Abstract
Background
The aim of this work is to evaluate pattern of care and clinical outcome in a large series of patients with in-breast recurrence (IBR), after quadrantectomy and intraoperative radiation therapy with electrons (IOERT) as partial breast irradiation.
Patients and Methods
Patients with IBR after IOERT, treated with salvage surgery ± adjuvant reirradiation (re-RT), were selected from a multiinstitution database. Disease-free survival (DFS), overall survival (OS), cumulative incidence of second IBR, and distant metastases (DM) were estimated.
Results
A total of 224/267 patients from seven institutions were included. Primary tumors received 21 Gy. Median time to first IBR was 4.3 years (range 2.6–6.1 years). Salvage mastectomy and repeat quadrantectomy were performed in 135 (60.3%) and 89 (39.7%) patients, followed by adjuvant re-RT in 21/135 (15.5%) and 63/89 (70.8%), respectively. Median follow-up after salvage treatment was 4.1 years. Overall, 5- and 8-year outcomes were as follows: cumulative incidence of second IBR: 8.4% and 14.8%; cumulative incidence of DM: 17.1% and 22.5%; DFS: 67.4% and 52.5%; OS: 89.3% and 74.7%. The risk of second IBR was similar in the salvage mastectomy and repeat quadrantectomy + RT groups [hazard ratio (HR) 1.41, p = 0.566], while salvage mastectomy patients had greater risk of DM (HR 3.15, p = 0.019), as well as poorer DFS (HR 2.13, p = 0.016) and a trend towards worse OS (HR 3.27, p = 0.059). Patients who underwent repeat quadrantectomy alone had worse outcomes (second IBR, HR 5.63, p = 0.006; DFS, HR 3.21, p = 0.003; OS, HR 4.38, p = 0.044) than those adding re-RT.
Conclusions
Repeat quadrantectomy + RT represents an effective salvage approach and achieved local control comparable to that of salvage mastectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Accelerated partial breast irradiation (APBI), as a component of breast conservation therapy, was an emerging paradigm in the early 2000s and has contributed to the radical change in the adjuvant treatment of women with early breast cancer over the past 2 decades.1,2 Since its introduction, use of APBI has increased sharply, with a nearly 10-fold increase noted between 2002 and 2007.3 often including patients who would have been later identified as poor candidates according to the dedicated guidelines on APBI first released in 2009 and 2010.4,5,6 Given the growing number of patients undergoing APBI, the best clinical management and outcome of in-breast recurrences (IBRs) are topics of great interest.
Salvage mastectomy plays a major role after IBR following breast conserving surgery,7 although in selected cases a second breast conservation can be considered with the recommendation of evaluating reirradiation.8,9 When IBR occurs after APBI, the choice of treatment options is even more challenging since limited published data are available.10,11 The two study groups (SGs), namely “Brachytherapy, Interventional Radiotherapy, and Intraoperative Radiotherapy (IORT)” and “Reirradiation,” of the Italian Radiotherapy and Clinical Oncology Society (AIRO) promoted and supported a multicentric study including women who experienced locoregional failure after being treated with conservative surgery and intraoperative electron radiotherapy (IOERT), one of the modalities currently used to perform APBI, to investigate the type of salvage treatment and analyze the outcome.
Patients and Methods
A dedicated database was initiated on behalf of the two AIRO dedicated SGs in 2013 to collate data of patients who experienced IBR after having received breast-conserving surgery and APBI with IOERT as part of the treatment of the primary breast tumor. The database was open to all the Italian centers equipped with IOERT and was managed by the European Institute of Oncology (IEO) as coordinating study center. Participation was on a voluntary basis and did not have any financial support. Each institution identified patients through their own tumor registries or databases and provided the data of interest both retrospectively and prospectively. The first medical records went back to March 2000. Follow-up was regularly performed, and systemic adjuvant therapies varied according to each institutional policy.
To allow the current analyses, the continuous data entry process was temporarily frozen in March 2016.
At the time of analysis, data from 267 patients were collected. For the purpose of the current study, only patients presenting IBR, with or without concomitant axillary involvement, excluding those having regional recurrence alone or metastatic disease as first event (N = 43), were considered. The aim is to analyze the type of salvage local treatment and the clinical outcome.
Overall, 7313 patients, of whom 967 entered clinical trials, were reviewed at seven Italian radiotherapy centers, and data from 224 patients were available. Considering the rate of relapsed patients out of the total number treated at each institution, the figures were the following: Bergamo-Ospedale Papa Giovanni XXIII (N = 58/778), Città di Castello-Ospedale di Città di Castello (N = 5/200), Genova-IRCC Azienda Ospedaliero/Universitaria San Martino IST (N = 7/380), Milano-IEO (N = 140/5249), Pavia-ICS Maugeri (N = 1/138), Roma-San Filippo Neri (N = 2/28), and Trento-Ospedale Santa Chiara (N = 11/540).
All patients gave consent for use of anonymized data for research and training purposes. The data collection and analyses were approved by the IEO Ethics Committee.
Participating centers were asked to classify IBRs by their clinical location in relation to the primary tumor site, according to the criteria described by Recht et al.12 A true recurrence/marginal miss (TR/MM) was defined as an IBR within or immediately adjacent to the primary tumor site; otherwise, it was considered as an elsewhere failure (E).
Primary tumor and IBR were also grouped according to molecular classification. In case of moderate overexpression of human epidermal growth factor receptor 2 (HER2) status 2+, HER2 gene amplification by fluorescence in situ hybridization test was applied.
When adjuvant reirradiation (re-RT) was given, modalities varied. After salvage mastectomy, re-RT was delivered either to the chest wall ± axillary nodes, in locally advanced stages, or to the nipple–areola complex, in case of nipple-sparing mastectomies. After repeat quadrantectomy, re-RT included either whole breast irradiation (WBI) or APBI by means of IOERT or external-beam RT.
Statistical Methods
The primary objective of the present study is to assess the clinical outcome following IBR after IOERT in terms of in situ or invasive disease-free survival (DFS) and overall survival (OS), both measured from date of salvage surgery. DFS and OS were defined by the standardized efficacy endpoints (STEEP) criteria.13 The other endpoints evaluated were cumulative incidence of second IBR and distant metastasis. The definition of second IBR included either in situ or invasive event. Acute and late toxicity were scored according to the Radiation Therapy Oncology Group (RTOG) scale and late effects in normal tissues–subjective, objective, management, and analytic (LENT-SOMA) scales, respectively. A four-point scale was used for cosmetic outcome (excellent, good, fair, or poor), and agreement between physicians and patients was measured using the weighted Cohen’s kappa coefficient.
Associations between types of salvage treatment and risk factors were evaluated using the Chi squared test for categorical variables and the t test for continuous variables.
DFS and OS functions were estimated using the Kaplan–Meier method. The log-rank test was used to assess differences between groups.
Cumulative incidence of second IBR and distant metastases curve functions was estimated according to methods described by Kalbfleisch and Prentice,14 taking into account the competing causes of recurrence. Gray’s test was used to assess differences between groups.
Univariable and multivariable Cox proportional hazard regression models were used to assess the association of type of salvage treatment with clinical and histopathologic characteristics, measured at the first IBR, on survival outcomes.
All analyses were performed using SAS software v. 9.4 (SAS Institute, Cary, NC). All tests were two-sided, and p values < 0.05 were considered statistically significant.
Results
Primary Tumor and First IBR Characteristics
Primary tumor, first IBR, and treatment characteristics are summarized in Table 1. At the time of primary tumor, 84.4% of the patients were > 50 years old. All patients presented initially with invasive breast cancer and underwent quadrantectomy, IOERT as the sole radiation treatment, and tailored systemic therapy. The IOERT dose consisted of 21 Gy at 90% isodose, in nearly all of them (with only 1.3% of women receiving less than 21 Gy), and was mainly delivered with applicators of 4-cm (41%) and 5-cm (43%) diameter.
The median interval between primary tumor and first IBR was 4.3 years. First IBR was defined as TR/MM in 124 patients [55.4%; median interval: 4.6 years; interquartile range (IQR): 2.4–7.0 years] and E in 75 patients (33.5%: median interval: 4.1 years; IQR: 2.7–6.1 years). The remaining sites were multicentric or not categorizable (inflammatory, angiosarcoma, unknown, etc.).
The median size was 15 mm (range 1–60 mm) for primary tumor and 12 mm (range 1–25 mm) for first IBR. In one-third of the first IBRs, repeat surgical axillary investigation was omitted on the basis of absence of clinical involvement (cNx: 33%).
Salvage Treatment
Salvage surgery consisted of either mastectomy, in most of the cases (60%), or repeat quadrantectomy, with or without axillary lymph node investigation (sentinel node/axillary dissection, according to previous surgery). Re-RT with different modalities was offered in a number of cases. After the second attempt at breast-conserving surgery, reirradiation was performed in 63 patients, mainly with WBI (N = 46, median dose 45 Gy, range 28.5–60 Gy, boost in only 8 cases) and, to a lesser extent, with a second APBI (N = 17), consisting of IOERT in most cases (N = 13), and external-beam RT in the remaining 4 women (median dose 47.5 Gy, range 37.05–50 Gy). Second IOERT was delivered with 21 Gy in 69% of cases and 16–18 Gy in the remaining ones, and smaller collimator size (4 cm in 92% of cases) compared with primary tumors. The main criterion for delivering WBI rather than second APBI was the site of recurrence in the breast: when first IBR was TR/MM, WBI was offered in 70% of the cases (32/46), whereas APBI in 29% (5/17).
Postmastectomy re-RT to the chest wall ± nodal basin was delivered to locally advanced stages. IOERT to the parenchyma behind the nipple–areola complex was reserved for ten patients undergoing nipple-sparing mastectomy. Analyzing the type of salvage treatment (Table 2), salvage mastectomy was given preferably in cases of earlier IBR onset, locally advanced stages, and estrogen receptor negative status. The salvage mastectomy rate increased as the tumor extension increased and was not found to be significantly related to histology, grade, or nodal status. Repeat quadrantectomy alone was mainly reserved for older patients (median age 73 years) with hormone-responsive tumors and was more frequently associated with lack of axillary investigation (54% pNx).
Survival Rates and Local Control
At median time of 4.1 years (min: 3 months, max: 13.5 years) from first IBR, 72 patients had further events. In more detail, there were 21 second IBRs, 4 isolated axillary node metastases, 34 distant metastases, 3 contralateral breast cancer, 4 primary tumors in other sites, and 6 deaths as first event. Earlier IBR occurrence indicated poor DFS and OS, while IBR size and nodal status were prognostic factors for DFS and triple-negative molecular subgroup for OS (Supplementary Tables 1S, 2S). The cumulative incidence of second IBR, DFS, and OS at 5 and 8 years is detailed in Table 3. Clinical outcome was different when analyzed according to type of salvage treatment: salvage mastectomy group showed a statistically significant greater risk of events (DFS, HR 2.13; Table 3, Fig. 1a) and distant metastases (HR 3.15, p = 0.019; Table 3, Fig. 1d) than repeat quadrantectomy + RT group, with a trend towards worse OS as well (HR 3.27, p = 0.059; Table 3, Fig. 1b). Cumulative incidence of second IBR was comparable between salvage mastectomy and repeat quadrantectomy + RT groups (HR 1.41; Table 3, Fig. 1c). Compared with repeat quadrantectomy + RT, repeat quadrantectomy alone was burdened with significantly lower outcome with respect to local control and survival (Table 3). In the repeat quadrantectomy + RT group, comparison between the two salvage radiation modalities showed statistically higher second IBRs rate for APBI compared with WBI (three events versus one event, the latter occurring after 10 years of follow-up), but no difference in terms of DFS, OS, or distant metastases incidence (Figure 1S). No difference in terms of DFS or OS was observed between TR/MM and E recurrences (Supplementary Table 1S, 2S).
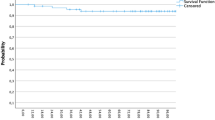
Since the worse prognosis observed in patients receiving salvage mastectomy can be easily explained by more advanced disease, subgroup analysis was performed comparing salvage mastectomy alone (without re-RT) and repeat quadrantectomy + RT groups, for small unicentric IBRs (≤ 2 cm) with absence of or limited nodal involvement. The comparison of 63 patients treated with salvage mastectomy alone with 56 with repeat quadrantectomy + RT showed no statistically significant difference in terms of DFS or cumulative incidence of second IBR between the two groups (Fig. 2). The hazard ratio for DFS in the salvage mastectomy alone group compared with repeat quadrantectomy + RT was 2.01 [95% confidence interval (CI) 0.89–4.52, p = 0.091] and dropped to 1.18 (95% CI: 0.46–2.90, p = 0.733) when time to first relapse, site across the breast, size, grade, and lymphovascular invasion were included in a multivariable model.
Disease-free survival (a) and cumulative incidence of second in-breast reappearances (IBR) (b), observed from date of salvage surgery, by type of salvage treatment (mastectomy and repeat quadrantectomy + radiotherapy), in a subgroup of patients having favorable characteristics (tumor size at first relapse ≤ 20 mm and absent/limited nodal involvement)
Toxicity
Toxicity was investigated in the 63 patients who underwent repeat quadrantectomy + RT, but information on acute and late side effects was available for only 35 and 33 patients, respectively. Regarding acute toxicity, no grade 3 (according to RTOG scale) was observed, while grade 2 toxicity included breast edema (11%), patchy desquamation (3%), and erythema (3%). Regarding late toxicity, according to LENT-SOMA, one patient (3%) complained of grade 3 breast pain, and four patients (12%) of grade 1 breast pain. Grade 2 fibrosis occurred in two patients (6%), and grade 1 in ten women (30%). Skin discoloration was observed in seven cases (21%), without telangiectasia. Radiological liponecrosis was seen in four cases (12%). Comparing toxicity between patients retreated with APBI and those reirradiated with WBI, there was no statistically significant difference (two-sided Fisher’s exact test) for any item of the LENT-SOMA scale (Supplementary Table 3S), although the small number of events and subjects did not lend sufficient statistical power to the tests to detect such a difference, if present.
Twenty-four and 18 cosmetic evaluations were available from physicians and patients, respectively, in the group of 46 women treated with repeat quadrantectomy + WBI. Cosmesis was rated as excellent/good in 22 cases by physicians and in 13 cases by patients. The interobserver agreement was low (0.3, 95% CI 0–0.70).
Discussion
To the best of the authors’ knowledge, the present work is one of the few published on the outcome of patients who locally relapsed after APBI, and represents the largest such series. The two other reports existing in current literature were based on a small number of IBRs.10,11 In both of those studies, almost all the local relapses were treated with salvage mastectomy, although a second conservative approach, reserved for a small minority, seemed to provide equivalent clinical outcome. In the series presented herein too, most salvage treatments consisted of mastectomy (135/224, 60%), but repeat quadrantectomy + RT was delivered to a considerable number (63/224, 28%). The paucity of published data must not overshadow the importance of the subject. The use of APBI with increasingly well-defined selection criteria has been expanding15 over time, resulting in a growing pool of patients who may face an IBR due to the tumor natural history and response to treatments. This new scenario is bound to ignite the debate on local management, which is still intensively ongoing in case of IBR following breast-conserving surgery and WBI.8,16
It is controversial whether the outcome after APBI local failure follows the same pattern as after WBI. Both clinical scenarios shared higher incidence of TR/MM IBRs and many well-known risk factors of poor prognosis, such as shorter time to recurrence.17,18 IBR after WBI may be an expression of biologic aggressiveness and intrinsic resistance to RT. In case of APBI failure, in addition to tumor biology, other factors may play a crucial role, such as inadequate target coverage and undetected distant tumor foci.19 This observation may explain the lack of difference in terms of DFS and OS in the current series between IBRs likely to be TR/MM and those occurred in separate quadrants (E), which are generally interpreted as new second primaries with better prognosis.20
The most important finding of this analysis is that addition of re-RT to a second quadrantectomy resulted in a statistically significant improvement not only in local control but also in DFS and OS compared with repeat quadrantectomy alone. Even more notable is the finding that repeat quadrantectomy + RT provided local control and survival outcomes comparable to salvage mastectomy. This findings are in line with the results of a recent systematic review on the feasibility of repeat quadrantectomy + RT for IBR after breast-conserving surgery followed by WBI,9 suggesting that the conservative option can also be offered to patients who locally relapse after APBI. In the aforementioned review, analyses of the pooled data revealed an oncological advantage of adding RT to the repeat quadrantectomy, with acceptable toxicity and reduction of second IBR rates by an estimated 18%, so that this approach might be considered as a feasible alternative to salvage mastectomy. Therefore, small IBRs can potentially be successfully treated with repeat quadrantectomy, as long as it is technically feasible and reirradiation is planned. However, since such a conservative approach might impair the cosmetic outcome and increase the risk of potential complications, the final decision on the treatment of choice must be shared with well-informed patients.21,22
It is widely accepted that IBR is an independent predictor of worse OS.23 As a whole, in the present study, OS was 89.3% at 5 years, which is more favorable than the incidences of 59.9% and 76.6% found respectively for positive- and negative-node patients included in five National Surgical Adjuvant Breast and Bowel Project randomized studies24,25 analyzing outcome after WBI failure. The survival results provided by the current study, especially in the repeat quadrantectomy + RT group, where salvage treatment of small operable tumors was maximized, showed that IBR after APBI failure might be associated with better prognosis than IBR after WBI failure. A similarly good OS rate of 88.7% was also observed when reirradiation was combined with repeat quadrantectomy after WBI failure, in the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology Breast Cancer Working Group multicentric study.26
The impact of salvage local treatment on OS has long been studied in case of IBR after WBI. While in some studies the extension of salvage surgery (either mastectomy or second quadrantectomy) did not have any impact on OS,27,28 in others salvage mastectomy conferred better survival outcome compared with repeat quadrantectomy alone,29,30 as described by Chen et al. using the Surveillance, Epidemiology, and End Results database. In their analysis, those authors emphasized that combining a second conservative surgery with re-RT may improve survival, although they could not further elaborate the finding due to concerns regarding selection bias. In the series presented herein, salvage mastectomy was mainly offered to more aggressive tumors, and as a consequence, it was followed by postmastectomy re-RT in 21 out of 135 cases and burdened by a higher incidence of distant metastases. Receptor-positive tumors (luminal A and B) are generally felt to have a more indolent evolution and were more frequently treated with repeat quadrantectomy. The tendency of being driven by molecular subtypes in the choice of type of salvage treatment has already been documented throughout literature,31 although the biologic aggressiveness of triple-negative and HER2-enriched subtypes goes somewhat beyond the local treatment.32 In the current series, salvage mastectomy and repeat quadrantectomy + RT did not show any statistically significant difference in survival, when considering either the overall population or the selected group with small IBRs.
It must be highlighted that the worst outcomes occurred in patients undergoing repeat quadrantectomy alone, which was given to IBRs with more favorable profile (later onset, older age, positive hormonal receptors) and receiving hormonal therapy. Therefore, in line with the recommendations of the DEGRO guidelines8 when a second breast conservation is planned, evaluation of additional reirradiation should always be considered.
The findings of the present work reinforce the concept that IBRs must be treated with curative intent, since minimizing the extension of salvage local treatment appeared to be detrimental.
Besides the benefit of adding RT to a second breast conservation, the extension of RT fields plays a crucial role in achieving good local control. In fact, patients retreated with WBI experienced fewer further local events than those receiving second APBI. Therefore, reirradiation with WBI should be preferred, although this approach can pose technical challenges when IBR occurs distant to the primary tumor site and the previously irradiated area is still in place. It can be speculated that, as reirradiation with APBI after previous WBI has proven to be relatively safe, with weighted estimates for grade III–IV acute and late complications ranging from 9% to 18%,9 a similar argument can be made for reirradiation with WBI after APBI. The choice of salvage mastectomy depends on many factors, mainly being based on tumor characteristics and stage,33 as the current study showed, but also on patient preference and physician attitude, especially at the time when conservative mastectomies, namely skin or nipple sparing, are on the rise.34
The present work has a number of limitations. Some data regarding histologic and biomolecular relapse features were missing. The distinction between TR/MM and E recurrences was based only on the location across the breast. Although this is the largest series reported in current literature, retreatment of first IBR was not homogeneous. This resulted in analyses of subgroups whose small size might have not shown statistically significant differences. Only 7 out of more than 40 Italian centers equipped with IOERT agreed to pool their data, and they had different follow-up policies. Considering that the study was partly conducted retrospectively, it is not possible to comment on breast toxicity given the paucity of available data after the second course of RT. In addition, the findings were related to a single modality of APBI. However, this study remains unique and provides some interesting information on the outcome and management of patients who relapse after IOERT. The finding of the efficacy of repeat quadrantectomy + RT for local control and survival outcomes suggests that IBR after APBI failure could be effectively treated with a second conservative approach.
References
Mannino M, Yarnold J. Accelerated partial breast irradiation trials: diversity in rationale and design. Radiother Oncol. 2009;91(1):16–22.
Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90(1):1–13. Review. Erratum in: Radiother Oncol. 2011;99(2):254.
Husain ZA, Mahmood U, Hanlon A, Neuner G, Buras R, Tkaczuk K, et al. Accelerated partial breast irradiation via brachytherapy: a patterns-of-care analysis with ASTRO consensus statement groupings. Brachytherapy 2011;10(6):479–85.
Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol. 2010;94(3):264–73.
Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7(2):73–9.
Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74(4):987–1001.
Halyard MY, Harris EE, Bailey L, Bellon JR, Freedman GM, Goyal S, et al. ACR appropriateness criteria local-regional recurrence (LRR) and salvage surgery-breast cancer. Oncology (Williston Park) 2014;28(2):157–64, C3.
Harms W, Budach W, Dunst J, Feyer P, Fietkau R, Haase W, et al. Breast cancer expert panel of the German society of radiation oncology (DEGRO). DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences. Strahlenther Onkol. 2016;192(4):199–208.
Walstra CJEF, Schipper RJ, Poodt IGM, van Riet YE, Voogd AC, van der Sangen MJC, et al. (2019). Repeat breast-conserving therapy for ipsilateral breast cancer recurrence: a systematic review. Eur J Surg Oncol. 2019. https://doi.org/10.1016/j.ejso.2019.02.008.
Shah C, Wilkinson JB, Jawad M, Wobb J, Berry S, Mitchell C, et al. Outcome after ipsilateral breast tumor recurrence in patients with early-stage breast cancer treated with accelerated partial breast irradiation. Clin Breast Cancer. 2012;12(6):392–7.
Shah C, Vicini F, Keisch M, Kuerer H, Beitsch P, Haffty B, et al. Outcome after ipsilateral breast tumor recurrence in patients who receive accelerated partial breast irradiation. Cancer. 2012;118(17):4126–31.
Recht A, Silen W, Schnitt SJ, Connolly JL, Gelman RS, Rose MA, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1988;15(2):255–61.
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–32.
Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 1st ed. New York: Wiley; 2011.
Hoopes DJ, Kaziska D, Chapin P, Weed D, Smith BD, Hale ER, et al. Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):674–81.
Montagne L, Gal J, Chand ME, Schiappa R, Falk AT, Kinj R, et al. GEC-ESTRO APBI classification as a decision-making tool for the management of 2nd ipsilateral breast tumor event. Breast Cancer Res Treat. 2019;176(1):149–57.
Gentilini O, Botteri E, Veronesi P, Sangalli C, Del Castillo A, Ballardini B, et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: criteria for selecting the best candidates. Ann Surg Oncol. 2012;19(12):3771-6.
Gentilini O, Botteri E, Rotmensz N, Santillo B, Peradze N, Saihum RC, et al. When can a second conservative approach be considered for ipsilateral breast tumour recurrence? Ann Oncol. 2007;18(3):468–72.
Kirby AM, Coles CE, Yarnold JR. Target volume definition for external beam partial breast radiotherapy: clinical, pathological and technical studies informing current approaches. Radiother Oncol. 2010;94(3):255–63.
Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48(5):1281–9.
Kaidar-Person O, Oldenborg S, Poortmans P. Re-irradiation and hyperthermia in breast cancer. Clin Oncol. (R Coll Radiol) 2018;30(2):73–84.
Al-Hilli Z, Grobmyer SR. Management strategies for locally recurrent breast cancer: redo-lumpectomy, redo-sentinel node biopsy, redo-radiation. Ann Surg Oncol. 2019. https://doi.org/10.1245/s10434-019-07545-y.
Botteri E, Bagnardi V, Rotmensz N, Gentilini O, Disalvatore D, Bazolli B, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol. 2010;21(4):723–8.
Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028–37.
Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27(15):2466–73.
Hannoun-Levi JM, Resch A, Gal J, Kauer-Dorner D, Strnad V, Niehoff P, et al. GEC-ESTRO Breast Cancer Working Group. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother Oncol. 2013;108(2):226–31.
Alpert TE, Kuerer HM, Arthur DW, Lannin DR, Haffty BG. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys. 2005;63(3):845–51.
Fodor J, Major T, Polgár C, Orosz Z, Sulyok Z, Kásler M. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast 2008;17(3):302–8.
Galper S, Blood E, Gelman R, Abner A, Recht A, Kohli A, et al. Prognosis after local recurrence after conservative surgery and radiation for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;61(2):348–57.
Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg. 2008;196(4):495–9.
Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–41.
Montagna E, Bagnardi V, Rotmensz N, Viale G, Renne G, Cancello G,et al. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2012;23(2):324–31.
Botteri E, Rotmensz N, Sangalli C, Toesca A, Peradze N, De Oliveira Filho HR, et al. Unavoidable mastectomy for ipsilateral breast tumour recurrence after conservative surgery: patient outcome. Ann Oncol. 2009;20(6):1008–12.
van Huizum MA, Hage JJ, Rutgers EJ, Hoornweg MJ. Immediate breast reconstruction with a myocutaneous latissimus dorsi flap and implant following skin-sparing salvage mastectomy after irradiation as part of breast-conserving therapy. J Plast Reconstr Aesthet Surg. 2016;69(8):1080–6.
Acknowledgement
The abstract was presented in the poster viewing session at ESTRO 36 Vienna, 5–9 May 2017 (Abstract Number PV 0237) and as an oral presentation (Abstract Number 0221) at AIRO Congress, 11–13/2017 Rimini, Italy. This work was partially supported by a research grant from Accuray Inc. The sponsor did not play any role in the study design, collection, analysis, and interpretation of data, nor in the writing of the manuscript or the decision to submit the manuscript for publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Disclosure of potential conflict of interest
All the authors declare that there is no actual or potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leonardi, M.C., Tomio, L., Radice, D. et al. Local Failure After Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy with Electrons: An Insight into Management and Outcome from an Italian Multicentric Study. Ann Surg Oncol 27, 752–762 (2020). https://doi.org/10.1245/s10434-019-08075-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08075-3