Abstract
Background
Neoadjuvant chemotherapy (NAC) represents a promising alternative to pancreatic ductal adenocarcinoma (PDAC) planned resection, but the survival impact remains undefined. To assess the feasibility and survival outcomes of NAC with gemcitabine and S1 (GS) for PDAC planned resection by prospective study.
Methods
Patients with resectable or borderline resectable PDAC received 2 cycles of NAC-GS and were offered curative resection followed by gemcitabine adjuvant. The primary endpoint was 2-year overall survival (OS). Adverse events during NAC, radiological and tumor marker responses, resection rate, and surgical safety were evaluated as secondary endpoints (UMIN000004148).
Results
We enrolled 104 patients between 2010 and 2012, with 101 patients treated using NAC-GS as the full analysis set (FAS). Of the 101 patients, 88% received the planned 2 cycles of NAC. Grade 3 neutropenia was common (35%). Radiological partial response and decreased carbohydrate antigen 19-9 concentration (> 50% decrease) were noted in 13% and 41%, respectively. R0/1 resections with M0 were performed in 65 patients without surgical mortality. Of the 65 patients, 44 received planned gemcitabine adjuvant for 6 months as the on-protocol cohort. The primary endpoint for the 2-year OS rate was 55.9% in the FAS (n = 101) and 74.6% in the on-protocol cohort (n = 44).
Conclusions
NAC-GS was feasible and actively prolonged survival following PDAC planned resection. Randomized control trials are needed to further clarify the survival benefit of NAC-GS in addition to surgery followed by adjuvant therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survival rates of patients with pancreatic ductal adenocarcinoma (PDAC) have improved only marginally during the last 30 years, with the 5-year survival rate of only 8 % [1] attributable to late clinical manifestation and the systemic nature of the disease at presentation. Recent advances in systemic chemotherapy have improved median survival for metastatic PDAC [2], but long-term survival remains rare. The only chance for long-term survival is complete resection for localized PDAC [3]. Estimated 5-year survival rates following resection alone are around 10% [4,5,6]. Resection alone provides cure for only a small population with PDAC, and systemic therapy is now typically added to surgery. Postoperative adjuvant chemotherapy has been tested and has shown survival improvements in a series of phase III trials [5,6,7]. Results from the JASPAC-01 trial were recently reported, showing promising survival benefit for adjuvant S1 monotherapy over adjuvant therapy with gemcitabine [8]. More recently, the ESAPC-04 trial showed that the adjuvant combination of gemcitabine and capecitabine was significantly superior to adjuvant gemcitabine [9].
The outcome of initial resection followed by adjuvant shows several limitations in terms of clinical interpretation. First, up to 30% of clinically resectable tumors cannot actually be resected due to undetected metastases or underestimated tumor invasion to peri-pancreatic major vessels at the time of surgery [10]. Second, up to 25% of patients cannot receive adjuvant therapy because of poor postoperative performance status following surgical morbidity [11]. Neither group of patients is included in adjuvant trials due to ineligibility, so the improved overall survival (OS) described in recent randomized controlled trials [5,6,7] does not hold true for all patient with clinically resectable (R) or borderline resectable (BR) disease for which initial resection is planned.
Neoadjuvant treatment offers several theoretical advantages over upfront surgery followed by adjuvant, including early delivery of systemic therapy for almost all patients, high tolerance of multi-agent regimens by patients, a higher negative margin resection rate and decreased nodal involvement, leading to improved survival. Conversely, neoadjuvant treatment carries a risk of disease progression during therapy if the tumor biology is unfavorable and/or treatment is ineffective [12]. A review of select trials for patients with localized pancreas cancer has suggested a benefit for neoadjuvant therapy by showing an increased median survival time and potentially higher resectability rates with neoadjuvant treatment [13, 14].
The phase II trial reported herein evaluated the efficacy of neoadjuvant therapy with gemcitabine and S1 (GS) in patients with R or BR PDAC. The rationale behind this regimen was based on a phase III trial that showed significantly longer progression-free survival and a higher objective response rate for GS therapy than for gemcitabine monotherapy [15]. In addition, pooled analysis of GS therapy for locally advanced pancreatic cancer reported improved survival compared to gemcitabine alone [16]. A previous study of GS therapy in the neoadjuvant setting showed acceptable feasibility and a high R0 resection rate [17]. We, therefore, undertook this non-randomized phase II study of GS in patients with radiographically R or BR PDAC.
Patients and methods
Eligibility criteria and patient evaluation
Between October 2010 and September 2012, a total of 104 patients from 12 participating institutions were enrolled in this trial. Inclusion criteria were as follows: (1) newly diagnosed invasive PDAC with histological or cytological confirmation; (2) age > 20 years; (3) Eastern Cooperative Oncology Group performance status, 0–1; (4) complete history and physical examination, and staging evaluation requiring multidetector-row computed tomography (MD-CT); (5) no distant metastases; (6) tumor considered as potentially or borderline resectable; (7) no previous antitumor treatment other than biliary drainage; and (8) tolerable curative resection with adequate hematological, hepatic, renal, and cardiopulmonary functions. Tumor with encasement of the porto-mesenteric vein and/or abutment of major arteries (hepatic or mesenteric artery) within 180° was defined as borderline. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of each participating hospital. The registration number of this clinical trial is UMIN000004148.
Study design and treatment regimen
This study was designed as a multi-institutional, open-label, single-arm phase II trial of neoadjuvant GS in patients with radiographically R or BR PDAC. The primary endpoint was 2-year OS. Secondary endpoints included adverse event, dose intensity, resection rate, residual tumor (R), nodal involvement (N), recurrence-free survival, and tumor marker response. Serum tumor marker response was determined by comparing pretreatment and preoperative concentrations of carbohydrate antigen (CA)19-9. In the patients with biliary obstruction, pretreatment bilirubin level was recorded as a total bilirubin level < 3.0 mg/dL after biliary drainage. Gemcitabine was provided at a dose of 1000 mg/m2 on days 1 and 8 of each cycle. Oral S-1 was administered twice daily at a dose of 40 mg/m2 for the first 14 consecutive days, followed by a 7-day rest. Each cycle was repeated every 21 days. Patients received two cycles of this regimen. During preoperative treatment, an interim medical history was elicited and patients underwent physical examination and laboratory studies. Toxicity of the treatment was evaluated using Common Toxicity Criteria (CTCAE, version 3.0). Relative dose intensity for each individual drug was calculated and defined as the dose intensity achieved relative to the standard schedule for each drug. After completion of neoadjuvant therapy, all patients underwent restaging studies with MD-CT to assess resectability, and surgery was planned to occur at 1–6 weeks after completing GS. Patients who were deemed to have unresectable disease showing local progression or distant metastasis were considered as off-protocol and further treatment was provided as recommended by the treating institution. Information regarding surgery after protocol completion included the type and duration of operation, estimated blood loss, complications, and 30-day mortality rate. Designated pathologists at each institution examined resected specimens, and reviews included size of the primary tumor, resection margins, and lymph node status. Tumor grade and stage were reported according to the American Joint Committee on Cancer staging. Post-resectional serum tumor marker (CA19-9) was assessed in all patients who underwent R0/1 resection with M0. To compare the results of gemcitabine adjuvant obtained CONKO-001 study [6], patients with postoperative CA19-9 < 2.5 times the upper normal limit of normal (ULN) and recovery sufficient for chemotherapy within 10 weeks after surgery, who were excluded from CONKO-001 study [6], defined as the on-protocol cohort, received six cycles of standard adjuvant gemcitabine (1000 mg/m2 on days 1, 8, and 15, every 4 weeks). Patients with R2 resection, M1, postoperative CA19-9 > 2.5 times ULN, or insufficient recovery within 10 weeks after surgery were considered off-protocol and received adequate treatment in each institution. Following completion of therapy, all patients were followed up with MD-CT and determination of tumor marker concentrations every 3 months for the first 2 years, then every 6 months for years 3–5 (detail protocol is provided as Supplemental material).
Biostatistical plan
All eligible patients were included in the intention-to-treat (ITT) population for efficacy analyses. The primary endpoint of 2-year OS was evaluated both for the ITT population and on-protocol patients. The historical 2-year survival of upfront surgery followed by standard adjuvant gemcitabine was 47.5% [6]. The sample size calculation was based on the assumption that the 2-year OS of on-protocol patients (R0/1 resection with M0, postoperative CA19-9 ≤ 2.5 times ULN, and sufficient recovery within 10 weeks after surgery) would be 45% (null survival probability) to 65% (alternative survival probability). A sample size of 41 on-protocol patients was required to detect an improvement in 2-year OS, with a bilateral 5% type I error and a power of 90%. A total sample size of 90 patients was required, assuming: (1) 30% unresectability including M1; (2) 10% sustained elevation of CA19-9 at > 2.5 times the ULN; and (3) 15% insufficient recovery after surgery (Fig. 1). OS was defined as the duration from provision of written consent to the protocol to death, and was estimated using the Kaplan–Meier method, with Greenwood’s formula used to calculate the standard error of the Kaplan–Meier estimate and the 95% confidence interval. For patients who underwent resection, recurrence-free survival was defined as the time from surgery to first recurrence or death, whichever occurred first. Variables were compared using Student’s t test by JMP version 10.0 software (SAS Inc. Cary, NC, USA).
Schematic diagram of the pathway to the study hypothesis. Incompletion due to the operative findings* means distant metastases or unresectable local extent of the tumor diagnosed at the time of surgery. The 2-year OS for the on-protocol cohort with upfront surgery was reported as 47.5% according to the CONKO-01 study [6]. The 2-year OS for the on-protocol cohort with the neoadjuvant strategy was estimated from 45% as the null probability to 65% as the alternative probability
Results
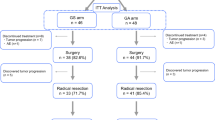
This trial enrolled 104 patients between 2010 and 2012. Three patients were excluded: one showed metastases before starting the protocol; one had intraductal papillary-mucinous non-invasive carcinoma confirmed from a resected specimen; and one patient did not meet the starting criteria for the protocol. As a result, 101 patients received the protocol-specified neoadjuvant therapy (full analysis set). Median age was 68 years, with 58 men (57%) and 80 patients (79%) showing the primary tumor located in the head of the pancreas. Patient flow for the study is illustrated in Fig. 2 and demographic information is summarized in Table 1.
Feasibility of neoadjuvant therapy
Of the 101 patients who started neoadjuvant treatment, 89 (88%) completed the planned 2 cycles of GS chemotherapy. Twelve patients (12%) received incomplete planned intervention related to adverse events. All patients were assessable for adverse events. Neoadjuvant treatment-related adverse events are listed in Table 2. In terms of hematological G3/4 toxicity, neutropenia was commonly noted for both all-grade (n = 58; 57%) and for grade 3/4 (n = 35; 35%) events. Nausea and/or anorexia (28%), and skin rash (20%) were observed relatively in common. Severe non-hematological toxicity (≥G3) was infrequent, namely, skin rash (4%), nausea/vomiting (2%), fatigue (1%), mucositis oral (1%), and cholangitis (1%).
Radiological and tumor marker response
Radiologically, objective tumor response was observed with 13 (13%) partial responses. The majority of patients showed stable disease (73%) and 10 patients (10%) displayed disease progression. Median tumor reduction rate was 9% (range, − 67 to 90%) as shown by waterfall plot (Fig. 3a). A serum CA19-9 decrease after neoadjuvant treatment was seen in 78%, with a median decrease of 38% (range, − 300 to 97.7%) as shown by waterfall plot (Fig. 3b). CA19-9 decrease > 50% was observed in 41%.
Surgical and pathological outcomes
Following neoadjuvant treatment, 85 patients proceeded to surgery. Surgery was not performed for 16 patients due to disease progression (n = 12) or patient refusal (n = 4). Of those 85 patients, 11 did not undergo resection. In 74 resections, 3 patients underwent macroscopic incomplete resection (R2, n = 3). In 71 patients with macroscopic curative resection (R0/1), 6 patients underwent locally R0 resection but with positive peritoneal cytology (n = 5), and para-aortic nodal metastases (n = 1). R0/1 with M0 resection was performed for 65 patients including 61 R0 resections and 4 R1 resections (Fig. 1). Local R0 resection was performed for 67 patients (including 6 patients with M1), comprising 91% of resections (n = 74). Postoperative morbidity was observed in 39%, without surgical mortality. Pancreatic fistula was noted in 9 patients (12%). Surgical procedures and perioperative outcomes are summarized in Table 3. In subset analysis according to the resectability status, blood loss was significantly greater in patients with BR tumor than in patients with R tumor (p = 0.0080). Vascular resection was required more frequently in the resection for BR tumor than in the resection for R tumor (p = 0.043). Although no significant difference was observed for resection rate in both subsets, R0 resection rate for R tumors was significantly higher than that for BR tumors (p = 0.029).
Planned adjuvant therapy
Postoperative tumor markers were assessed for planned adjuvant therapy for patients with R0/1 with M0 resection (n = 65), consisting of 60 patients from the completion of neoadjuvant treatment (n = 89) and 5 patients from the incomplete set of neoadjuvant treatment (n = 12). Sustained elevation of CA19-9 at > 2.5-fold above the ULN (> 92.5 U/ml) within 2 months after resection was recorded in 8 patients. These patients received chemotherapy on an off-protocol basis. Of the 57 patients planned to receive adjuvant gemcitabine for 6 months, 13 patients did not receive adjuvant therapy due to incomplete recovery after surgery (n = 8) or patient refusal (n = 5). A final total of 44 patients started the planned adjuvant therapy as the on-protocol cohort (Fig. 2). In the on-protocol cohort, 30 patients received 4 and over cycles of gemcitabine adjuvant (26 patients received full cycles), but remaining 14 patients received less than 4 cycles of adjuvant.
Survival and patterns of failure
The cutoff for data analysis was September 18th, 2015. Median duration of follow-up was 22 months. The primary endpoint of the 2-year OS was 55.9% in the FAS (n = 101) and 74.6% in the on-protocol cohort (n = 44). Median OS in the FAS was 30.8 months (95% confidence interval, 20–43 months). The 2-year survival rate of the off-protocol cohort (n = 57) was 40.9%, significantly lower than that in the on-protocol cohort (Fig. 4, p < 0.0001). In subset analysis according to the resectability status, median OS of the patients with R PDAC (n = 63, 39.2 months) was longer, but not statistically significant, than that of the patients with BR PDAC (n = 38, 21.1 months) by intention-to-treat analysis (p = 0.35). In the on-protocol cohort, the 2-year OS of R PDAC was 78.9%, which was higher than that of BR PDAC (66.7%). The difference was also not statistically significant (Supplemental Fig. 1, p = 0.70). Although completion or not of adjuvant did not affect the survival outcome for on-protocol cohort significantly (p = 0.22), 2-year OS of the patients receiving 4 and over cycles of adjuvant gemcitabine (n = 30) was significantly higher than that of the patients receiving less than 4 cycles of adjuvant gemcitabine (n = 14, p = 0.0067) in the on-protocol cohort (Supplemental Fig. 2). At the time of analysis, 48 patients experienced recurrence. Median recurrence-free survival was 15.8 months. Frequent patterns of recurrence were hepatic (n = 17), locoregional (n = 17), peritoneal (n = 9), and pulmonary (n = 11) recurrence.
Overall survival curve for PDAC planned NAC-GS. Overall survival in the on-protocol cohort (n = 44, solid line) was significantly longer (p < 0.0001) than that in the off-protocol cohort (n = 57, broken line). The 2-year OS and median OS in FAS (n = 101, bold line) was 55.9% and 30.8 months, respectively
Discussion
Although several reports have suggested benefits of neoadjuvant therapy for pancreatic cancer [13, 14, 18], the standard strategy for patients with pancreatic cancer for which resection is planned remains upfront surgery followed by postoperative adjuvant treatment, as described in the guidelines [19, 20]. To obtain definitive evidence for the benefits for neoadjuvant therapy, accurate comparison of efficacy and feasibility between neoadjuvant and upfront surgery is needed. Several obstacles exist to comparisons of outcomes between upfront surgery and neoadjuvant strategy. First, most of the published literature pertaining to neoadjuvant therapy has involved small, single-center case series, with insufficient data for comparison with upfront surgery [13, 14, 18]. Second, many of the studies with neoadjuvant therapy have included patients of unresectable disease before treatment, representing inadequate candidates for upfront surgery. Third, the most important difficulty in comparison is the difference in participants of both strategies. Participants in studies with postoperative adjuvant therapy were patients with completely resected PDAC following full recovery after surgery [5,6,7,8,9]. Since neoadjuvant treatment is applied to patients with PDAC for which resection was planned (before surgery), both patients diagnosed as unresectable at the time of surgery and patients with insufficient recovery from surgery (off-protocol cohort) are included in ITT analysis. Although the completion rate of neoadjuvant part in this study was 88%, which was high enough to accept the main part of this protocol, the completion rate of total protocol treatment including neoadjuvant and surgery was apparently low (44%). That was because the patients must be enrolled before selection by surgery. With regard to upfront surgery, as well as neoadjuvant intervention, which is applied to PDAC planned resection, most reports have described outcomes for resected patients [21, 22], representing only the on-protocol cohort of the upfront surgery strategy. Freiss et al. [10] found that only 70% of patients with PDAC planned resection underwent curative surgery, and resection was not possible intraoperatively for the remaining 30%. A similar result was obtained from a prospective study in which the resection rate for planned resection of pancreatic head adenocarcinoma was 56–67% [23]. In the view point of intention-to-treat for PDAC planned resection, recent advances in adjuvant trials could influence survival only in selected patients such as those with resected and fully recovered PDAC. The benefit of adjuvant therapy might be limited for the off-protocol cohort, including patients with unresected tumor at the time of surgery or insufficient recovery after surgery.
Our phase II trial explored the efficacy of neoadjuvant therapy with GS in patients with R or BR PDAC. This study met the primary endpoint in that 2-year OS of the on-protocol cohort reached 74.6%, superior to the result obtained from previously reported trials with adjuvant gemcitabine [6, 7]. Moreover, the 2-year OS of the FAS (ITT), which included the off-protocol cohort with poor prognosis, reached 55.9%, comparable to the survival outcome of gemcitabine adjuvant trials [6, 7]. Adjuvant treatment still contributed the favorable outcome for the patients of curatively resected PDAC after NAC. The result that the survival of patients after NAC was significantly improved by 4 and over cycles of adjuvant (≥ 50% of planned treatment) also suggested that NAC might complement the adjuvant treatment as a part of perioperative therapy (Supplemental Fig. 2). Perioperative factors were also evaluated for surgical feasibility after neoadjuvant treatment. Surgical outcomes after NAC-GS were comparable with previously reported findings for upfront surgery [24, 25]. No significant increase was observed for morbidities, including specific complications such as pancreatic fistula and mortality (Table 3). As shown by a multi-center survey comparing short-term outcomes [26], neoadjuvant therapy might not increase mortality and morbidity rates, supported by the multi-institutional setting. These results obtained from a single-arm, but representing the largest prospective cohort (n = 101) for neoadjuvant chemotherapy against PDAC, demonstrated that NAC-GS prior to surgery might be feasible and prolong survival for PDAC planned resection. As well as other active regimens including gemcitabine with oxaliplatin [27] or gemcitabine with nab-paclitaxel [28], and FOLFIRINOX [29], NAC-GS [17] should be tested in further investigations.
In summary, this trial showed that neoadjuvant treatment with GS was active and provided survival benefits for R and BR PDAC. The findings from this study are suitably encouraging for us to conduct further trials exploring the efficacy of NAC-GS. We have conducted a multi-center randomized controlled trial for R and BR PDAC to demonstrate the superiority of NAC-GS against upfront-surgery (Prep-02/JSAP05, UMIN-CTR#000009634).
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Saung MT, Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin Ther. 2017;39:2125–34.
Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80.
Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–75.
Neoptolemos JP, Stocken DD, Freiss H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;351:1200–10.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–56.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Friess H, Kleeff J, Silva JC, et al. The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg. 1998;186:675–82.
Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–62.
D’Angelo F, Antolino L, Farcomeni A, et al. Neoadjuvant treatment in pancreatic cancer: evidence-based medicine? A systematic review and meta-analysis. Med Oncol. 2017;34:85.
Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–8.
Yanagimoto H, Ishii H, Nakai Y, et al. Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci. 2014;21:761–6.
Motoi F, Ishida K, Fujishima F, et al. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreaticductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. 2013;20:3794–801.
Gillen S, Schuster T, Zum Büschenfelde CM, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Pancreatic Adenocarcinoma Version 3. 2017. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 19 Oct 2017.
Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–68.
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210.
Luberice K, Downs D, Sadowitz B, et al. Has survival improved following resection for pancreatic adenocarcinoma? Am J Surg. 2017;214:341–6.
Eshuis WJ, van der Gaag NA, Rauws EA, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840–9.
Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265–73.
Kang MJ, Jang JY, Kim SW. Surgical resection of pancreatic head cancer: what is the optimal extent of surgery? Cancer Lett. 2016;382:259–65.
Motoi F, Unno M, Takahashi H, et al. Influenceof preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: project study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:148–58.
OʼReilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260:142–8.
Ielpo B, Duran H, Diaz E, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394–400.
Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22:1153–9.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research 24592018 from the Japan Society for the Promotion of Science. We would like to express sincere appreciation to Drs. M. Kurata, H. Yanagimoto, H. Toyama, Y. Nagakawa, K. Maemura, Y. Mataki, T. Akahori, S. Kinoshita, H. Terashima, A. Horiguchi, Y. Ohtsuka, A. Nanashima, K. Kanemitsu, H. Ohigashi, M. Tani, T. Takahara, H. Shiomi, I. Endo, H. Suzuki, T. Rikiyama, H. Ikoma, M. Yasunaga, K. Nakamura, S. Egawa, Y. Katayose, K. Nakagawa, K. Okada, and S. Ottomo as clinical investigators in the study group of preoperative therapy for pancreatic cancer (PREP).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2018_1506_MOESM1_ESM.pptx
Supplemental Figure 1. Overall survival curve for PDAC planned NAC-GS according to the resectability status. 1A. Survival comparison by intention-to-treat analysis. Median OS of the patients with R PDAC (n=63, solid line) was 39.2 months, which was longer but not significant than that of the patients with BR PDAC (n=38, 21.1 months, broken line)(p=0.35). The 2-year OS of R and BR PDAC were 60.4% and 48.6%, respectively. 1B. Survival comparison by on-protocol analysis. The 2-year OS of R PDAC (n=29, solid line) and BR PDAC (n=15, broken line) were 78.9% and 66.7%, respectively (p=0.70) (PPTX 123 kb)
535_2018_1506_MOESM2_ESM.pptx
Supplemental Figure 2. Overall survival curve for PDAC planned NAC-GS according to cycles receiving postoperative adjuvant treatment. 1A. Survival comparison of on-protocol cohort with or without completion of postoperative adjuvant treatment. The 2-year OS of the patients receiving 6 cycles (n=26, solid line) and less than 6 cycles (n=18, broken line) of gemcitabine adjuvant were 84.4% and 60.6%, respectively (p=0.22). 2B. Survival comparison of on-protocol cohort with 4 and over cycles or less than 4 cycles of postoperative adjuvant treatment. The 2-year OS of the patients receiving 4 and over cycles (n=30, solid line) and less than 4 cycles (n=14, broken line) of gemcitabine adjuvant were 86.5% and 49.0%, respectively (p=0.0067) (PPTX 114 kb)
Rights and permissions
About this article
Cite this article
Motoi, F., Satoi, S., Honda, G. et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol 54, 194–203 (2019). https://doi.org/10.1007/s00535-018-1506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1506-7