Abstract
Background
Several randomized trials have been performed comparing partial breast irradiation (PBI) and whole breast irradiation (WBI) though controversy remains, including regarding differences by PBI technique. We performed a meta-analysis to compare results between WBI versus PBI and between PBI techniques.
Methods
A systematic review was performed to identify modern randomized studies listed in MEDLINE from 2005 to 2020. PBI trials were divided into external beam radiation and brachytherapy techniques, with intraoperative radiation excluded. A Bayesian logistic regression model evaluated the risk of ipsilateral breast tumor recurrence (IBTR) and acute and chronic toxicities. The primary outcome was IBTR at 5 years with WBI compared with PBI.
Results
A total of 9758 patients from 7 studies were included (4840-WBI, 4918-PBI). At 5 years, no statistically significant difference in the rate of IBTR was noted between PBI (1.8%, 95% HPD 0.68–3.2%) and WBI (1.7%, 95% HPD 0.92–2.4%). By PBI technique, the 5-year rate of IBTR rate for external beam was 1.7% and 2.2% for brachytherapy. Rates of grade 2 + acute toxicity were 7.1% with PBI versus 47.5% with WBI. For late toxicities, grade 2/3 rates were 0%/0% with PBI compared with 1.0%/0% with WBI.
Conclusions
IBTR rates were similar between PBI and WBI with no significant differences noted by PBI technique; PBI had reduced acute toxicities compared to WBI. Because studies did not provide toxicity data in a consistent fashion, definitive conclusions cannot be made with additional data from randomized trials needed to compare toxicity profiles between PBI techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Radiation therapy is an appropriate standard of care for the majority of patients with early-stage breast cancer following breast-conserving surgery (BCS), providing a reduction in both local recurrence and breast cancer-specific mortality.1,2.Traditionally, adjuvant radiation therapy has consisted of whole breast irradiation (WBI) initially delivered over 5–6 weeks, but more recently over 3 weeks with hypofractionated approaches.3 During the past few decades, partial breast irradiation (PBI) has emerged as an alternative to WBI. PBI targets radiation to the area of the lumpectomy bed with a limited margin. This results in potentially less normal breast tissue irradiated and allows for a treatment duration of 1–3 weeks.4,5 PBI can be delivered with multiple techniques, including external beam radiation therapy (3-dimensional conformal radiation therapy [3D-CRT], intensity modulated radiation therapy [IMRT]/volumetric modulated arc therapy [VMAT]), or brachytherapy (interstitial catheters or applicator-based devices), with randomized and/or prospective trials evaluating each of these techniques.5
The comparative efficacy and toxicity profiles of PBI compared with WBI have been published in multiple randomized trials with follow-up periods ranging from 5 to 10 years. We therefore performed a meta-analysis of these trials to evaluate rates of local recurrence and toxicity with PBI compared with WBI. In addition, we evaluated outcomes with external beam PBI techniques compared with brachytherapy PBI techniques.
Methods
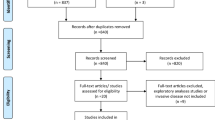
For this meta-analysis, a systematic literature review was performed to identify modern randomized studies comparing PBI to WBI listed in MEDLINE from 2005 to 2020 in conjunction with PRISMA guidelines (no formal protocol).6 Studies were identified using PubMed and Google searches (to identify abstracts) as well as clinicaltrials.gov and major conference proceedings (ASTRO, ASCO, ESTRO, San Antonio Breast Cancer Symposium) with the terms “partial breast irradiation” or “IMRT” and “randomised/randomized,” as well as through use of previous reviews and citations from identified articles (Fig. 1). We initially considered all published randomized trials comparing PBI to WBI therapy from the time period (2005–2020). Once identified, inclusion criteria included randomized trials with greater than 100 patients comparing WBI and PBI as well as IBTR outcomes reported at a minimum of 5 years. PBI techniques were sorted into brachytherapy and external beam radiation therapy.
Exclusion criteria included studies initiated before 1995. Trials from Guy’s Hospital, the Yorkshire Breast Cancer Group, and Christie Hospital were excluded, because they did not apply modern breast-conserving techniques, such as compute tomography (CT)-based treatment planning as well as 3D-CRT or IMRT/VMAT and included patients who would not generally be considered acceptable for breast conservation (larger tumors, positive margins) compared with modern PBI trials. Additionally, randomized trials evaluating intraoperative radiation therapy (IORT) were excluded; modern radiation techniques are based on accurately documenting the dose received to a particular target volume with dose volume histogram (DVH) analyses. IORT, by nature of how it is delivered, does not accurately provide documentation (dosimetry) of the actual dose delivered to a defined target structure or normal tissues (i.e., dose-target analysis) and therefore does not represent a standard approach as to how every other disease site is treated with modern radiation therapy. This is consistent with current evidence-based guidelines regarding PBI and IORT.4,5
PBI trials were divided into external beam techniques and brachytherapy. The majority of PBI patients in the NSABP B39/RTOG 0413 trial were treated with 3D-CRT PBI (73%, n = 1536). For the purpose of the analyses comparing IBTR rates between WBI and PBI, all PBI patients were considered. For the PBI analyses by technique, all PBI patients on the NSABP B39/RTOG 0413 trial were considered external beam as outcomes have not been published by PBI technique. Data were extracted from each trial (when available) including age, menopausal status, tumor size, nodal status, grade 3 disease, lymphovascular space invasion (LVSI), receptor status, endocrine therapy receipt, and chemotherapy receipt.
Outcomes extracted included ipsilateral breast tumor recurrence (IBTR) at 5 years as well as acute (Grade 2 skin toxicity, Grade 3 skin toxicity, Grade 2 + skin toxicities) and late (Grade 2 skin toxicity, Grade 3 skin toxicity, Grade 2 + skin toxicities) toxicities and cosmetic (excellent/good) outcomes when reported. Data regarding cardiac/pulmonary toxicities and second malignancies were not consistently available and not included. This was a study level rather than a patient level meta-analysis. A Bayesian logistic regression model evaluated the risk of above outcomes for evidence of deviation by WBI and PBI techniques. The margin cohort-specific random intercepts characterizing the log-odds of 5-year IBTR and acute or late toxicities assumed hierarchical Gaussian prior distributions with random means and standard deviations. The random hierarchical means followed Gaussian prior distributions centered at 0 and variance = 200. Hierarchical standard deviations followed uniform prior distributions over the interval [0, 10]. Bayesian computation used Markov chain Monte Carlo sampling implemented by JAGS software (version 4.3.0) with the JAGS package in R (version 3.6.2). Statistical estimation reports risk of rate by posterior median and 95% highest posterior density (HPD) interval.
Results
Initially, 1164 studies were identified using our search strategy. Ultimately, seven randomized trials met all inclusion criteria and were included in this study. These included trials from the National Institute of Oncology (Hungary), the Groupe Européen de Curiethérapie (GEC-ESTRO), the University of Florence, Barcelona, the Randomized Trial of Accelerated Partial Breast Irradiation (RAPID), the Intensity Modulated Partial Organ Radiotherapy-LOW (IMPORT-LOW), and National Surgical Adjuvant Breast and Bowel (NSABP) B39/Radiation Therapy Oncology Group (RTOG) 0413 trials (Table 1), as noted in the methods trials using intraoperative radiation were excluded.7,8,9,10,11,12,13,14,15,16,17 A total of 9758 patients were included with 4840 receiving WBI and 4918 PBI.
Table 2 provides a summary of IBTR rates and toxicity outcomes by trial. At 5 years, no statistically significant difference in IBTR was noted between PBI (1.8%, 95% HPD 0.68–3.2%) and WBI (1.7%, 95% HPD 0.92–2.4%). Figure 2 presents rates of IBTR for PBI and WBI for each study, while Fig. 3 presents forest plots for IBTR for PBI and WBI. When evaluating rates of IBTR by PBI technique, the 5-year rate of IBTR for 3DCRT/IMRT was 1.7% (95% HPD 0.10–4.2%) and 2.2% (95% HPD 0–71.1%) for brachytherapy.
With respect to acute skin toxicities, grade 2/3 rates were 7.1% (95% HPD 0–62.5%)/0% (95% HPD 0–1.0%) with PBI compared with 40.0% (95% HPD 0–87.5%)/3.6% (95% HPD 0–59.5%) with WBI. Figure 4 presents rates of acute skin toxicities for PBI and WBI for each study included in the analysis, while Fig. 3 presents the forest plot. Rates of grade 2 or greater acute skin toxicity were 7.1% with PBI (95% HPD 0–63.4%) compared with 47.5% (95% HPD 0–93.4%) with WBI, although wide HPDs were noted. For late skin toxicities, grade 2/3 rates were 0% (95% HPD 0–36.9%)/0% (95% HPD 0–15.6%) with PBI compared with 1.0% (95% HPD 0–49.3%)/0% (95% HPD 0–6.0%) with WBI (Figs. 3 and 5). Rates of late grade 2 or greater skin toxicity were 0% (95% HPD 0–39.3%) with PBI compared with 1.0% (95% HPD 0–52.7%) with WBI. The rate of excellent or good cosmetic outcomes were 89% (95% HPD 40–100%) with PBI compared with 88% (95% HPD 34–100%) with WBI (Fig. 3).
Discussion
The results of the present meta-analysis demonstrate no statistically significant difference in rates of IBTR at 5 years when comparing PBI and WBI. When comparing PBI techniques rates of IBTR, a nonsignificant increase with brachytherapy (2.2%) compared with external beam radiation techniques (1.7%) was noted.
While a previous meta-analysis found inferior IBTR with PBI compared with WBI, it should be noted that the analysis did not include the publication of several of the modern, randomized trials used in the present study and included older trials, which were excluded from the present analysis, because they are inconsistent with modern PBI techniques.4,5,18. The previous meta-analysis had similar results to a 2016 Cochrane review, which also found higher rates of IBTR with PBI. However, the Cochrane review also did not include modern PBI studies, which were only recently published and for this reason stated that definitive conclusions were not possible until ongoing trials (included in the present study) were available.19
A more recent meta-analysis included modern trials but also included studies evaluating IORT and unsurprisingly found higher rates of IBTR. As noted previously, IORT is uniquely different than modern PBI techniques with respect to dose and image guidance such that randomized trials including IORT (TARGIT-A and ELIOT,) demonstrated higher rates of recurrence compared with WBI (TARGIT-A: 3.3% vs. 1.3%; ELIOT: 4.4% vs. 0.4%).4,5,20 Therefore, modern PBI guidelines do not treat IORT in the same category as other PBI techniques, hence our rationale for excluding from the present meta-analysis.4,5 For this reason, the results of the present meta-analysis are consistent with the individual randomized trials and support the use of PBI (external beam and brachytherapy) in appropriately selected patients when applying modern PBI techniques.4,5 Importantly, the techniques used in current meta-analysis are widely available; with respect to external beam techniques, three-dimensional conformal radiation and IMRT are widely available. With brachytherapy, many centers offer applicator brachytherapy (used in NSABP B-39), although interstitial brachytherapy is less commonly available in the United States.
The present analysis focused on rates of IBTR rather than survival. Since rates of IBTR have consistently been shown to be similar between radiation therapy techniques, it is unlikely that a survival difference would exist between PBI and WBI, particularly at 5 years. In addition, no modern, randomized trial has reported a statistically significant difference in overall or breast cancer-specific survival between PBI and WBI. A previous meta-analysis comparing PBI and WBI did identify a reduction in nonbreast cancer mortality and overall mortality with PBI. However, the rationale for the reduction in nonbreast cancer mortality with PBI was not clear. Additionally, the analysis included IORT randomized trials, which as noted above, limits comparison to the present analysis.21,22
A major rationale for utilization of PBI, beyond reducing treatment duration, has been the potential to decrease toxicities compared with WBI. The results of this analysis demonstrate that PBI is associated with a reduction in acute skin toxicities, similar to a previous meta-analysis, with marginal differences in late toxicities.19 However, it is important to recognize that not all studies included in the present analysis provided toxicity data in a consistent fashion (Table 2). The ranges presented were large in some cases, particularly acute skin toxicities. Additionally, acute toxicities may have been higher with WBI due to the longer courses of treatment with WBI; as such, acute toxicities associated with PBI may not be noted until the patient has completed treatment, whereas with WBI, the patient would still be on treatment. As such, definitive conclusions regarding toxicity cannot be drawn from this analysis, particularly without late toxicity outcomes at 10 years. Importantly, toxicity profiles have been shown to vary widely between PBI techniques. For example, the RAPID trial (which utilized 3D-CRT PBI delivered twice daily) demonstrated higher rates of chronic toxicity with PBI despite reduced acute toxicities, whereas the NSABP B-39/RTOG 0413 found no difference with respect to acute and chronic toxicities.11,12 Recently, an analysis of quality of life and cosmetic outcomes from the NSABP B-39/RTOG 0413 trial found similar patient and physician (blinded, standardized digital photo review) rated cosmetic outcomes.23 In contrast, trials utilizing IMRT PBI have found reduced acute and chronic toxicities compared with WBI. This difference (compared with 3D-CRT PBI) may be attributed to improved conformality with IMRT/VMAT as well as differences in fractionation schedules used with IMRT PBI (daily or every other day) compared with 3D-CRT PBI (twice daily).14,15 The inclusion of both techniques in the PBI cohort may explain why our meta-analysis found no difference in cosmetic outcomes as compared to WBI, while a previous meta-analysis found a trend for worse cosmetic outcomes with PBI.20 Long-term follow-up from the trials assessing cardiac and pulmonary toxicities as well as second malignancies would add additional information regarding differences in toxicity profiles between WBI and PBI.
At this time, limited data are available comparing PBI techniques, as most individual randomized trials evaluating PBI (with the exception of the NSABP B-39/RTOG 0413 and the Hungarian NIO trials) included only one PBI technique. The results of our analysis found similar rates of IBTR by PBI technique although there were insufficient data to compare PBI techniques with respect to toxicity and cosmetic outcomes.
When comparing PBI techniques, one confounding factor is that each PBI technique can be associated with different target margins with smaller margins generally associated with brachytherapy and larger margins with external beam radiation therapy. Therefore, future studies are required to elucidate the minimum margin of adjacent, noninvolved breast tissue incorporated into target volumes in order to maximize local control while minimizing normal tissue toxicity. Similarly, the absolute minimum dose required to optimize local control requires further study as doses can correlate with normal tissue toxicity, particularly when hypofractionated regimens (such as those delivered with accelerated courses of PBI) are utilized.
A question facing clinicians is appropriate patient selection for PBI as compared to WBI. With respect to patient and tumor characteristics, the median age for all seven trials collectively was greater than 60 years old, with only the Hungarian NIO and NSABP B39/RTOG 0413 trials having a mean/median age less than 60 years old. The majority of tumors in all of the trials were less than 2 cm, with fewer than 300 cases between 2 and 3 cm in patients undergoing PBI. Similarly, although the NSABP B39/RTOG 0413 trial included more than 200 patients with node-positive disease, approximately 95% of patients treated with PBI in the randomized trials were node-negative. With respect to receptor status, 84% of patients were hormone receptor-positive (83% of those received endocrine therapy) with limited information regarding lymphovascular space invasion. Collectively, these results support that PBI is an appropriate option to WBI for patients older than 50 years with T1, node-negative, hormone-positive tumors.4,5 Importantly, many trials in this analysis excluded patients with lobular cancers; as such, patients with invasive lobular carcinomas are underrepresented in this analysis. At this time, ASTRO guidelines list lobular cancers in the cautionary category for PBI with further study needed. With respect to systemic therapy, 17% of PBI patients received chemotherapy, supporting that PBI may be appropriately utilized in patients receiving systemic treatment. At this time, more data are needed to determine whether PBI can be applied in patients with higher risk features.
There are limitations to the present analysis. We performed a study-based meta-analysis rather than a patient-level meta-analysis, with outcomes derived from data published in the included studies. For example, the NSABP B39/RTOG 0413 trial included approximately 600 patients treated with brachytherapy. However, as outcomes were not published by PBI technique in the final manuscript, PBI results were included with external beam PBI cases as 73% of all PBI patients were treated with this technique.11 While publication bias can exist with meta-analyses, use of randomized trials has limited this effect. However, there were a small number of randomized trials meeting criteria and therefore large HPD intervals. Also, while the meta-analysis followed PRISMA guidelines, a formal protocol was not completed or registered; also, while we completed a search, we did not include sources such as EMBASE, Scopus, and CINAHL. As such, additional studies may have been missed. Finally, there is heterogeneity within PBI techniques with outcomes potentially impacted by use of different radiation target expansions and planning techniques.
Finally, follow-up beyond 5–10 years may be required to assess long-term equivalence and toxicity profiles between PBI and WBI as previous studies found that 20–30% of recurrences occurred beyond Year 5 of follow-up. Although 10-year results were provided in the NSABP B39/RTOG 0413 and Hungarian trials, additional long-term results may be needed to elucidate any small differences between PBI and WBI that may exist. Finally, we did not address differences in surgical techniques and margin definitions in this analysis based on guidelines for invasive cancers supporting no tumor on ink following breast-conserving surgery.
At this time, based on the randomized trials presented, PBI is an appropriate option for appropriately selected patients. Each of the randomized trials included in the analysis showed no difference or less than a 1% difference (NSABP B-39) in local control with three trials (NSABP B-39, RAPID, and National Institute of Oncology) having 8-10 year follow-up supporting the clinical efficacy of PBI. Moving forward, trials are underway to further reduce the duration of PBI schedules as well as utilizing new techniques (e.g., MRI-guided radiation therapy) to reduce target volumes and therefore potential toxicities.
Conclusions
The rates of IBTR at 5 years with PBI were similar to WBI with no differences noted by PBI technique. Overall, acute and chronic skin toxicities were lower with PBI compared with WBI; comparison of toxicity profiles between PBI techniques requires further study.
References
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Early Breast Cancer Trialists’ Collaborative Group (EBCTG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data on 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
Hasan Y, Waller J, Yao K, Chmura SJ, Huo D. Utilization trend and regimens of hypofractionated whole breast radiation therapy in the United States. Breast Cancer Res Treat. 2017;162:317–28.
Correa C, Hariss EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;17:73–9.
Shah C, Vicini F, Shaitelman SF, et al. The American Brachytherapy Society consensus statement for accelerated partial breast irradiation. Brachytherapy. 2018;17:154–70.
Liberati A, Altman DG, Tatzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Polgar C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108:197–202.
Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma- 5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702.
Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–38.
Polgar C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:259–68.
Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–64.
Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): aa randomised controlled trial. Lancet. 2019;394:2165–72.
Olivotto I, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: A randomized trial of accelerated partial breast irradiation using 3D conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–45.
Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51:451–63.
Meattini I, Saieva C, Lucidi S, et al. Accelerated partial breast or whole breast irradiation after breast conservation surgery for patients with early breast cancer: 10-year follow up results of the APBI IMRT Florence randomized phase 3 trial. Cancer Res. 80:GS4–06.
Coles C, Agarwal R, Ah-See ML, et al. Partial breast radiotherapy for women with early breast cancer: first results of local recurrence data for IMPORT LOW (CRUK/06/003). Eur J Cancer. 2016;57:S4.
Rodriguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;87:1051–7.
Marta GN, Macedo CR, Carvalho Hde A, et al. Accelerated partial breast irradiation for breast cancer: systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother Oncol. 2015;114:42–9.
Hickey BE, Lehman M, Francis DP, et al Partial breast irradiation for early breast cancer. Cohrance Database Syst Rev. 2016;7:CD007077.
Korzets Y, Fyles A, Shepshelovich D, et al. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;175:531–45.
Vaidya JS, Bulsara M, Wenz F, et al. Reduced mortality with partial-breast irradiation for early breast cancer: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2016;96:259–65.
Shah C, Wobb J, Khan A. Intraoperative radiation therapy in breast cancer: still not ready for prime time. Ann Surg Oncol. 2016;23:1796–8.
White JR, Winter K, Cecchini RS, et al. Cosmetic outcome from post-lumpectomy whole breast irradiation (WBI) versus partial breast irradiation (PBI) on the NRG Oncology/NSABP B39-RTOG0413 Phase III Clinical Trial. Int J Radiat Oncol Biol Phys. 2019;105:S5.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Chirag Shah- Consultant Impedimed and PreludeDx, Grant and Travel Expenses Varian Medical Systems, Grant- VisionRT, Grant- PreludeDx; Brian Hobbs- Research Funding Amgen, Scientific Advisor Presagia; Martin Keisch- Consultant Hologic; Neilendu Kundu- Consultant 3 M/Acelity; Rahul Tendulkar - honoraria from Varian Medical Systems for educational talks; Simona Shaitelman - Grant from Varian; David Wazer - Medical Advisory Board, Advanced Radiation Therapy, Inc; Frank Vicini - Consultant for ImpediMed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, C., Jia, X., Hobbs, B.P. et al. Outcomes with Partial Breast Irradiation vs. Whole Breast Irradiation: a Meta-Analysis. Ann Surg Oncol 28, 4985–4994 (2021). https://doi.org/10.1245/s10434-020-09447-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09447-w