Abstract
Purposes
Tumor regression grade (TRG) of the primary tumor after neoadjuvant therapy is one of the most sensitive prognostic factors among patients with locally advanced esophageal cancer, although no TRG system is fully accepted. The Ryan score was proposed in 2005 to evaluate TRG in rectal cancer and could be adaptable for pathological evaluation of esophageal cancer. The objective of this study is to evaluate the prognostic value of the Ryan score for esophageal cancer in the setting of trimodal therapy.
Methods
We performed a retrospective cohort study in which patients with locally advanced esophageal cancer, submitted to neoadjuvant therapy followed by surgical resection, were selected. One hundred thirty-four patients were selected. All tissue specimens were assessed as per the TRG system proposed by Ryan et al. Survival curves were assessed by the Kaplan–Meier method and log-rank test. Chi-square test or likelihood-ratio test was used for absolute and relative variables. Kruskal–Wallis and analysis of variance tests were used to assess significant differences on a continuous dependent variable by a categorical independent variable.
Results
Of the 134 included patients, 94 (70.1%) had squamous cell carcinoma, and 40 (29.9%) adenocarcinoma. Ryan score was correlated with histological type (p < 0.001), and clinical (p = 0.044) and pathological (p < 0.001) staging. Mean follow-up was 31.1 months. Multivariate analysis showed that Ryan score can safely predict survival, and systemic and lymphatic recurrence (p < 0.05).
Conclusions
Ryan score is an effective system to evaluate TRG and can predict risk for lymph node or distant metastasis, overall survival, and disease-free survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgical resection is the primary treatment for localized esophageal carcinoma. However, since the CROSS Group1 reported good results of neoadjuvant therapy, preoperative chemoradiation has become the standard treatment among most patients with potentially curable esophageal cancer.1,2 Many systematic reviews favor preoperative chemoradiotherapy for locally advanced esophageal cancer, since it improves survival rates and reduces margin-positive resections and long-term recurrence.3 Complete pathologic response has been associated with lower recurrence rates and higher survival rates and is, therefore, an important prognostic factor.4
Many studies have proposed scores to evaluate pathological response as a way to predict long-term prognosis.3 In 1994, Mandard et al. first published a five-tier system for evaluating TRG in esophageal carcinoma. At the time, it had already shown a significant association with overall survival and disease-free survival.5 Many other systems were proposed in the following years: Chirieac et al. suggested, in 2005, a three-tier system with percentage cut-points,6 and, in the same year, Schneider et al. published a four-tier system that considers lymph node involvement.7 Each one of these systems emphasizes determinate histological features, evaluating the presence/absence of residual cancer cells differently, as described in Table 1.
Although many systems evaluate TRG in esophageal cancer, none of them is entirely accepted.3 One study attempted to compare which system was the most accurate in predicting prognosis, with inconclusive results.3
In 2005, Ryan et al. published a practical three-point system to evaluate TRG of patients with locally advanced rectal adenocarcinoma who underwent neoadjuvant treatment.8 Compared with other systems, it is associated with more concordance between pathologists, with better reproducibility and similar prognostic significance.8,9
Theoretically, Ryan score could be adaptable for pathological evaluation of other tumors, e.g., in the esophagus. The prognostic value of the Ryan score for evaluating TRG in esophageal cancer, considering trimodal therapy, with neoadjuvant chemoradiotherapy using platinum and taxane-based regimen followed by curative-intent esophagectomy, has not been reported and is the aim of this study.
Methods
Study Subjects
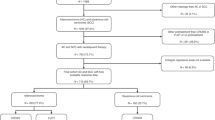
We performed a retrospective cohort study in which patients at a single institute with completion of neoadjuvant chemoradiotherapy using platinum- and taxane-based regimens, followed by curative-intent esophagectomy, were selected.
Recruitment was performed from 2009 to 2019. Patients were staged with endoscopy, computed tomography (CT) scan, and positron emission tomography (PET) scan before neoadjuvant therapy and classified as per the eight edition of the Union for International Cancer Control (UICC) staging.10 Neoadjuvant therapy was indicated for any clinical stage with T3–4 or N1–3, and M0. Radiation field was defined as a 4-cm superior/inferior clinical target volume expansion (CTV) and 1.0–1.5-cm radial CTV expansion.
Patients were followed with clinical evaluations, peripheral blood analysis, CT scans, and endoscopies. The local ethics committees approved the study.
All surgical specimens were assessed by two pathologists for pathological response to neoadjuvant chemoradiotherapy, and the primary tumor was graded accordingly to Ryan score8 (Fig. 1):
Examples of esophageal carcinoma scored accordingly to Ryan score after neoadjuvant chemoradiotherapy. Score 1: microscopic evaluation demonstrating complete response, with extensive fibrosis (a), microcalcifications (b), and pools of acellular mucin (c); rare small groups of cancer cells (arrow; d); Score 2: squamous cell carcinoma with evident tumor regression, fibrosis, chronic inflammatory infiltrate, and microcalcifications, but more than single cells or rare small groups of cancer cells (left side; e); Score 3: poor or no response with extensive adenocarcinoma residual (f). Hematoxylin and eosin
Score 1: complete response (no viable cancer cells) or near-complete response (single cells or rare small groups of cancer cells)
Score 2: partial response (residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells)
Score 3: poor or no response (extensive residual cancer with no evident tumor regression)
The modified Ryan score (a four-tier system) was also evaluated for prognosis. In this system, the pathological complete response (no viable cancer cells) was taken as a score apart from near-complete response (single cells or rare small groups of cancer cells).
Statistical Analysis
Chi square test or likelihood-ratio test was used for each outcome concerning absolute and relative variables. Kruskal–Wallis and ANOVA tests were used to assess significant differences on a continuous dependent variable by a categorical independent variable. Data were assessed using IBM-SPSS software version 20.0, and a significance level of 0.05 was adopted.
Results
One hundred thirty-four patients underwent neoadjuvant chemoradiotherapy using platinum- and taxane-based regimens, followed by esophagectomy, and were included. The mean follow-up was 31.1 months (SD 22 months). The mean age was 60.1 years (SD 8.5 years), with male predominance (75%). In total, 104 transthoracic (video-assisted thoracoscopic) procedures and 30 transhiatal procedures were identified, all of them with cervical anastomosis. The median time from completion of neoadjuvant chemoradiotherapy to surgery was 13.4 weeks [interquartile range (IQR) 8 weeks]. The two chemotherapy regimens adopted were carboplatin and paclitaxel (74%), and cisplatin and paclitaxel (26%). The radiation therapy dosage was 41.4 cGy (73%), 45 cGy (16%), or 50.4 cGy (11%).
Ryan score was associated with histology. Squamous cell carcinoma was more likely to achieve Ryan score 1 than adenocarcinoma (p < 0.001). The clinical pretreatment stage was also correlated to Ryan score, and early disease (cStage I or II) was more likely to show pathological response to neoadjuvant therapy (Table 2).
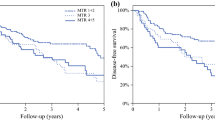
Ryan score could predict long-term survival. Ryan score 1 was more likely to evidence overall survival and disease-free survival than Ryan 2 or 3 (p < 0.001). Ryan score 3 was more likely to show recurrence (p < 0.001), mainly systemic (p = 0.045) and lymph nodal recurrence (p = 0.027), but was not related to higher probability of locoregional (p = 0.279) or peritoneal recurrence (p = 0.126) (Table 2; Figs. 2, 3).
Ryan score (three-tier system) was not different from modified Ryan score (four-tier system) in terms of prognosis. Modified Ryan score 1 was not different than score 2, both for overall survival [hazard ratio (HR) 0.53; 95% confidence interval (CI) 0.16–1.79; p = 0.308] and for disease-free survival (HR 0.62; 95% CI 0.21–1.79; p = 0.372) (Table 3).
Discussion
The three-point system described by Ryan et al., initially proposed for application in rectal cancer,8 has shown to be correlated with prognosis and can be an alternative to evaluate TRG in esophageal cancer. Other studies have also shown an association of TRG and prognosis using different TRG score systems, although they used heterogenic neoadjuvant therapies.3,4,5,11
Statistical analysis of our sample showed that Ryan score shows association with overall survival and disease-free survival, with statistical significance. Patients who had complete pathological response (score 1) had considerably longer overall survival than those with persistent neoplasm in the surgical specimen (score 2 or 3). Also, patients with inadequate response to neoadjuvant therapy and classified as Ryan 3 had worse survival, with 100% mortality in up to 48 months of follow-up.
Ryan score also showed association with recurrence rate, especially for systemic and lymphatic metastasis. However, there was no statistically significant association between Ryan score and locoregional or peritoneal recurrence.
This study evidences that pathological response tends to be better among patients with earlier disease. Most of the patients classified as Ryan 1 were at pretreatment clinical stages I (all T1N1) or II, while most of the patients classified as Ryan 3 were at advanced disease stages (stages III and IV). As a result, the disease tends to assume more or less aggressive behavior as per the pathological response: Those with better pathological response (Ryan 1) tend to show less risk for lymph node metastasis and distant metastasis than patients with inadequate response (Ryan 3). These results may be due to the tumor volume, which may influence the radiotherapy outcome.12
As in other works,13,14 the results of this study indicate that the dose of radiation used in the neoadjuvant protocol did not show association with TRG. Consequently, lower-radiation regimens, such as 41.4 cGy, would avoid the potential risk of toxicity and would not interfere with the Ryan score. Of note, our data show that 11 of 15 (73.3%) patients who were treated with 50.4 Gy had significant tumor response (Ryan 1), although this was not statistically significant, possibly due to the small sample size.
The interval from completion of neoadjuvant therapy to surgery was not related to Ryan score, although some studies observed a tendency concerning higher pathological complete response in patients with longer time interval, and moreover a higher probability of response in adenocarcinoma15,16 than squamous cell carcinoma.17 In our study, long queues for surgical procedures at the institute led to relatively long interval, however the median was close to 12 weeks, as suggested in recent trials.
As in the CROSS trial,2 our sample also showed higher pathological response in patients with squamous cell carcinoma (SCC), when compared with those with adenocarcinoma, although the CROSS trial used Mandard score to evaluate pathological specimens.5 In this study, more than 90% of the patients classified as Ryan 1 were diagnosed with SCC.
The use of Ryan score for esophageal cancer and its association with overall survival, disease-free survival, and recurrence of disease is currently unprecedented. Ryan score enables easier and more clear-cut scoring than other scores and may predict survival and recurrence. However, the results of this study should be interpreted in the context of certain inherent limitations. The survey was conducted at a single center, retrospectively, and with a relatively low number of included patients. Controlled prospective studies, with larger samples, and studies evaluating interexaminer concordance, are expressly warranted to validate Ryan score for esophageal cancer. Also, future studies should evaluate different neoadjuvant regimens other than platinum and taxane based, as well as different radiation field setting and different type of surgery, and also compare neoadjuvant therapy and definitive intention chemoradiotherapy.
Conclusions
Despite all its limitations, this study is the first to assess the prognostic value of Ryan score for esophageal cancer, in the setting of trimodal therapy with neoadjuvant chemoradiotherapy with platinum- and taxane-based regimen followed by curative-intent esophagectomy. Ryan score predicts survival and recurrence rates.
References
Van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Mota FC, Cecconello I, Takeda FR, Tustumi F, Sallum RAA, Bernardo WM. Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. Int J Surg. 2018;54(Pt A):176–81.
Lerttanatum N, Tharavej C, Chongpison Y, Sanpavat A. Comparison of tumor regression grading system in locally advanced esophageal squamous cell carcinoma after preoperative radio-chemotherapy to determine the most accurate system predicting prognosis. J Gastrointest Oncol. 2019;10(2):276–82.
Andreollo NA, Beraldo GC, Alves IPF, Tercioti-Junior V, Ferrer JAP, Coelho-Neto JS, et al. Pathologic complete response (ypT0 ypN0) after chemotherapy and radiotherapy neoadjuvant followed by esophagectomy in the squamous cell carcinoma of the esophagus. Arq Bras Cir Dig. 2018;31(4):e1405.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6.
Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–55.
Schneider PM, Baldus SE, Metzger R, Kocher M, Bongartz R, Bollschweiler E, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005;242(5):684–92.
Ryan R, Gibbons D, Hyland JM, Treanor D, White A, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–6.
Kim SH, Chang HJ, Kim DY, Park JW, Baek JY, Kim SY, et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat. 2016;48(3):998–1009.
Rice TW, Patil DT, Blackstone EH. 8th Edition AJCC/UICC Staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–30.
Takeda FR, Viyuela MS, da Cruz Junior JB, Tustumi F, Braghiroli OFM, Karolyne Ernesto Luiz Nobre KEL, et al. Variables associated to pathologic complete response, overall survival and disease-free survival in the neoadjuvant setting for esophageal cancer: a retrospective cohort analysis. Int Surg. 2018;103:214–21.
Dubben HH, Thames HD, Beck-Bornholdt HP. Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol. 1998;47(2):167–74.
Ji KSY, Thomas SM, Roman SA, Czito B, Anderson KL Jr, Frakes J, Adam MA, et al. Low- vs. high-dose neoadjuvant radiation in trimodality treatment of locally advanced esophageal cancer. J Gastrointest Surg. 2019;23(5):885–94. https://doi.org/10.1007/s11605-018-4007-3. Epub 2018 Oct 29.
Buckstein M, Rhome R, Ru M, Moshier E. Neoadjuvant chemoradiation radiation dose levels for surgically resectable esophageal cancer: predictors of use and outcomes. Dis Esophagus. 2018;31(5).
van der Werf LR, Dikken JL, van der Willik EM, van Berge Henegouwen MI, Nieuwenhuijzen GAP, Wijnhoven BPL; Dutch Upper Gastrointestinal Cancer Audit (DUCA) group. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. Eur J Cancer. 2018;91:76-85.
Shapiro J, van Hagen P, Lingsma HF, Wijnhoven BP, Biermann K, ten Kate FJ, Steyerberg EW, van der Gaast A, van Lanschot JJ; CROSS Study Group. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg. 2014;260(5):807–13; discussion 813–4.
Chiu CH, Chao YK, Chang HK, Tseng CK, Chan SC, Liu YH, Chen WH. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol. 2013;20:4245–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There are no relevant conflicts of interest or disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takeda, F.R., Tustumi, F., de Almeida Obregon, C. et al. Prognostic Value of Tumor Regression Grade Based on Ryan Score in Squamous Cell Carcinoma and Adenocarcinoma of Esophagus. Ann Surg Oncol 27, 1241–1247 (2020). https://doi.org/10.1245/s10434-019-07967-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07967-8