy strategies employed in TNT have involved combination regimens administered either before (induction therapy) or after (consolidation therapy) radiotherapy. In most studies, the median interval time to surgery was 4–11 weeks after last dose of treatment. However, it should be noted that those studies that employed consolidation TNT resulted in a longer wait from completion of radiotherapy to surgery. For example, the RAPIDO trial by Bahadoer et al., which reported a median time of 20–22 weeks,31 and Marco et al. with a reported median time of 14–25 weeks from completion of radiotherapy.28 Hence, the efficacy and safety profile of the new TNT strategy (resulting in a longer wait but receiving consolidation chemotherapy) specific to surgery was assessed in this metaanalysis. First, the longer wait from the two above-mentioned studies resulted in much higher pCR rates of 28.4% and 38.5%, respectively. Critically important, Bahadoer et al. showed that, despite the long interval wait for surgery, the TNT group achieved an equivalent R0 resection margin and a significant survival advantage in terms of the risk of distant recurrence.31 It is worth reminding that in the GRECCAR-6 trial, a longer interval time to surgery after CRT (11 vs. 7 weeks) had resulted in similar pCR rates but increased postoperative complications and worse TME quality. Reassuringly, TNT in the RAPIDO trial does not increase postoperative morbidity despite a longer interval time to surgery. The rates of anastomotic leak, wound complications, bleeding, and deep surgical site infection were equivalent between the two groups. Because of the paucity and heterogeneity of the reported data, other perioperative safety metrics of the TNT approach could not be evaluated in this metaanalysis. Nevertheless, data from the unpublished PRODIGE23 trial presented at ASCO2020 demonstrates a similar median hospital stay and rate of overall postoperative morbidity whether TNT or standard LCCRT was used. Moreover, in this study, patients in the TNT group were less likely to undergo a nontherapeutic laparotomy either because of metastatic disease or unresectable primary. Postoperative mortality was also higher in the CRT group (2.8%) versus the TNT group (0%).
One of the interesting observations of our metaanalysis is that, when pooled together, the studies that used short course radiation in their consolidation-type TNT protocol had an 86% increase in local recurrences compared with CRT. Overall, the rate of local recurrence was still low for LARC, with 9.3% versus 5.3% in the pooled SRT TNT and CRT groups, respectively. Nevertheless, this suggests that the additional downstaging expected with long-course CRT is probably the preferred radiation protocol for rectal cancer at higher risk of local recurrence such as T4 disease. On the other hand, short course radiation has its advantages in terms of health costs and patient convenience. Therefore, it is thus a valid option for tumors at lower risk of local recurrence. Since all the studies with local recurrence data in the consolidation TNT subgroup all used short course radiation, it is difficult to ascertain whether the increased local recurrences are due to the radiation protocol used or because of the consolidation sequence with the inherent longer wait time between the end of radiation and surgery. In the unpublished OPRA trial (ClinicalTrials.gov identifier: NCT02008656) the only difference between the two TNT groups was the sequencing of chemotherapy and CRT. Preliminary data from this trial do not suggest that the sequence of chemotherapy affects the rate of local recurrences, although pCR rates were higher with consolidation chemotherapy.
Although this metaanalysis demonstrates improvements in pCR, nodal downstaging, anal preservation, and resection margin with TNT, it should be remembered that the current practice remains a blanket approach for all patients with locally advanced rectal cancer regardless of their risk of distant metastasis. Without pathological information such as tumor morphology, nodal positivity, and lymphovascular invasion (high-risk features for distant metastasis), current stratification relies on radiological evidence such as T4a/b, EMVI positivity and nodal positivity in the mesorectum or lateral pelvic sidewall on MRI as eluded in the RAPIDO trial.31 Therefore, further validation in selecting the most appropriate patient for the TNT approach is required to balance the risk of distant metastasis against the morbidity associated with overtreatment in the form of additional chemotherapy. On the other hand, if safety can be demonstrated, an organ-preservation approach is possible in a higher proportion of patients who receive TNT compared with standard CRT, and this could counterbalance the effects of overtreating some patients who are overstaged by preoperative MRI.
As more data emerges, it is clear that any long-term survival advantage of TNT over standard CRT is through a lower rate of distant recurrences. Our induction and consolidation subanalyses (Table 2) found that only consolidation-type TNT was associated with a statistically significant lower rate of distant recurrences when compared with non-TNT neoadjuvant protocols. The German CAOAROAIO-12 phase II randomized trial compared induction to consolidation-type TNT in stage II and III rectal cancer to pick the best approach for their subsequent CAOAROAIO-18 phase 3 trial comparing TNT with standard of care. In CAOAROAIO-12, only the consolidative chemotherapy group met the prespecified primary end point of a 25% pCR rate (17% pCR with induction chemotherapy and 25% pCR with consolidative chemotherapy). In the unpublished OPRA trial, with a 2.1-year median follow-up, DFS did not seem to be affected by the chemotherapy and radiation sequence. Overall, 77% of the patients in the consolidation chemotherapy group were free of disease at 3 years, compared with 78% in the induction group. There was a significant difference in organ preservation between the two groups, again in favor of the radiation first approach (58 versus 43%, p = 0.01).
There are several limitations to this metaanalysis. First is the TNT protocol heterogeneity, including three studies that used short-course radiotherapy, and hence it is not clear which regimen would give the maximum oncological benefit. Second is the inclusion of nonrandomized observational studies. Third, the analysis only included 3-year oncological outcomes as this was the most commonly reported data point, therefore more mature data will need to be accumulated to determine the true effect on overall survival. Fourth, is that there were only a few studies that had reported on anastomotic leak, with no reported definitions on how anastomotic leak was diagnosed. As an anastomotic leak is a key surgical outcome, particularly with extended chemotherapy with potential immunosuppressive side effects, this would be an important endpoint to document in future studies. Fifth, the only postoperative complication that was consistently reported was anastomotic leak. Other operative outcomes such as surgical site infections, estimated blood loss, medical complications, and length of stay are a few indicators that are equally important and were rarely reported.
Conclusions
The current pooled data have shown that TNT has an immediate positive effect on rectal cancers, in particular downstaging of both the primary site and nodal basin with the added benefit in the rate of anal preservation, distant recurrences, disease-free survival, and 3-year overall survival. Our subanalyses have given some idea on the optimal TNT protocol. Long-course chemoradiotherapy followed by consolidation chemotherapy appears to be a preferable protocol for patients with LARC. In fact, this is the protocol that will be compared with standard CRT in the awaited CAOAROAIO-18 trial as well as the watch and wait JANUS trial. As more RCTs mature, we will have better clarity regarding the potential benefit of TNT on long-term survival. The favorable oncological outcomes of TNT combined with its practical advantages, such as reducing the rate of APR, the time with a stoma,18 and testing the tumor’s sensitivity to chemotherapy agents, potentially makes it the favored approach for locally advanced rectal cancer. TNT is already becoming much more prevalent, and some argue that it should be considered the new standard of care for LARC.38 As we await long-term survival data, it is important that we work on identifying the patient group that will benefit most from the TNT approach. Like in breast cancer, categorizing rectal cancer according to several biomarkers like MMR status and RAS/RAF mutation could potentially help us to tailor neoadjuvant therapy for each patient, thereby maximizing outcomes and limiting unnecessary toxicity.
References
Heald RJ. The “Holy Plane” of rectal surgery. J R Soc Med. 1988;81(9):503–8.
Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30(31):3827–33.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90.
Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223–9.
Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial†. Ann Oncol. 2015;26(4):696–701.
Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and metaanalysis of individual patient data. Lancet Oncol. 2015;16(2):200–7.
Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. JNCCN. 2018;16(7):874–901.
Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–40.
Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56(7):921–30.
Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18(3):336–46.
Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a metaanalysis of published studies. Ann Surg. 2016;263(3):458–64.
Lefevre JH, Mineur L, Kotti S, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016;34(31):3773–80.
Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23(10):2627–33.
Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525–30.
Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–8.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071.
Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and metaanalysis of treatment outcomes. Ann Surg. 2020;271(3).
Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC Multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34(27):3300–7.
Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38(15_suppl):4007.
Quezada-Diaz F, Jimenez-Rodriguez RM, Pappou EP, et al. Effect of neoadjuvant systemic chemotherapy with or without chemoradiation on bowel function in rectal cancer patients treated with total mesorectal excision. J Gastrointest Surg. 2019;23(4):800–7.
Zhu S, Brodin NP, English K, et al. Comparing outcomes following total neoadjuvant therapy and following neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. E Clin Med. 2019;16:23–9.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2020.
Viechtbauer W. Conducting Meta-Analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48.
Liang HQ, Dong ZY, Liu ZJ, et al. Efficacy and safety of consolidation chemotherapy during the resting period in patients with local advanced rectal cancer. Oncol Lett. 2019;17(2):1655–63.
Bhatti AB, Waheed A, Hafeez A, et al. Can induction chemotherapy before concurrent chemoradiation impact circumferential resection margin positivity and survival in low rectal cancers? APJCP. 2015;16(7):2993–8.
Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. 2018;61(10):1146–55.
Markovina S, Youssef F, Roy A, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys. 2017;99(2):417–26.
Calvo FA, Sole CV, Serrano J, et al. Preoperative chemoradiation with or without induction oxaliplatin plus 5-fluorouracil in locally advanced rectal cancer. Long-term outcome analysis. Strahlenther Onkol. 2014;190(2):149-157.
Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: the randomized RAPIDO trial. J Clin Oncol. 2020;38(15_suppl):4006.
Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223–33.
Kim SY, Joo J, Kim TW, et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14–03. Int J Radiat Oncol Biol Phys. 2018;101(4):889–99.
Moore J, Price T, Carruthers S, et al. Prospective randomized trial of neoadjuvant chemotherapy during the “wait period” following preoperative chemoradiotherapy for rectal cancer: results of the WAIT trial. Colorect Dis. 2017;19(11):973–9.
Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26(8):1722–8.
Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859–65.
Healey Bird BRJ. Total neoadjuvant therapy for locally advanced rectal cancer: the fuse is lit. Br J Surg. 2020;107(13):1705–7.
Author information
Authors and Affiliations
Contributions
Joseph Kong, Mikael Soucisse and Alexander Heriot: Substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data. All authors: Drafting and revising the article. All authors: Final approval of the version to be published.
Corresponding author
Ethics declarations
Disclosures
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kong, J.C., Soucisse, M., Michael, M. et al. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann Surg Oncol 28, 7476–7486 (2021). https://doi.org/10.1245/s10434-021-09837-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09837-8